Abstract
Due to their bioaccumulation and biomagnification pathways, inorganic elements can accumulate in high-level aquatic organisms in the food web. Then, this species can be used to monitor the quality of the environment. Blood concentration of nine inorganic elements, including possible toxic metals (An, Cu, Mn, Se, As, Ni, Cd, Pb, and Hg), in 20 males and 20 females from eight different locations with high industry and agriculture activities in Iran were evaluated in this work. Additionally, size, sex, condition index, and locations were also included and analyzed. Among the essential elements, Zn and Se presented very high concentrations (56.14 ± 2.66 and 8.44 ± 0.77 μg/g ww, respectively) in all locations. Regarding possible toxic elements, Pb and Cd presented concerning concentrations as well (0.52 and 0.58 μg/g ww); this is especially true for Pb, an element found in very high concentrations in tissues of turtles from the same area in a previous study. The sex and the size of the individuals also had significant differences in concentration of Pb, Cd, As, and Hg.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, the rapid development of industry and agriculture has resulted in the use and release of many chemical elements in the environment, including trace elements (Olowu et al. 2010). Inorganic elements, including possible toxic metals, occur as trace constituents of primary minerals in igneous rocks (which crystallize from molten magma) (Alloway 1995). The concentration of trace elements in a living organism first depends on their presence in the environment from natural (geological) or anthropogenic (pollution) sources. Second, it depends on the intake of this element in the organism, generally through food, thus impacting the ecology of the organism. Lastly, it depends on the assimilation and excretion of the element as well as on the physiology of the organism (Rainbow 2002). Metals accumulate in aquatic animals almost exclusively through their diet (Clemente et al. 2011; da Silva et al. 2016; Langston and Spence 1995; Yu et al. 2011 or maternal transfer to the eggs (Guirlet et al. 2008; Paez-Osuna et al. 2010a).
Possible toxic metals, such as cadmium (Cd), mercury (Hg), and lead (Pb) are important to be monitored due to their toxicity and bioaccumulative behavior in aquatic organisms (Kalantzi et al. 2013). These elements are released in the environment in different ways, for example, through extraction, diffusion, combustion, and industrial activities. They subsequently enter the aquatic environment through the discharge of domestic, industrial, and agricultural waste water, accidental leakage, ballast water discharges by ships, and soil erosion (Bahnasawy et al. 2009; Javed 2005). These metals can be strongly accumulated along water and aquatic food chains, thus resulting in sublethal effects or death in local fish populations and, eventually, in humans (Grillitsch and Schiesari 2010; Pérez-López et al. 2008).
The increasing concerns about global declines in reptilian populations have derived investigations to evaluate the effects of contaminants on this group of species (Martínez-López et al. 2017). Additionally, their high trophic-level position of carnivorous and omnivorous species (such as Mauremys caspica) and their long life spans make them of particular risk to environmental contaminants. In turtles, metals have been shown to correlate with health biomarkers (Day et al. 2010; Day et al. 2007) and hatching success (Perrault et al. 2011). Pb, for instance, influences survival and righting (ability to turn over from their back) of hatchling slider turtles (Burger et al. 1998). Cd can disrupt reproduction in turtles by decreasing germ cell numbers and increasing oocyte apoptosis (Kitana andCallard 2008). However, few studies have investigated the population-concentration impact of environmental contamination in turtles. Albers et al. (1986a) observed a lower abundance of common snapping turtles (Chelydra serpentine) at sites with high concentrations of metals (Hg, Cd, Cr, Cu, Ni, Pb, and Zn) compared to sites with lower concentrations, but a similar effect was not detected in a more recent study (Yu et al. 2013). Inorganic elements, defined as those not present among the 10 most frequent elements in the earth’s crust (Alloway 1995), have been measured in the seven marine turtle species from several populations, but only in five freshwater turtle species worldwide.
Caspian pond turtle (M. caspica) is a freshwater turtle from the family Geoemydidae (Guillon et al. 2012), from the Eastern Mediterranean region, located in the Northwester Saudi Arabia, Iraq, Bahrain, Turkey, Caucasus, and Tbilisi to the northern, central, and southwestern parts of Iran (Vamberger et al. 2013). This species is widely dispersed in different provinces of Iran, such as Mazandaran, Golestan, Guilan, Ardabil, Azerbaijan, Kurdistan, Fars, and Khuzestan (Honda et al. 2002). There are two previous works done in M. caspica from Iran, where Pb, Cd, and Hg were determined in the liver, muscle, and shell (Adel et al. 2015; Yadollahvand et al. 2014). Pb in the liver and muscle of these turtles was found to be extremely high compared with other turtle species, such as Aspideretes gangeticus previously reported by Malik et al. (2013) or Trachemys scripta elegans (Yu et al. 2011) from other contaminated areas. However, the use of blood to assess the level of exposure to elements is an attractive option for studies on wildlife health because blood can be non-lethally collected and allows the determination of many environmental pollutants with a small blood sample.
The aims of this work were (1) to assess the concentrations of nine inorganic elements (Zn, Cu, Mn, Se, As, Ni, Cd, Pb, and Hg) in the blood of free-ranging male and female turtles from 8 different locations of Caspian pond turtles from the Southern basin of Caspian Sea and (2) to evaluate the influence of location, sex, size, and corporal index on metal concentrations.
Material and methods
Sampling
Permission to collect of Caspian pond turtle was authorized by the Iran Department of Environment (Permission Number: 1184-N/14/2; 2014; 22th September). The sample sites are located in Northern Iran within the Kopet Dagh and Alborz mountain ranges in Mazandaran and Golestan provinces (Fig. 1a). These mountains form a barrier between the South Caspian Sea and the Iranian plateau (Ghorbani 2013). A total of 40 Caspian pond turtles were collected from the southern basin of the Caspian Sea during September and October 2014. Individuals (males and females) were caught in eight different continental aquatic systems (Fig. 1b); three males and three females originated from each Noor, Babolsar, Sari, and Miankaleh locations and two males and two females originated from each Alagol, Aliabad, Gorgan, and Gonbad-e Qabus locations.
Turtles were transported alive to the central laboratory of the Caspian Sea Ecology Research Centre, and their carapace length (cm) and total mass (g) were measured. A 3-ml blood sample was then collected from the dorsal coccygeal veins and transferred into tubes containing heparin according to the protocol described in Ley-Quiñónez et al. (2011). At the end of the study, turtles were set free. Samples were kept at 4 °C and sent immediately to be analyzed.
For each individual, sex, carapace length, and mass were recorded. Carapace length is use as age indicator in turtles (Suzuki et al. 2012) or age at which individual has reached maturity (Tucek et al. 2014). Mass relates to both the age and health of individuals. To eliminate the strong relationship between length and mass, a condition index (CI) was estimated.
Condition index analysis
From mass and length to CI
The model linking mass and length is an exponential model with two fitted parameters, a and b: mass = a × lengthb. Values a and b were estimated using a generalized linear model (GLM). Likelihood (L) was estimated with a Gaussian distribution of mass and an identity link. The CI for each individual was the residual of the fitted relationship between mass and length (Stevenson and Woods 2006).
Differences between males and females for the relationship between mass and length were tested by the fit of males (m), females (f), and then both sexes together (mf) along with the estimation for each model using the Akaike information criteria (AIC). The AIC (Akaike 1974) for each model (AICm, AICf, and AICmf, respectively) was calculated as AIC = − 2 ln L + 2 p, where p is the number of parameters in the model and L the likelihood of the data for the model. The AIC is a measure of the quality of the fit that penalizes the number of parameters. It reduced the risk of over-parameterization. The Akaike weight (Burnham and Anderson 2002) between AICm + AICf and AICmf estimated the probability of a single model for males and females being sufficient to model the data.
Metal analysis
Trace element concentrations in blood samples were determined using the procedure described previously by Ley-Quiñónez et al. (2013). Briefly, all laboratory materials were acid and deionized water washed to prevent samples contamination. Approximately 0.5 g of the homogenized wet sample was added to 5 ml of concentrated (65%) ultra-pure HNO3 (Merck, Germany), HCl, and H2O2 (proportion of 2:2:1). Each sample was subjected to acid digestion in a microwave system for 40 min. A repetition of two blood samples with added standard multielement SIGMA 6000 (Perkin-Elmer) (0.06 μL) and another with deionized water were used to evaluate the efficiency of the method and observe whether the analysis matrix could generate interference. The digested material including residues obtained from the blood digestions, blanks, and standard was dissolved in 25 mL of deionized water. Calibration curves were made for the spectrophotometer using SIGMA 3000 (Perkin-Elmer). The detection and reading limits for each metal were achieved using the wavelength (nm) recommended by the distributor. The limit of detection (LOD) was 0.01 μg/g for Pb, Cd, As, and Ni; 0.001 μg/g for Hg; and 0.04 μg/g for Zn, Cu, Mn, and Se. To determine the percentage of recovery and evaporation during the digestion process, replicates were used in the reference material (RM) TORT-2. Recovery of RM (TORT-2) and standard repetition were added with SIGMA 3000, and the percentage of recovery was between 85 and 97%. Concentrations of trace elements are expressed as micrograms per gram of blood on wet weight basis.
Exploration of the data
Univariate exploration
The distribution of each metal concentration in the blood of individuals was tested for normality using histogram visual inspection and the Shapiro test for normality.
Multivariate exploration
Permutational MANOVA (also known as PERMANOVA) is a method used for partitioning sums of squares using semimetric and metric distance matrices (Anderson 2001). The distance matrix using Euclidean distance for all metal concentrations between individuals was regressed against sex, location, length, and CI. The advantage of this method is that it allows all metal concentrations to be tested in a single step. If a significant effect is detected, univariate analyses must be performed to determine which factors are involved. PERMANOVA has the advantage over other multivariate analysis in producing a test with a significance level. Before this analysis, the concentration of each metal C i was standardized (named SC i ) from 0 to 1 (see Warton et al. 2012 for justification).
Metal concentration spatial pattern
The Mantel test was used to test for a possible relationship between metal concentration and the great-circle distances (geographic distance) between individual locations. The Harversine formula was used to produce the geographic distance matrix, and Euclidean distance with standardized metal concentrations was used for the metal concentration matrix. Distances were computed with all elements in a single metric or on an element-by-element basis.
Statistical analysis
The same analysis was done for each element: A GLM with Gaussian distribution and identity link was fitted against sex, sites, length, and CI. All first-order interactions were initially included:
Model simplification was based on the AIC. At each step, the AIC was calculated with one factor being removed, and the model was retained when the removed factor led to a better AIC. A factor could not be removed if it was used in an interaction. All statistical analyses were performed with R 3.3.0 and the MASS, visreg, HelpersMG, mapdata, fields, ppcor, vegan, fossil, and FactoMine R packages.
Results
Biometry of animals
Males presented a length mean (± SD) of 11.78 ± 1.78 cm, mass mean of 279.6 ± 100.6 g, and a condition index mean of − 0.07 ± 0.23. Female’s biometrics in the same order were 10.87 ± 1.52 cm, 272.5 ± 63.5 g, and 0.07 ± 0.14 CI. For both sex biometrics were 11.36 ± 1.71 cm, 273.9 ± 83.0 g, and 0.0 ± 0.20 CI. As expected, length and mass were strongly correlated (r = 0.74, p < 0.000), and thus both factors cannot be simultaneously analyzed. The fit of mass = a × lengthb for males, females, and both sexes together led to AIC being 1.99, − 18.26, and − 11.11 respectively; a single model for both sexes is thus sufficient (Akaike weight, p = 0.07).
Exploration of metal concentration
A visual inspection of histograms and Shapiro tests do not show a strong deviation from the Gaussian distribution for the concentration of the nine measured elements. Two elements (Ni and Hg) show a slightly significant deviation from the Gaussian distribution (p values between 0.02 and 0.05), but further analyses are fairly robust against such a difference (Atkinson 1984).
PERMANOVA was used to test for sex, sites, length, and CI effects on the standardized distribution of the nine elements in a single analysis. A very significant effect of sex (p = 0.002) and sites (p = 0.008) was observed. However, a Mantel test on the Euclidean distances matrix according to geographic distances between sites does not reveal any spatial structuration by distance when all elements are tested simultaneously (p = 0.10) or separately (all p > 0.14).
Analysis of metal concentration
Each element was tested against sex, sites, length, and CI, including all first-order interactions. The model and graphical representation of the significant effects and interactions is shown in Figs. 2, 3, and 4.
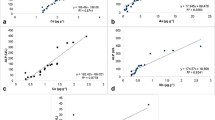
Figure 2 shows the element concentrations by site for Zn, Cu, Ni, and As. Partial correlations for the concentrations of these four elements at the eight sites show only a significant relationship for Zn and Ni (p = 0.001) with a negative correlation (r = − 0.84). Figure 3 shows the two significant effects found for Ni and Cd with the turtle’s length and describes sex effect observed for Cd (M > F) and Se (M < F).
An interaction between sex and length was observed for As (Fig. 4), with the concentration being higher for larger males and lower for larger females. Pb and Hg showed an effect for sex, length, and CI but in the opposite direction: Pb concentration was higher for smaller males with a high CI and for larger females with a low CI (Fig. 4), whereas Hg concentration was higher for smaller males with a low CI and for larger females with a high CI (Fig. 4). Only Mn showed no significant effect for any of the tested factors.
Discussion
There is an important lack of information about the concentration of trace elements in freshwater turtles and even less on their effects. A very high concentration of inorganic elements was reported in two previous studies in M. caspica from Iran (Adel et al. 2015; Yadollahvand et al. 2014). However, these results were difficult to interpret because of the lack of comparison with other sites. We therefore sampled the same species in eight locations around the sampled site used in those previous studies. For ethical reasons, we sampled trace elements just in the blood, which is a physiologic medium of exchange and transport of substances among the tissues of organisms; its flux in the different organs and tissues varies significantly (Chang 1996). When trace metals are ingested in organisms, they are rapidly distributed from the gastrointestinal tract by the sanguineous torrent, and the final concentration in organs and tissues depends on the relative velocities of the distribution processes and elimination (Chang 1996). Day et al. (2005) proposed the use of blood for monitoring trace metal exposure in turtles because it is possible to predict the load in internal tissues; moreover, sanguineous material is considered a good parameter for diagnostic indexes of recent exposure in organisms.
Elements concentrations
We were able to detect the nine trace elements in all samples (Table 1), in males and females. Element concentrations (μg/g wm) in decrease order were as follows: Zn (53.01 ± 2.66) > Se (8.66 ± 0.80) > As (2.95 ± 0.38) > Mn (2.06 ± 0.24) > Cu (1.39 ± 0.16) > Ni (1.10 ± 0.21) > Pb (0.55 ± 0.08) > Cd (0.52 ± 0.11) > Hg (0.011 ± 0.003). This elements order is relatively normal since the first five elements are considered as essential elements in trace concentration; all these elements are essential and involved in growth, proper functioning of the immune system, and reproduction of all vertebrates and have been described as elements that are relatively easily absorbed and physiologically important in tissues of reptiles (Grillitsch and Schiesari 2010). In the other hand, Ni, Pb, Cd, and Hg are considered as non-essential and potential toxic elements in very low concentrations in different species (ATSDR 2003, 2007a, b, 2012, 2015; Cortés-Gómez et al. 2014; da Silva et al. 2016).
The comparison of trace elements is complicated. Firstly, there are very few works about these pollutants in freshwater turtles. Secondly, when different species are used, their ecology and physiology could have an effect, because trophic levels or ingested food differ or the excretion pattern varies (Meyers-Schöne 1989). Even within a species, the situation can be complicated, because CI, age, and sex can differ depending on the location. Furthermore, the number of eggs or nesting events per female could vary for different populations, thus changing the excretion concentration in eggs (Guirlet et al. 2010). Even if we found more than 30 studies about these elements in turtles (Table 2), it was very difficult to compare our results with all of them because the different tissues analyzed (most of them in organs than in the blood). Moreover, no two studies use the same tissues, species, trace elements, and protocols. Meta-analysis is impossible in such a situation, or very strong hypotheses must be done to convert one value (species, tissue, and dry or wet weight) to another metric. Even more, the values below the detection limit are sometimes not included in the final estimates, thus creating a bias in the reported metal concentration. Often, the standard error is reported without indicating the sample size, and thus the standard deviation cannot be estimated.
In Table 3, all the works found regarding inorganic elements in the blood of freshwater turtles and selected works in marine turtles to be compared with the present study in wet and dry mass are shown. Regarding just freshwater turtles and tortoises, we found six representative works: two from Rio Negro in Brazil (Cd, Pb, As, Se, and Hg (Burger et al. 2010) and Hg (Schneider et al. 2011)), three from the USA (Hg (Bergeron et al. 2007); Zn, Cu, As, Se, and Pb (Allender et al. 2015), both in four different species; and Pb (Bishop et al. 2010)), and one work from Spain in freshwater turtles (Zn, Cu, Cd, Pb, and Hg (Martínez-López et al. 2017)).
Concentrations of Cd, Pb, As, and Se reported by Burger et al. (2010); Zn, Cu, Cd, Pb, and Hg concentrations reported by Martínez-López et al. (2017); and Zn, Cu, Se, As, and Pb concentrations reported by Allender et al. (2015) were low and even very low compared with those found in this study (Table 3). The locations selected for this study also present a high agriculture activity. Moreover, Martínez-López et al. (2017) studied mostly adults Mauremys leprosa turtles (more than 140 mm), species closely related to M. caspica, and turtles in this study were mostly juveniles (108–117 mm (Tok 1999)). Then, these higher concentrations in our turtles cannot be explained only for bioaccumulation, but for a higher exposition to most of these elements. Pesticides and fertilizer are commonly and widely used in the areas where turtles were collected; this could be an important source of metal pollution in aquatic environments in these locations. Additionally, Mazandaran wood, paper industry, antibiotic companies, cattle and poultry industries, MDF factories, and fisheries farms, among others, are developed in these locations. All these factors combined may be the reason of these high concentrations found in the blood of these turtles compared with other studies (pers. comm.).
Regarding potentially toxic elements, Pb is considered one of the most toxic heavy metals, causing alterations in vascular, nervous, renal, immune, reproductive, and hematological systems, as well as behavioral abnormalities (Bishop et al. 2010; Martínez-López et al. 2017). Martínez-López et al. (2010) suggested blood Pb levels above 15 μg/dL (0.15 μg/g ww) as inductors of sublethal effects on tortoises (Testudo graeca). Turtles from this study had 0.54 μg/g ww, high compared with those concentrations and compared with other freshwater turtles but similar to those found in some sea turtles (Table 3). Similar results were found for Cd, and concentrations on this study were high (0.58 μg/g ww) compared with other freshwater species, but similar or lower compared with sea turtles. This could mean that marine turtles are highly exposed to those metals, but Caspian turtles from the north of Iran are highly exposed than other freshwater species. Since turtles are from different areas, the concentration of these elements seems to be quite widespread in the north of Iran.
On the other hand, Hg in this study presented low concentrations compared with other species (Table 3). This element was especially high for the four species studied for Bergeron et al. (2007), presenting one of the highest concentrations found in turtles (ranking from 0.080 to 0.960 against 0.01 μg/g wm found in the present work). Bergeron et al. (2007) mention that these high concentrations are due to the very high persistence of this metal in the environment (more than 50 years since the use of mercuric sulfate was banned in the river where the turtles were found). He also found that Hg concentrations vary among the sites (more or less contaminated) and among the species (due to their feeding ecology). We can then suppose that the environment of the turtles from this study is not very highly contaminated with Hg.
We also compared our results with studies in marine turtles (Table 3); even if their environment is very different, they occupy a similar ecological niche depending on their feeding habits than freshwater turtles. Zn was the element with the higher concentration, and one of the highest compared with other species, but it was also highly variable among species and populations. Cu, Ni, Mn, and As tend to be in low concentrations in most turtle species, and concentrations of these elements found in this study are medium compared with other populations (Table 3). Although, Se was among the highest concentrations among other species (8.44 μg/g wm). Se is a necessary detoxifying element that can become toxic in high concentrations; in some species, Se (along with Hg) has been related with reduced reproductive success (Perrault et al. 2013). Higher Se concentrations have been reported in carnivorous than in herbivorous turtles (Aguirre et al. 1994). Caspian pond turtle are carnivorous as juveniles becoming more omnivorous as adults (Fritz and Havaš 2007); this could explain, at least in part, these high Se concentrations found in this study.
On the other hand, we have a previous work (Adel et al. 2015) in the same species but in different locations. This work showed a very high Pb concentration in the three tissues they analyzed (liver, muscle, and shell) with higher concentration in the liver (35.46 μg/g dm) but also high in the other organs (muscle, 23.06; shell, 29.5 μg/g dm). This means that this population is exposed to Pb in a chronic but also a constant form (in the liver, Pb accumulates in short–medium term and calcified tissue in a chronic exposure where it can accumulate for many years; ATSDR 2007a). Thus, and the concentration we found in the blood in this study, we hypothesized that the season could be another important variable to consider when you make a biomonitoring program. Especially for blood sampling as the rain or temperatures could influence on the bioavailability of the metals for the turtles (for the reason that food could be more or less contaminated).
Site differences
The places where turtles were collected (Fig. 1) are characterized by the dominance of platform-type sediments, with volcanic rocks rich in trace elements and particularly potentially toxic metals (Maanijou et al. 2013; Moazzen and Oberhänsli 2008; Movahedi et al. 2005). Agriculture is also observed at all sampling sites with the use of pesticides like organophosphates. In addition, the Sari, Noor, and Gorgan regions have high industrial activities. Fossil fuel diffusion from the Shahid Salimi (Neka) power plant, oil storage reservoirs, merchant vessels in Amir Abad port, and agricultural activities could increase the presence of inorganic elements in Sari region.
For Zn, Cu, Ni, and As, we found significant differences among the sampling sites (Fig. 2). Two major sources of metals are natural (e.g., volcanoes, erosion, and natural cycling of elements) and anthropogenic (e.g., mining, industry, agriculture, wastes, nuclear plants or smelters, and fossil fuels). When these significant effects for sites were observed, individuals from the Gorgan and Gonbad-e Qabus sites were always among the most contaminated (especially with Cu and As). Gorgan is an industrial site close to the Gharehsu River sampled by Yadollahvand et al. (2014) and Gonbad-e Qabus City located at 70 km on the same river basin. Here, Aliabad, at 3 km from Gorgan City, showed high concentrations of Ni and Cu. This observation highlights the heterogeneity of the environment for trace elements. This heterogeneity could derive not only from the geology of drainage basin but also from human activities.
Size, sex, and CI effects
Factors influencing the concentration of trace elements differ according to the element, thus reflecting the complexity of the metabolism for these elements. Ni had a significant positive relationship with the length of individuals (Fig. 3). Ni is an essential micronutrient with increased bone strength in birds as one proposed positive effect (Wilson et al. 2001). Some authors have showed that both low and high concentrations of Ni can affect bone development; it has been also noticed that the absorption of Ni increases during reproductive processes (Berglund et al. 2011; Wilson et al. 2001). We also observed a concentration of Ni in the blood within the rates compared with other turtle species (Table 3); thus, we can suggest that elder turtles absorb more Ni due to reproduction stages and/or bone physiological needs.
On the contrary, Cd had a negative significant relationship with the turtle’s length (Fig. 3), meaning that older turtles have less Cd concentration in the blood. Since Cd intake in turtles is mainly through the food (Andreani et al. 2008; Gardner et al. 2006; Maffucci et al. 2005), this decrease could be due to the change of diet during these turtles life spam, passing from carnivorous to omnivorous (Fritz and Havaš 2007). Compared with the other four freshwater species (Table 3), Cd concentration in this work is higher, but compared with marine turtles (Table 3), these concentrations can be considered medium/low; this difference must be due to the difference in their ecosystem and Cd bioavailability. Cd is a very toxic element that can cause many physiological alterations; many authors also suggest that it has a very low metabolic regulation in turtles (Barbieri 2009; Camacho et al. 2013; Cortés-Gómez et al. 2014). Cd also showed a significant relationship between males and females (Fig. 2), males being more contaminated with Cd than females. This could be explained with the fact that turtles tend to transfer metals to eggs during the reproduction processes (Aguirre et al. 2006; Ehsanpour et al. 2014; Guirlet et al. 2008).
Selenium had a significant effect between males and females (Fig. 3); females showed significantly higher concentrations of this element than males. Se concentration in the blood of these turtles was relatively high compared with other species (Table 3). However, it has been reported that turtles seems to be more tolerant against high Se concentrations compared with other animal species (Hopkins et al. 2005). The full role of Se in reproduction has yet to be established; though, it also appears to be important in reproduction and oxidative stress protection (Perrault et al. 2013). With this in mind, this higher Se concentration in females could be whether females have more demand and then more absorption of this element for reproductive processes or their intake is higher than their excretion capacities than males.
Finally, we found three significant differences between sexes after the GLM-AIC selection in As, Pb, and Hg (Fig. 4). Arsenic showed significant relationships among the size and sex against this element concentration in the blood (Fig. 4). These relationships showed that smaller (younger) female turtles had higher As concentration; on the contrary in males, bigger (older) turtles had higher As concentration. This decrease in bigger females could be explained due to maternal transfer during egg formation (Martínez-López et al. 2017). On the other hand, Pb and Hg concentrations had significant relationships with sex, index condition (IC), and size. Males with higher Pb concentrations were smaller and with higher IC, and larger females with lower IC had higher Pb concentration in the blood (Fig. 4). Regarding Hg, shorter males with lower IC had higher concentration in the blood, and larger females with higher IC had higher Hg concentration (Fig. 4). Different from As, in Pb and Hg, it seems that maternal transfer to eggs is not an important detoxification pathway in these turtles. Moreover, the accumulation of these two metals seems to be related to the size in females but not in males.
All these above-observed differences among male and females can reflect the divergence in element intake, taking into account the difference in the amount of food ingested between males and females, as it has been previously observed by Lovich (1996). In this case, the relationship with CI would be also expected (Fig. 4). These relationships with CI can also reflect the effect of hematocrit (ratio between red cells and plasma). Hematocrit can indicate a number of factors that may influence chemical concentrations in the blood, such as dehydration, malnutrition, and chronic disease (Frye 1991). Hematocrit can affect blood metal concentrations as red cells preferentially bind metals, compared to plasma (Chang 1996). The difference could also arise from the elimination of some elements in the eggs of nesting females (Guirlet et al. 2008; Paez-Osuna et al. 2010b). In this case, we should observe lower concentrations in females with a greater length (this effect was observed with As and Cd; Figs. 3 and 4). Of course, all of these factors could influence the concentration of elements and produce complex patterns (e.g., Hg and Pb).
The conclusion of Witkowski andFrazier (1982) about metal concentration in turtles was thus: “it is difficult to interpret the significance of these findings because so little is known about baseline concentrations and physiological effects of potentially toxic metals in these animals.” Indeed, this is still true after more than 30 years! These authors called for more “basic descriptive studies.” Yet we now have more than 30 descriptive studies (Table 2) from which we can conclude that we still know very little. Thus, more experimental studies with precise protocol, as being done for the impact of cadmium on reproduction (Kitana and Callard 2008), or more ecosystem studies are needed. It is also very important to standardize work protocols to be able to compare the results with others. Hence, the importance to always publish the humidity percentage found in samples, either if the results are published in wet or dry weight, could be even better if publications includes both results (e.g., Table 3).
We also observed that many researchers still consider that high dispersion in their data indicates that they have done poor laboratory work, which they try to hide using SE rather than SD. A contrario, high standard deviations indicate more interesting patterns that reveal complex individual life history. For instance, comparing two different studies often involve choosing, from among all of the combinations available, those that allow the initial hypotheses to be confirmed. Furthermore, the shape of the distribution of concentrations cannot be inferred from the mean and SD when the distribution is not symmetrical. In such a situation, the best is to report median and 2.5–97.5% centiles (see Table 1). The range of minimum and maximum values is also not informative, because these are not robust statistics for dispersion since they are very sensitive to aberrant values.
Conclusions
This is the first work on assessing inorganic elements in Caspian pond turtles’ blood. This work can be used as baseline dataset for future works in this species that has been scarcely studied. We can see that these turtles are useful as bioindicators of the environmental pollution. Most of the studied elements were found in high concentrations compared with other locations and spices. The importance of sex in the accumulation of inorganic elements in this species was also observed. For Cd, males showed higher concentrations than females; on the contrary, for Se, females showed significantly higher concentrations than males; this may be for reproductive processes. Regarding As, smaller females had higher As concentrations; on the contrary, bigger males had higher As concentrations. Males with higher Pb concentrations were smaller and with higher IC, and larger females with lower IC had higher Pb concentration in the blood. Regarding Hg, shorter males with lower IC had higher concentration in the blood, and larger females with higher IC had higher Hg concentration. All these above-observed differences among male and females can reflect the divergence in their reproductive behavior and elements intake, taking into account the difference in the amount of food ingested. Additionally, the importance of standardizing sampling and statistical protocols for biomonitoring studies is also highlighted in this study. In the future, capture-mark-recapture to estimate the survivorship of individuals and follow their load in different trace elements could allow us to decipher the impact of inorganic elements and particularly those possible toxic metals for turtles in natural conditions.
References
Adel M, Saravi HN, Dadar M, Niyazi L, Ley-Quinonez CP (2015): Mercury, lead, and cadmium in tissues of the Caspian pond turtle (Mauremys caspica) from the southern basin of Caspian Sea. Environmental Science and Pollution Research
Aguirre AA, Balazs GH, Zimmerman B, Galey FD (1994) Organic contaminants and trace metals in the tissues of green turtles (Chelonia mydas) afflicted with fibropapillomas in the Hawaiian islands. Mar Pollut Bull 28:109–114
Aguirre AA, Gardner SC, Marsh JC, Delgado SG, Limpus CJ, Nichols WJ (2006) Hazards associated with the consumption of sea turtle meat and eggs: a review for health care workers and the general public. EcoHealth 3:141–153
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19:716–723
Albers PH, Sileo L, Mulhern BM (1986a) Effects of environmental contaminants on snapping turtles of a tidal wetland. Arch Environ Contam Toxicol 15:39–49
Albers PH, Sileo L, Mulhern BM (1986b) Effects of environmental contaminants on snapping turtles of a tidal wetland. Arch Environ Contam Toxicol 15:39–49
Allender MC, Dreslik MJ, Patel B, Luber EL, Byrd J, Phillips CA, Scott JW (2015) Select metal and metalloid surveillance of free-ranging eastern box turtles from Illinois and Tennessee (Terrapene carolina carolina). Ecotoxicology 24:1269–1278
Alloway BJ (ed) (1995) Heavy metals in soils. Blackie and Son Ltd, Glasgow and London, 329 pp
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecology 26:32–46
Andreani G, Santoro M, Cottignoli S, Fabbri M, Carpene E, Isani G (2008) Metal distribution and metallothionein in loggerhead (Caretta caretta) and green (Chelonia mydas) sea turtles. Sci Total Environ 390:287–294
Atkinson AC (1984): Simulation in research on linear models. In: Rasch D, Tiku ML (Editors), Robustness of statistical methods and nonparametric statistics Springer-Verlag, London, pp. 9–12
ATSDR (2003) Toxicological profile for selenium. U.S. Department of Health and Human Services. Agency for Toxic Substances and Disease Registry, Atlanta, Georgia
ATSDR (2007a) Toxicological profile for lead. U.S. Department of Health and Human Services. Agency for Toxic Substances and Disease Registry, Atlanta, Georgia
ATSDR (2007b) Toxicological profile for arsenic. U.S. Department of Health and Human Services. Public Health Service. Agency for Toxic Substances and Disease Registry, Atlanta, Georgia
ATSDR (2012) Toxicological profile for cadmium. U.S. Department of Health and Human Services. Agency for Toxic Substances and Disease Registry, Georgia
ATSDR (2015): Support document to the 2015 priority list of hazardous substances. In: Sciences AfTSaDRDoTaHH (Hrsg.). ATSDR, Atlanta, GA
Bahnasawy M, Aziz khidr A, Dheina N (2009) Seasonal variations of heavy metals concentrations in mullet, Mugil cephalus and Liza ramada (Mugilidae) from lake Manzala, Egypt. J Appl Sci Res 5:845–852
Barbieri E (2009) Concentration of heavy metals in tissues of green turtles (Chelonia mydas) sampled in the Cananéia Estuary, Brazil. Braz J Oceanogr 57:243–248
Bergeron CM, Husak JF, Unrine JM, Romanek CS, Hopkins WA (2007) Influence feeding ecology on blood mercury concentrations in four species of turtles. Environ Toxicol Chem 26:1733–1741
Berglund AM, Koivula MJ, Eeva T (2011) Species- and age-related variation in metal exposure and accumulation of two passerine bird species. Environ Pollut 159:2368–2374
Bishop BE, Savitzky BA, Abdel-Fattah T (2010) Lead bioaccumulation in emydid turtles of an urban lake and its relationship to shell disease. Ecotoxicol Environ Saf 73:565–571
Burger J, Carruth-Hinchey C, Ondroff J, McMahon ME (1998) Effects of lead on behavior, growth, and survival of hatchling slider turtles. J Toxicol Environ Health Part A 55:495–502
Burger J, Jeitner C, Schneider L, Vogt R, Gochfeld M (2010) Arsenic, cadmium, chromium, lead, mercury, and selenium levels in blood of four species of turtles from the Amazon in Brazil. J Toxic Environ Health A 73:33–40
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer-Verlag, New York
Camacho M, Oros J, Boada LD, Zaccaroni A, Silvi M, Formigaro C, Lopez P, Zumbado M, Luzardo OP (2013) Potential adverse effects of inorganic pollutants on clinical parameters of loggerhead sea turtles (Caretta caretta): results from a nesting colony from Cape Verde, West Africa. Mar Environ Res 92:15–22
Chang LW (1996) Toxicology of metals. CRC Press, New York
Clemente Z, Castro V, Jonsson C, Fraceto L (2011) Ecotoxicology of nano-TiO2—an evaluation of its toxicity to organisms of aquatic ecosystems. International Journal of Environmental Research 6:33–50
Cortés-Gómez AA, Fuentes-Mascorro G, Romero D (2014) Metals and metalloids in whole blood and tissues of Olive Ridley turtles (Lepidochelys olivacea) from La Escobilla Beach (Oaxaca, Mexico). Mar Pollut Bull 89:367–375
da Silva CC, Klein RD, Barcarolli IF, Bianchini A (2016) Metal contamination as a possible etiology of fibropapillomatosis in juvenile female green sea turtles Chelonia mydas from the southern Atlantic Ocean. Aquat Toxicol 170:42–51
Davenport J, Wrench I, McEnvoy J, Carnacho-Ibar V. (1990) Metal and PCB concentrations in the “Harlech” leatherback. Marine Turtle Newsletter 48:l–6
Day RD, Christopher SJ, Becker PR, Whitaker DW (2005) Monitoring mercury in the loggerhead sea turtle, Caretta caretta. Environmental Science & Technology 39:437–446
Day RD, Segars AL, Arendt MD, Lee AM, Peden-Adams MM (2007) Relationship of blood mercury levels to health parameters in the loggerhead sea turtle (Caretta caretta). Environ Health Perspect 115:1421–1428
Day RD, Keller JM, Harms CA, Segars AL, Cluse WM, Godfrey MH, Lee AM, Peden-Adams M, Thorvalson K, Dodd M, Norton T (2010) Comparison of mercury burdens in chronically debilitated and healthy loggerhead sea turtles (Caretta caretta). J Wildl Dis 46:111–117
de Macedo GR, BT T, Barbosa IS, Pires TT, Rostan G, Goldberg DW, Pinto LF, Korn MG, Franke CR (2015) Trace elements distribution in hawksbill turtle (Eretmochelys imbricata) and green turtle (Chelonia mydas) tissues on the northern coast of Bahia, Brazil. Mar Pollut Bull 94:284–289
Ehsanpour M, Afkhami M, Khoshnood R, Reich KJ (2014) Determination and maternal transfer of heavy metals (Cd, Cu, Zn, Pb and Hg) in the hawksbill sea turtle (Eretmochelys imbricata) from a nesting colony of Qeshm Island, Iran. Bull Environ Contam Toxicol 92:667–673
Fritz U, Havaš P (2007) Checklist of chelonians of the world. Bonn and Dresden, German Federal Ministry of Environment. Nature Conservation and Nuclear Safety and Museum of Zoology Dresden
Frye FL (1991) Hematology as applied to clinical reptile medicine. In: Frye FL (ed) Reptile care: an atlas of diseases and treatments. TFH Publications, Neptune, pp 211–234
García-Fernández AJ, Gómez-Ramírez P, Martínez-López E, Hernández-García A, María-Mojica P, Romero D, Jiménez P, Castillo JJ, Bellido JJ (2009) Heavy metals in tissues from loggerhead turtles (Caretta caretta) from the southwestern Mediterranean (Spain). Ecotoxicol Environ Saf 72:557–563
Gardner SC, Fitzgerald SL, Vargas BA, Rodriguez LM (2006) Heavy metal accumulation in four species of sea turtles from the Baja California peninsula, Mexico. Biometals 19:91–99
Ghorbani M (2013) The economic geology of Iran: mineral deposits and natural resources. Springer Geology. Springer Science+Business Media, Dordrecht, 580 pp
Grillitsch B, Schiesari L (2010) The ecotoxicology of metals in reptiles. 337-448
Guillon J-M, Guéry L, Hulin V, Girondot M (2012) A large phylogeny of turtles (Testudines) using molecular data. Contrib Zool 81:147–158
Guirlet E, Das K, Girondot M (2008) Maternal transfer of trace elements in leatherback turtles (Dermochelys coriacea) of French Guiana. Aquat Toxicol 88(4):267–276
Guirlet E, Das K, Thome JP, Girondot M (2010) Maternal transfer of chlorinated contaminants in the leatherback turtles, Dermochelys coriacea, nesting in French Guiana. Chemosphere 79:720–726
Harris HS, Benson SR, Gilardi KV, Poppenga RH, Work TM, Dutton PH, Mazet JAK (2011) Comparative health assessment of Western Pacific leatherback turtles (Dermochelys Coriacea) foraging off the coast of California, 2005–2007. J Wildl Dis 4:321–337
Henny CJ, Beal KF, R Goggans (2003) Organochlorine pesticides, PCBs, trace elements, and metals in western pond turtle eggs from Oregon. Northwest Science 77:46-53
Honda M, Yasukawa Y, Ota H (2002) Phylogeny of the Eurasian freshwater turtles of the genus Mauremys Gray 1869 (Testudines), with special reference to a close affinity of Mauremys japonica with Chinemys reevesii. J Zool Syst Evol Research 40:195–200
Hopkins WA, Snodgrass JW, Baionno JA, Roe JH, Staub BP, Jackson BP (2005) Functional relationships among selenium concentrations in the diet, target tissues, and nondestructive tissue samples of two species of snakes. Environ Toxicol Chem 24:344–351
Ikonomopoulou MP, Olszowy H, Limpus C, Francis R, Whittier J (2011) Trace element concentrations in nesting flatback turtles (Natator depressus) from Curtis Island, Queensland, Australia. Mar Environ Res 71:10–16
Innis C, Tlusty M, Perkins C, Holladay S, Merigo C, Weber ES (2008) Trace metal and organochlorine pesticide concentrations in cold-stunned juvenile Kemp’s Ridley turtles (Lepidochelys kempii) from Cape Cod, Massachusetts. Chelonian Conservation and Biology 7:230–239
Javed M (2005) Heavy metal contamination of freshwater fish and bed sediment in the Ravi river stretch and related tributaries. Pakistan J Biol Sci 8:1337–1341
Kalantzi I, Shimmield TM, Pergantis SA, Papageorgiou N, Black KD, Karakassis I (2013) Heavy metals, trace elements and sediment geochemistry at four Mediterranean fish farms. Sci Total Environ 444:128–137
Kitana N, Callard IP (2008) Effect of cadmium on gonadal development in freshwater turtle (Trachemys scripta, Chrysemys picta) embryos. J Environ Sci Health A Tox Hazard Subst Environ Eng 43:262–271
Lam JC, Tanabe S, Chan SK, Lam MH, Martin M, Lam PK (2006) Levels of trace elements in green turtle eggs collected from Hong Kong: evidence of risks due to selenium and nickel. Environ Pollut 144:790–801
Langston WJ, Spence SK (1995): Biological factors involved in metal concentrations observed in aquatic organisms. In: Tessier A, Turner DR (Editors), Metal speciation and bioavailability in aquatic systems. John Wiley and Sons, Chichester, pp. 407–478
Ley-Quiñónez C, Zavala-Norzagaray AA, Espinosa-Carreón TL, Peckham H, Marquez-Herrera C, Campos-Villegas L, Aguirre AA (2011) Baseline heavy metals and metalloid values in blood of loggerhead turtles (Caretta caretta) from Baja California Sur, Mexico. Mar Pollut Bull 62:1979–1983
Ley-Quiñónez CP, Zavala-Norzagaray AA, Rendon-Maldonado JG, Espinosa-Carreon TL, Canizales-Roman A, Escobedo-Urias DC, Leal-Acosta ML, Hart CE, Aguirre AA (2013) Selected heavy metals and selenium in the blood of black sea turtle (Chelonia mydas agasiizzi) from Sonora, Mexico. Bull Environ Contam Toxicol 91:645–651
Lovich JE (1996) Possible demographic and ecologic consequences of sex ratio manipulation in turtles. Chelon Conserv Biol 2:114–117
Maanijou M, Aliani F, Miri M, Lentz DR (2013) Geochemistry and petrology of igneous assemblage in the south of Qorveh area, west Iran. Chemie der Erde - Geochemistry 73:181–196
Maffucci F, Caurant F, Bustamante P, Bentivegna F (2005) Trace element (Cd, Cu, Hg, Se, Zn) accumulation and tissue distribution in loggerhead turtles (Caretta caretta) from the Western Mediterranean Sea (Southern Italy). Chemosphere 58:535–542
Malik RN, Ghaffar B, Hashmi MZ (2013) Trace metals in Ganges soft-shell turtle (Aspideretes gangeticus) from two barrage: Baloki and Rasul, Pakistan. Environ Sci Pollut Res 20:8263–8273
Martínez-López E, Sousa AR, María-Mojíca P, Gómez-Ramírez P, Guilhermino L, García-Fernández AJ (2010) Blood delta-ALAD, lead and cadmium concentrations in spur-thighed tortoises (Testudo graeca) from Southeastern Spain and Northern Africa. Ecotoxicology 19:670–677
Martínez-López E, Gómez-Ramírez P, Espin S, Aldeguer MP, García-Fernández AJ (2017): Influence of a former mining area in the heavy metals concentrations in blood of free-living Mediterranean pond turtles (Mauremys leprosa). Bull Environ Contam Toxicol
Meyers-Schöne L (1989) Comparison of two freshwater turtle species as monitors of environmental contamination. PhD thesis Thesis, University of Tennessee, Knoxville, TN
Moazzen M, Oberhänsli R (2008) Whole rock and relict igneous clinopyroxene geochemistry of ophiolite-related amphibolites from NW Iran—implications for protolith nature. Neues Jahrbuch für Mineralogie - Abhandlungen 185:51–62
Movahedi M, Chitgari E, Yazdi M, Khakzad A (2005) Geochemical exploration in igneous rocks of the Tootyzar area, Aran, central Iran, mineral deposit research: meeting the global challenge. Springer-Verlag, London, pp 1019–1020
Olowu RA, Ayejuyo OO, Adewuyi GO, Adejoro IA, Denloye AAB, Babatunde AO, Ogundajo AL (2010) Determination of heavy metals in fish tissues, water and sediment from Epe and Badagry lagoons, Lagos, Nigeria. E-Journal of Chemistry 7:215–221
Paez-Osuna F, Calderon-Campuzano MF, Soto-Jimenez MF, Ruelas-Inzunza JR (2010a) Lead in blood and eggs of the sea turtle, Lepidochelys olivacea, from the Eastern Pacific: concentration, isotopic composition and maternal transfer. Mar Pollut Bull 60(3):433–439
Paez-Osuna F, Calderon-Campuzano MF, Soto-Jimenez MF, Ruelas-Inzunza JR (2010b) Trace metals (Cd, Cu, Ni, and Zn) in blood and eggs of the sea turtle Lepidochelys olivacea from a nesting colony of Oaxaca, Mexico. Arch Environ Contam Toxicol 59(4):632–641
Paez-Osuna F, Calderon-Campuzano MF, Soto-Jiménez MF, Ruelas-Inzunza J (2011) Mercury in blood and eggs of the sea turtle Lepidochelys olivacea from a nesting colony in Oaxaca, Mexico. Mar Pollut Bull 62:1320–1323
Pérez-López M, Mendoza MH, López-Beceiro A, Soler F (2008) Heavy metal (Cd, Pb, Zn) and metalloid (As) content in raptor species from Galicia (NW Spain). Ecotoxicol Environ Saf 70:154–162
Perrault J, Wyneken J, Thompson LJ, Johnson C, Miller DL (2011) Why are hatching and emergence success low? Mercury and selenium concentrations in nesting leatherback sea turtles (Dermochelys coriacea) and their young in Florida. Mar Pollut Bull 62:1671–1682
Perrault JR, Miller DL, Garner J, Wyneken J (2013) Mercury and selenium concentrations in leatherback sea turtles (Dermochelys coriacea): population comparisons, implications for reproductive success, hazard quotients and directions for future research. Sci Total Environ 463-464:61–71
Rainbow PS (2002) Trace metal concentrations in aquatic invertebrates: why and so what? Environ Pollut 120:497–507
Schneider L, Belger L, Burger J, Vogt RC, Jeitner C, Peleja JRP (2011) Assessment of non-invasive techniques for monitoring mercury concentrations in species of Amazon turtles. Toxicol Environ Chem 93:238–250
Stevenson RD, Woods WA Jr (2006) Condition indices for conservation: new uses for evolving tools. Integr Comp Biol 46:1169–1190
Storelli MM, Ceci E, Marcotrigiano GO (1998) Distribution of heavy metal residues in some tissues of Caretta caretta (Linnaeus) specimen beached along the Adriatic Sea (Italy). Bull Environ Contam Toxicol 60(4):546–552
Suzuki K, Noda J, Yanagisawa M, Kawazu I, Sera K, Fukui D, Asakawa M, Yokota H (2012) Relationships between carapace sizes and plasma major and trace element status in captive hawksbill sea turtles (Eretmochelys imbricata). J Vet Med Sci 74:1677–1680
Tok CV (1999) The taxonomy and ecology of Mauremys caspica rivulata Valenciennes, 1833 (Testudinata: Bataguridae) and Testudo graeca ibera Pallas, 1811 (Testudinata: Testudinidae) on Reşadiye (Datça) Peninsula. Turkish Journal of Zoology 23:17–21
Tryfonas AE, Tucker JK, Brunkow PE, Johnson KA, Huseein HS, Lin Z-Q (2006) Metal accumulation in eggs of the red-eared slider (Trachemys scripta elegans) in the Lower Illinois River. Chemosphere 63:39–48
Tucek J, Nel R, Girondot M, Hughes G (2014) Age−size relationship at reproduction of South African female loggerhead turtles Caretta caretta. Endanger Species Res 23:167–175
Vamberger MH, Stuckas H, Ayaz D, Gracia E, Aloufi AA, Els J, Mazanaeva LF, Kami HG, Fritz U (2013) Conservation genetics and phylogeography of the poorly known Middle Eastern terrapin Mauremys caspica (Testudines: Geoemydidae). Organisms Diversity & Evolution 13:77–85
van de Merwe JP, Hodge M, Olszowy HA, Whittier JM, Lee SY (2010) Using blood samples to estimate persistent organic pollutants and metals in green sea turtles (Chelonia mydas). Mar Pollut Bull 60:579–588
Wang H-C (2005) Trace metal uptake and accumulation pathways in Kemp’s Ridley. A&M University, Texas, 275 pp
Warton DI, Wright TW, Wang Y (2012) Distance-based multivariate analyses confound location and dispersion effects. Methods Ecol Evol 3:89–101
Wilson JH, Wilson EJ, Ruszler PL (2001) Dietary nickel improves male broiler (Gallus domesticus) bone strength. Biol Trace Elem Res 83:239–249
Witkowski SA, Frazier JG (1982) Heavy metals in sea turtles. Mar Pollut Bull 13:254–255
Yadollahvand R, Kami HG, Mashroofeh A, Bakhtiari AR (2014) Assessment trace elements concentrations in tissues in Caspian pond turtle (Mauremys caspica) from Golestan Province, Iran. Ecotoxicol Environ Saf 101:191–195
Yu S, Halbrook RS, Sparling DW, Colombo R (2011) Metal accumulation and evaluation of effects in a freshwater turtle. Ecotoxicology 20(8):1801–1812
Yu S, Halbrook RS, Sparling DW (2013) Correlation between heavy metals and turtle abundance in ponds near the Paducah Gaseous Diffusion Plant, Kentucky, USA. Arch Environ Contam Toxicol 65:555–566
Zavala-Norzagaray AA, Ley-Quiñónez CP, Espinosa-Carreon TL, Canizalez-Roman A, Hart CE, Aguirre AA (2014) Trace elements in blood of sea turtles Lepidochelys olivacea in the Gulf of California, Mexico. Bull Environ Contam Toxicol 93:536–541
Acknowledgements
This study was supported by the Iranian Fisheries Science Research Institute. The authors acknowledge the anonymous reviewers who have contributed their time and expertise to improve this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Capsule
Ecology and physiology interplay to explain the dynamic of inorganic elements, including possible toxic metals, in the freshwater turtle Mauremys caspica from Iran.
Highlights
• Concentration of nine elements was determined in 40 individuals from eight populations of the Caspian pond turtle.
• Concentration of the nine elements have been tested against location, sex, length, and condition index using the PERMANOVA, Mantel test, and generalized linear model.
• Significant differences between sex and size were found for Pb, Cd, Hg, and As.
• Pb, Zn, and Se had very high concentration compared with other populations and species.
Rights and permissions
About this article
Cite this article
Adel, M., Cortés-Gómez, A.A., Dadar, M. et al. A comparative study of inorganic elements in the blood of male and female Caspian pond turtles (Mauremys caspica) from the southern basin of the Caspian Sea. Environ Sci Pollut Res 24, 24965–24979 (2017). https://doi.org/10.1007/s11356-017-0067-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0067-2