Abstract
Characteristics and gel properties of sardine and mackerel surimi produced by conventional washing process and alkaline solubilising process were investigated. The decrease in Ca2+-ATPase activity with the changes in the surface hydrophobicity was found in surimi produced by alkaline solubilising process (p<0.05), suggesting the denaturation of protein induced by this process. The alkal-ine solubilising process with prewashing could remove myoglobin most effectively from sardine muscle, whereas the process without prewashing resulted in the greatest myoglobin removal in mackerel muscle (p<0.05). Surimi conventionally prepared by water or NaCl washing showed the gel with greater breaking force and deformation than that from alkaline solubilising process (p<0.05). The hig-her expressible moisture was found in the gels of surimi pre-pared by alkaline process, indicating the poor water holding capacity of the gel matrix. The highest whiteness was found in the gel of sardine surimi produced by alkaline process with prewashing but the highest whiteness was obtained in the gel of mackerel surimi washed with distilled water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of under-utilised small pelagic fish species, such as sardine and mackerel, for surimi production is limited, mainly due to the large quantity of lipids and myoglobin in the muscle tissue. Generally, high-quality surimi with the improved gel strength and whiteness can be obtained when dark muscle is removed as much as possible [1]. However, abundant dark muscle in red-fleshed fish such as mackerel and sardine is difficult to remove with a meat separator [1]. Basically, the washing process is necessary for colour improvement and gel strengthening of surimi produced from whole muscle. Conventional surimi production is aimed to concentrate myofibrillar proteins by removing sarcoplasmic proteins, fat, blood and pigments through continuous washing of the fish mince. Recently, Chaijan et al. [2] reported that the lowest residual myoglobin content and the best colour improvement were found in the minces washed with 0.2% NaCl (w/v) and 0.5% NaCl (w/v) for sardine and mackerel minces, respectively.
A new approach to obtain the functional protein isolates from dark muscle fish has been developed by Hultin and Kelleher [3]. Acid or alkaline solubilising process potentially overcomes some of the problems caused by the nature of the pelagic species [4]. The recovered proteins from these processes retain their functionality including their ability to form a gel. This process consists of isolating the protein component of fish muscle tissue by acid or alkaline and subsequent precipitating of all soluble proteins at their isoelectric point [5–7]. Gels prepared from rockfish and Atlantic croaker solubilised proteins at alkaline pH exhibited better gel quality than those prepared from the acid-aided process [6, 7]. To improve the colour of dark-fleshed mince, the alkaline solubilisation process, especially in combination with prewashing, can be an alternative means to enhance the removal of myoglobin associated with the flesh tissue. The present study aimed to investigate the effect of alkaline solubilising process and conventional process on the physicochemical properties, gel-forming ability and myoglobin content of surimi from sardine and mackerel whole muscles.
Materials and methods
Chemicals
Sodium dodecyl sulphate (SDS), dithiothreitol (DTT) and β-mercaptoethanol (βME) were purchased from Sigma (St. Louise, MO, USA). Trichloroacetic acid was obtained from Merck (Darmstadt, Germany). Acrylamide, N,N,N′,N′-tetramethylethylenediamine (TEMED) and bis-acrylamide were obtained from Fluka (Buchs, Switzerland). Sodium dithionite was purchased from Riedel (Seeize, Germany).
Fish samples
Sardine (Sardinella gibbosa) with an average weight of 55–60 g and mackerel (Rastrelliger kanagurta) with an average weight of 85–90 g were caught from Songkhla-Pattani Coast along the Gulf of Thailand during March and April, 2004. The fish, off-loaded approximately 12 h after capture, were placed in ice with a fish/ice ratio of 1:2 (w/w) and transported to the Department of Food Technology, Prince of Songkla University, Hat Yai within 2 h. The fish were immediately washed and filleted. The muscle was kept on ice during preparation and analysis. The pH values of sardine and mackerel muscle were 6.53–6.58 and 6.24–6.27, respectively.
Surimi and surimi gel preparation
To prepare surimi by the conventional washing process (CWPS), fish mince was washed with cold distilled water (4°C) or cold NaCl solution (0.2% NaCl (w/v) for sardine and 0.5% NaCl (w/v) for mackerel) [2] using a washing media/mince ratio of 3: 1 (v/w). The mixture was stirred gently for 10 min in a cold room (4°C) and the washed mince was filtered with a layer of nylon screen. Washing was performed for three times. Finally, the washed mince was centrifuged at 700×g for 15 min using a basket centrifuge (Model CE 21K, Grandiumpiant, Belluno, Italy). To produce the surimi by alkaline solubilisation process (ASPS), the method of Undeland et al. [4] was used. The mince (250 g) was homogenised for 1 min with 2,250 ml of cold distilled water (4°C) using an IKA homogeniser (Selangor, Malaysia). The homogenate was adjusted to the pH of 10.8 using 2N NaOH. The soluble proteins were then precipitated by adjusting the pH to 5.5 using 2N HCl. Precipitated proteins were collected and their pH was adjusted to 7.0 using 2N NaOH. Both CWPS and ASPS were added with 4% sucrose and 4% sorbitol, mixed well and frozen using an air-blast freezer. The frozen samples were kept at −18°C until used. The storage time was not more than 1 month.
To prepare the gels, the frozen surimi samples were thawed at 4°C until the core temperature reached 0°C. The samples were then cut into small pieces and the moisture content was adjusted to 80%. The samples were added with 2.5% (w/w) NaCl and chopped for 5 min in a walk-in cold room at 4°C to obtain the homogeneous sol. The sol was then stuffed into polyvinylidine casing with a diameter of 2.5 cm and both ends of the casing were sealed tightly. The sol was then incubated at 40°C for 30 min, followed by heating at 90°C for 20 min [2]. The gels were cooled in iced water and stored for 24 h at 4°C prior to analysis.
Determination of Ca2+-ATPase activity
The Ca2+-ATPase activity of natural actomyosin (NAM) from unwashed mince and different surimi was determined according to the method of Benjakul et al. [8]. NAM prepared as described by Benjakul et al. [8] was diluted to 2.5–8 mg/ml with 0.6 M KCl, pH 7.0. Diluted NAM solution (1 ml) was added to 0.6 ml of 0.5 M tris-maleate, pH 7.0. The mixture was added with 1 ml of 0.1 M CaCl2. Deionised water was added to make up a total volume of 9.5 ml. A 0.5 ml of 20 mM adenosine 5′-triphosphate (ATP) solution was added to initiate the reaction. The reaction was conducted for 8 min at 25°C and terminated by adding 5 ml of chilled 15% (w/v) trichloroacetic acid. The reaction mixture was centrifuged at 3,500×g for 5 min and the inorganic phosphate liberated in the supernatant was measured by the method of Fiske and Subbarow [9]. The Ca2+-ATPase activity was expressed as μmoles inorganic phosphate released/mg protein/min. A blank solution was prepared by adding chilled trichloroacetic acid prior to addition of ATP.
Determination of surface hydrophobicity
Surface hydrophobicity of NAM was determined as described by Benjakul et al. [8] using 1-anilinonaphthalene-8-sulphonic acid (ANS) as a probe. NAM dissolved in 10 mM phosphate buffer, pH 6.0 containing 0.6 M NaCl was diluted to 0.1, 0.2, 0.3, and 0.5% (w/v) protein using the same buffer. The diluted protein solution (2 ml) was added with 10 μl of 8 mM ANS in 0.1 M phosphate buffer, pH 7.0. The fluorescence intensity of ANS-conjugates was measured using a RF-1501 spectrofluorophotometer (Shimadzu, Kyoto, Japan) at an excitation wavelength of 374 nm and an emission wavelength of 458 nm. The initial slope of the plot of fluorescence intensity versus NAM concentration was referred to as surface hydrophobicity (SoANS).
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE)
Protein patterns of different surimi and unwashed mince were analysed using SDS-PAGE according to the method of Laemmli [10]. To prepare the protein sample, 27 ml of 5% (w/v) SDS solution were added to the sample (3 g). The mixture was homogenised for 1 min. The homogenate was incubated at 85°C for 1 h to dissolve total proteins. The sample was centrifuged at 8,500×g for 5 min at room temperature (26–28°C) using a Biofuge primo centrifuge (Sorvall, Hanau, Germany). Protein concentration was determined according to the Biuret method [11], using bovine serum albumin as a standard. Protein samples (20 μg) were applied into the gel. After separation, the proteins were stained in 0.125% (w/v) Coomassie brilliant blue R-250 and destained in 25% (v/v) ethanol and 10% (v/v) acetic acid.
Determination of myoglobin content
The extractable myoglobin content was determined by direct spectrophotometric measurement as described by Benjakul and Bauer [12]. A chopped sample (2 g) was weighed into a 50-ml polypropylene centrifuge tube and 20 ml of cold 40 mM phosphate buffer, pH 6.8 were added. The mixture was homogenised at 13,500 rpm for 10 s, followed by centrifuging at 3,000×g for 30 min at 4°C. The supernatant was filtered with Whatman No.1 filter paper. The supernatant (2.5 ml) was added with 0.2 ml of 1% (w/v) sodium dithionite to reduce the myoglobin. The myoglobin content was determined by direct spectrophotometric measurement at 555 nm. Myoglobin content was calculated from the millimolar extinction coefficient of 7.6 and a molecular weight of 16,110 [13]. The myoglobin content was expressed as mg/g sample.
Texture analysis
Texture analysis of the gels was performed using a TA-XT2 texture analyser (Stable Micro Systems, Godalming, Surrey, UK). Gels were equilibrated and evaluated at room temperature (28–30°C). Seven cylinder-shaped samples with a length of 2.5 cm were prepared and subjected to determination. Breaking force (gel strength) and deformation (elasticity/deformability) were measured using the texture analyser equipped with a spherical plunger (diameter 5 mm; depression speed 60 mm min−1).
Determination of whiteness
Surimi gel colour was determined using a JP7100F colourimeter (Juki Corp, Tokyo, Japan). L* (lightness), a* (redness/greenness) and b* (yellowness/blueness) were measured and whiteness was calculated as described by Park [14] as follows:
Determination of expressible moisture
Expressible moisture was measured according to the method of Ng [15]. A gel sample with a thickness of 0.5 cm was weighed and placed between two pieces of Whatman filter paper No. 1 at the top and three pieces of the same filter paper at the bottom. The standard weight (5 kg) was placed on the top of the sample and maintained for 2 min. The sample was then removed and weighed again. Expressible moisture was calculated and expressed as percentage of sample weight.
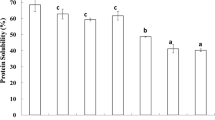
Ca2+-ATPase activity of NAM extracted from sardine (▪) and mackerel (□) surimi produced by conventional washing process and alkaline solubilising process. Bars indicate standard deviation from triplicate determinations. Different letters under the same species indicate significant differences (p<0.05).
Statistical analysis
Data were subjected to analysis of variance (ANOVA). Comparison of means was carried out by Duncan's multiple-range test [16]. Statistical analysis was performed using the Statistical Package for Social Science (SPSS 10.0 for windows, SPSS Inc., Chicago, IL).
Results and discussion
Ca2+-ATPase activity, surface hydrophobicity and protein pattern of CWPS and ASPS
The Ca2+-ATPase activity of NAM extracted from sardine and mackerel CWPS and ASPS is depicted in Fig. 1. Among all surimi from both species, the highest Ca2+-ATPase activity was found in CWPS prepared by NaCl washing (p<0.05). With the NaCl solution washing, sarcoplasmic proteins, lipid and unnecessary materials could be removed, resulting in the concentrated myofibrillar proteins [2]. This resulted in the greater content of myosin heavy chain with Ca2+-ATPase activity. However, no differences in Ca2+-ATPase activity were observed between mackerel unwashed mince and CWPS with NaCl washing (p>0.05). Roura et al. [17] reported that the myofibrillar ATPase activities have been widely used as a measure of actomyosin integrity. ASPS with and without prewashing had the lower Ca2+-ATPase activity than CWPS and unwashed mince for both sardine and mackerel (p<0.05). The result suggested that the denaturation of myosin was induced by alkaline solubilising process. From the result, prewashing prior to alkaline solubilisation resulted in the greater decrease in Ca2+-ATPase activity. It was postulated that soluble components might prevent the myosin from the denaturation caused by alkaline. Alkaline pH could modify the charge of proteins, resulting in the repulsion of molecules with subsequent conformational changes.
Surface hydrophobicity of CWPS and ASPS from sardine and mackerel is shown in Fig. 2. No differences in surface hydrophobicity were found between CWPS prepared using different washing media. Nevertheless, surface hydrophobicity of CWPS prepared by NaCl washing had the increased surface hydrophobicity (p<0.05), compared with that of unwashed mince and CWPS prepared by water washing. Obviously, mackerel ASPS with and without prewashing had the marked decrease in surface hydrophobicity. However, surface hydrophobicity of both sardine ASPS was greater than that of CWPS prepared by water washing (p<0.05). Increased surface hydrophobicity indicates an exposure of the interior of the molecule as well as conformational changes due to the denaturation or degradation of muscle proteins [18]. The decrease in surface hydrophobicity found in both mackerel ASPS might be due to the hydrophobic interaction between the exposed hydrophobic residues of denatured proteins. This interaction was reported to affect surface hydrophobicity [19]. Therefore, alkaline solubilising process induced the denaturation of both sardine and mackerel proteins as evidenced by the changes in surface hydrophobicity.
Surface hydrophobicity of NAM extracted from sardine (▪) and mackerel (□) surimi produced by conventional washing process and alkaline solubilising process. Bars indicate standard deviation from triplicate determinations. Different letters under the same species indicate significant differences (p<0.05).
Myoglobin content of CWPS and ASPS
The myoglobin contents of sardine and mackerel CWPS and ASPS are shown in Table 1. The myoglobin content of unwashed mince was 7.23 and 4.80 mg/g sample for sardine and mackerel, respectively. For sardine, the highest myoglobin removal was found in ASPS with prewashing. Thus, the removal of myoglobin with cold distilled water prior to alkaline treatment resulted in the higher myoglobin removed from the muscle. For mackerel, the greatest decrease in myoglobin content was obtained in ASPS without prewashing (p<0.05). From the result, washing with distilled water and NaCl solution removed 46.89 and 67.50% of myoglobin from sardine muscle and removed 22.71% and 29.37% of myoglobin from mackerel muscle, respectively. Alkaline solubilising process without and with prewashing removed 68.88 and 80.77% of myoglobin from sardine muscle and removed 76.25 and 71.25% of myoglobin from mackerel muscle, respectively.
In general, the sarcoplasmic proteins and other proteinous materials are not removed in the alkaline-aided process [7]. Those protein components are precipitated at their isoelectric point. Sarcoplasmic proteins in fish muscle include myoglobin, heamoglobin, enzymes and other albumins [20]. The differences in isoelectric point of various proteins in sarcoplasmic fraction might contribute to the differences in the precipitation of those proteins. Protein–protein interaction increases because the electrostatic forces of the molecules are at a minimum and less water interacts with the protein molecules [21]. Mackerel and sardine myoglobins had the isoelectric point of 5.8–5.9 [22]. At the pH above the pI, myoglobin possesses negative charge, resulting in the repulsion of molecules. At pH 5.5 used for muscle proteins recovery, some denatured myoglobin might not be precipitated, leading to the lowered myoglobin in the resulting precipitates. However, some myoglobin tightly bound with muscle proteins might be co-precipitated with those proteins during the recovery process, resulting in the presence of some myoglobin in the ASPS.
Breaking force (A) and deformation (B) of gels of sardine (▪) and mackerel (□) surimi produced by conventional washing process and alkaline solubilising process. Bars indicated standard deviation from seven determinations. Different letters under the same species indicate significant differences (p<0.05).
Gel forming abilities of CWPS and ASPS
The breaking force and the deformation of sardine and mackerel CWPS and ASPS gels are depicted in Fig. 3. CWPS prepared by water or NaCl washing showed the greater breaking force and deformation than ASPS with and without prewashing (p<0.05) (Fig. 2). The highest breaking force and deformation of gels from both species were found in CWPS prepared by NaCl washing. With the appropriate washing, sarcoplasmic proteins could be removed, resulting in the concentrated myofibrillar proteins, which play an essential role in gel formation. In the alkaline process, the sarcoplasmic protein fraction of the meat is retained [6]. Small quantities of sarcoplasmic proteins can have an adverse effect on the strength and deformability of myofibrillar protein gels [3, 20]. These proteins may interfere with myosin cross-linking during gel matrix formation because they do not form gels and have poorer water holding capacity [23]. Some sarcoplasmic proteins may bind to the myofibrils during the heat treatment, thus decreasing the strength of the gel [23]. However, with prewashing, ASPS gel showed the lower breaking force and deformation than the gels of other CWPS and unwashed mince.
The differences in gel forming ability might result from the differences in protein integrity and bonding formed during thermal process [24]. From the result, alkaline solubilising process might induce the denaturation of muscle protein as indicated by the lowered Ca2+-ATPase activity and the change in surface hydrophobicity (Figs. 1 and 2). This might result in the poor gel-forming ability. Additionally, differences in endogenous transglutaminase, which play a role in protein cross-linking, might contribute to the different gel strength [25, 26]. Setting occurred to a higher extent in unwashed mince and CWPS, compared to ASPS as evidenced by the much greater breaking force and deformation. It was postulated that alkaline solubilisation process might cause the loss in transglutaminase activity, leading to the poorer setting phenomenon. Pérez-Mateos et al. [6] reported that surimi treated by acid or alkaline had no endogenous transglutaminase activity. This enzyme might be denatured during acid or alkaline solubilisation process. From the result, alkaline solubilising process showed the adverse effect on gel-forming ability of surimi from mackerel and sardine caught in Thailand. The result was contradictory with those reported by Underland et al. [4] and Pérez-Mateos et al. [6] in which alkaline solubilising process rendered the surimi with the better gel properties, compared with conventional process. This might be due to the differences in the susceptibility of protein and endogenous enzyme involving in gelation to denaturation induced by alkaline treatment. Additionally, the different method used, including the rate of pH adjustment or the time the sample exposed to alkaline pH prior to neutralisation, might cause the different result.
Whiteness and expressible moisture of the gel from sardine and mackerel CWPS and ASPS are shown in Table 2. For sardine surimi gel, the highest whiteness was found in the gel of ASPS with prewashing (p<0.05). Removal of myoglobin with water prior to alkaline treatment improved the whiteness of surimi from sardine effectively, compared with other treatments. For mackerel, the highest whiteness was found in the gel of CWPS prepared by water washing (p<0.05) (Table 2). The lower whiteness in mackerel CWPS prepared by NaCl washing or alkaline treatment might be due to the oxidation of myoglobin retained in these samples, which was catalysed by NaCl or alkaline to yield the brown metmyoglobin. From the results, it was presumed that myoglobin from mackerel was more susceptible to oxidation than that from sardine, especially in presence of salt or alkaline.
The higher expressible moisture in both species was found in gels from ASPS than that of CWPS (p<0.05) (Table 2). This was possibly due to the poor gel network of ASPS caused by low gel-forming ability of denatured protein induced by alkaline treatment. Those gel matrixes could not imbibe water. Different expressible moisture suggested the difference in water holding capacity of gel network [27]. The lowest expressible moisture of surimi gels from both species was found in CWPS prepared by NaCl washing, indicating that protein network of these gels were higher in water holding properties. In general, the lower expressible moisture was coincidental with the increased breaking force.
Conclusion
Alkaline solubilising process induced the denaturation of the muscle proteins as evidenced by the decrease in the Ca2+-ATPase activity with the coincidental changes in the surface hydrophobicity. Marked decrease in myoglobin content was found in alkaline solubilising surimi, leading to the improved whiteness of sardine surimi. However, surimi prepared from conventional washing process showed a superior gel to that from alkaline solubilising process.
References
Ochiai Y, Ochiai L, Hashimoto K, Watabe S (2001) J Food Sci 66:1301–1305
Chaijan M, Benjakul S, Visessanguan W, Faustman C (2004) Food Res Intern 37:1021–1030
Hultin HO, Kelleher SD (2000) Surimi processing from dark muscle fish. In: Park JW (ed) Surimi and Surimi Seafood. Marcel Dekker, New York, pp 59–77
Underland I, Kelleher SD, Hultin HO (2002) J Agric Food Chem 50:7371–7379
Yongsawatdigul J, Park JW (2004) J Food Sci 69:499–505
Pérez-Mateos M, Amato PM, Lanier TC (2004) J Food Sci 69:328–333
Kim YS, Park JW, Choi YJ (2003) Fish Sci 69:1231–1239
Benjakul S, Seymour TA, Morrissey MT, An H (1997) J Food Sci 62:729–733
Fiske CH, Subbarow Y (1925) J Biol Chem 66:375–400
Laemmli UK (1970) Nature 227:680–685
Robinson HW, Hodgen CG (1940) J Biol Chem 135:707–725
Benjakul S, Bauer F (2001) Food Chem 72:207–217
Gomez-Basauri JV, Regenstein JF (1992) J Food Sci 57:1337–1339
Park JW (1994) J Food Sci 59:525–527
Ng CS (1978) In: Hasegawa H (ed) Laboratory manual on analytical methods and procedure for fish and fish products. Southest Asian Fisheries Development Center, Singapore, pp 1–2
Steel RGD, Torrie JH (1980) Principle and procedure of statistics, 2nd edn. MacGraw-Hill, New York
Roura SJ, Monteccia C, Coldemberg AL, Truco RE, Crupkin M (1990) J Food Sci 55:688–692
Multilangi WAM, Panyam D, Kilara A (1996) J Food Sci 61:270–274
Hill AR, Irvine DM, Bullock DH (1982) Can Inst Food Sci Technol J 15:155–160
Haard NF, Simpson BK, Pan BS (1994) In: Sikorski ZE, Pan BS, Shahidi F (eds) Seafood Proteins. Chapman & Hall, New York, pp 13–39
Vojdani F (1996) In: Hall GM (ed) Methods of testing protein functionality. Chapman & Hall, London, pp 11–60
Yamaguchi K, Takeda N, Ogawa K, Hashimoto K (1979) Bull Jap Soc Sci Fish 45:1335–1339
Sikorski ZE (1994) In: Sikorski ZE, Pan BS, Shahidi F (eds) Seafood Proteins. Chapman & Hall, New York, pp 40–57
Benjakul S, Visessanguan W, Ishizaki S, Tanaka M (2001) J Food Sci 66:1311–1318
Benjakul S, Visessanguan W (2003) Food Res Intern 36:253–266
Kumazawa Y, Numazawa T, Seguro K, Motoki M (1995) J Food Sci 60:715–717
Niwa E (1992) In: Lanier TC, Lee CM (eds) Surimi technology. Marcel Dekker, New York, pp 389–427
Acknowledgement
Financial support from Thailand Research Fund under the Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0129/2545) to Manat Chaijan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaijan, M., Benjakul, S., Visessanguan, W. et al. Physicochemical properties, gel-forming ability and myoglobin content of sardine (Sardinella gibbosa) and mackerel (Rastrelliger kanagurta) surimi produced by conventional method and alkaline solubilisation process. Eur Food Res Technol 222, 58–63 (2006). https://doi.org/10.1007/s00217-005-0091-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-005-0091-1