Abstract
The concentration of nine metals was measured in liver, kidney, heart, muscle, plastron, and carapace of Aspideretes gangeticus from Rasul and Baloki barrages, Pakistan. The results indicated that metal concentration were significant different among tissues of Ganges soft-shell turtles. However, higher concentrations of Co (5.12 μg/g) and Ni (1.67 μg/g) in liver, Cd (0.41 μg/g) in heart, Fe (267.45 μg/g), Cd (2.12 μg/g) and Mn (2.47 μg/g) in kidney, Cd (0.23 μg/g), Cu (2.57 μg/g), Fe (370.25 μg/g), Mn (5.56 μg/g), and Pb (8.23 μg/g) in muscle of A. gangeticus were recorded at Baloki barrage than Rasul barrage. Whereas mean concentrations of Pb (3.33 μg/g) in liver, Co (1.63 μg/g), Cu (11.32 μg/g), Pb (4.8 μg/g) and Zn (144.69 μg/g) in heart, Co (4.12 μg/g) in muscle, Ni (1.31 μg/g), Pb (2.18 μg/g), and Zn (9.78 μg/g) in carapace were recorded higher at Rasul barrage than Baloki barrage. The metals followed the trend Fe > Zn > Ni > Cu > Mn > Pb > Cr > Co > Cd. Metals of toxicological concern such as Cr, Pb, and Cd were at that level which can cause harmful effects to turtles. The results provide baseline data of heavy metals on freshwater turtle species of Pakistan.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Toxic effects of heavy metals have been reported for several marine vertebrates (Law 1996; Franson 1996). Recent reports have documented that marine pollution by plastic debris, tar balls, heavy metals, and persistent organochlorine compounds have been of great concern and that may have played a role in declining populations of sea turtles (Godley et al. 1999). Due to their long lifespan and high trophic level in the aquatic food web, turtles are vulnerable to heavy metals pollution. Studies on heavy metals or organic compounds bioaccumulation in turtles are limited and different organs and tissues such as in blood (Páez-Osuna et al. 2011), eggs (Páez-Osuna et al. 2010), eggshells (Sakai et al. 1995), liver, kidney, and bone (García-Fernández et al. 2009) to assess and monitor heavy metal accumulations (Sakai et al. 2000b; García-Fernández et al. 2009) and persistent organic pollutants (D’Ilio et al. 2011). However, there is no information on toxicological effects and detrimental threshold concentrations. Progress towards the understanding of the possible heavy metal impact on turtle health might be obtained with more data on accumulation and distribution of trace elements within their body.
The Ganges soft-shelled turtle is found in the Ganges, Indus, and Mahanadi river systems of Pakistan, northern India, Bangladesh, and southern Nepal. This turtle inhabits deep rivers, streams, large canals, lakes and ponds, with a bed of mud or sand. According to the International Union for Conservation of Nature, freshwater turtle species are vulnerable. It tends to prefer areas where the water is turbid. Ganges soft-shelled turtle spends more time eating aquatic plants and a large variety of smaller animals, such as fish, mollusks, insects, amphibians, and waterfowl (Bonin et al. 2006). Freshwater turtles have been evaluated in several studies as biomonitors to evaluate an array of environmental contaminants; however, most of the studies related to heavy metal accumulation have been carried out on marine turtles. Illegal trade of freshwater turtle’s body parts for traditional medicine products is amongst the major threats to freshwater turtles (Anan et al. 2009) in south Asia, in particular to China. Many species of Asian turtles are being used to make a popular “turtle jelly,” which endangers these species. The threats to freshwater turtles are further magnified by changes to their habitat resulting from human activities ranging from logging to slash-and-burn agriculture, pollution, and the damming and channeling of rivers (Anan et al. 2009).
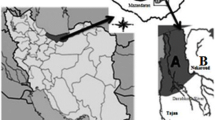
The Punjab province in Pakistan is most populated and is famous for its riverine system. A lot of barrages have been constructed in different rivers of the Punjab province for irrigation purposes. Rasul barrage (latitude 32 º42' and longitude 73 º31') was constructed on the Jehlum River at the confluence of River Chenab which receives water from Mangla dam, with catchments area of 24,069 Km2. The total irrigated area of Rasul barrage is 1,967 Km2 http://en.wikipedia.org/wiki/Rasul_Barrage. Rasul barrage has a water discharge capacity of 24,070 cubic meters per second. Rasul barrage receives untreated domestic and industrial waste from the Jehlum city. While Baloki barrage (latitude 31 º13' and longitude 73 º31') was constructed in the Ravi River with total irrigated area 1,965 Km2 and drainage basin 4,709 Km2. Baloki barrage receives untreated discharge from Lahore city, Degh Nala, and Hudiara drain in upstream region. The Hudiara drain is considered as a major source of pollution that carries industrial, municipal, and agricultural waste from both India and Pakistan (Farooq et al. 2011; Hashmi et al. 2013). Both barrages are highly urbanized with total inhabitants living in Rasul barrage at 18,374 and 208,475 people in Baloki barrage (District Census 2012). Cotton, wheat, and rice are the main crops in the vicinity of both barrages. Major agrochemicals used are herbicides, pesticides, and fertilizers. There is no information of heavy-metal accumulation in freshwater turtles inhabiting in Baloki and Rasul barrages. So, this study was conducted in these barrages as a part of preliminary assessment of presence/absence of freshwater soft-shell turtle species by the Pakistan Wetland Program conducted on November 2007 (Fig. 1). Therefore, this study aimed to determine the trace metals Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, and Zn in six different body organs of freshwater turtles (liver, kidney, heart, muscle, plastron, and carapace) in the Baloki and Rasul barrages
Materials and methods
Sampling
A total of 12.25 km upstream right bank and approximately 1 km downstream of the Baloki and Rasul barrages was surveyed in April–May 2008 in semi-rainy season. During the field visits, a large number of Ganges soft-shell turtle (Aspideretes gangeticus) was recorded. Although turtle hunting is illegal according to the Punjab Wildlife Act 1974, revised in November 2007, illegal killing was found common at two barrages during the study period due to poor enforcement of laws. A large group of local communities and turtle sellers were found involved in activities such as killing and selling of turtle plastrons during the field visits. During early stages of the study, the turtle seller did not cooperate to sell the turtles but later on agreed to provide the required samples if their names and identity will not be disclosed. A total of ten male adult turtle samples were purchased from the turtle sellers involved in illegal trade from each barrage. The juvenile turtles were caught using fishing nets by the sellers. The collection of sample size and further turtles handling was done as described by Anan et al. (2009). All these turtles were transported to the laboratory under fresh condition. In the laboratory, the turtle’s biometric characteristics were measured and then dissected to collect tissues and organs for heavy metal analysis. Average carapace length (cm) and width (cm) were 30.89 ± 5.30 and 29.19 ± 3.01, respectively. While plastron length (cm) was 20.11 ± 2.22. Heart, liver, muscles, kidneys, plastron, and carapace were separated from each turtle. Each organ was weighed to establish wet weight, washed with the deionized water, placed in pre-washed zipped plastic bags with HNO3 (10 %), tagged, and refrigerated at −20 °C before metal analyses.
Trace metal analysis
Each tissue and organ of the turtles was cut with the stainless steel knives washed with the 10 % nitric acid solution to avoid any contamination. The samples were cut into cubical pieces, chopped to make the homogeneous sample for metal analysis and oven dried at 70 °C. Dried samples measuring 1 g each weighed and digested with 4 ml of ultra pure nitric acid (HNO3) for microwave-assisted digestion. Digested samples were filtered with Whatman filter paper 1 and volume increased to 25 ml with deionized water. All samples were run in triplicate to measure Pb, Zn, Fe, Co, Cu, Cr, Mn, Ni, and Cd using Atomic Absorption Spectrophotometer (Varian FSAA-240). Procedural blanks and certified reference material, NIES No. 1 was also prepared using identical procedures as used for the samples. Each calibration curve was evaluated by determination of quality control standards before, during and after a set of sample measures. The recovery rates for the studied metals were within 90 ± 10 %.
All the chemicals used were of analytical grade (Merck Darmstadt, Germany). Deionized water was used throughout the analysis. Standard solutions of metals were prepared by dilution of 1,000 ppm certified standards solutions (Fluka Kamica Busch Switzerland) of corresponding metal. Glass and plastic ware used in the field and laboratory analyses was cleaned with washing detergent (Decon 90), rinsed repeated times with deionized water, soaked in HNO3 (10 %, v/v) for 24 h, and finally rinsed with deionized water. The level of metal accuracy with precision for studied heavy metals was 83 ± 2–96 ± 3 %.
Statistical analysis
Shapiro–Wilk normality test was used to check the normal distribution of data before analysis of variance (ANOVA). The data was distributed normal then parametric tests were employed for statistical analysis. One-way ANOVA was used to examine mean differences among the organs and sites. For all tests, P values of ≤0.05 were used to determine significant differences. Metal concentrations are reported in μg/g on dry weight basis. Statistical analysis was performed using Statistica Software (Statsoft Inc. 1999).
Results
The mean concentration of heavy metals in various tissues and organs of Ganges soft-shell turtle from both sites are presented in Table 1. Ganges soft-shell turtle samples from Baloki barrage showed higher concentrations of metals such as Cd, Co, Cr, Cu, Mn, Ni, and Pb. The concentrations of Cd (P = 0.00), Co (P = 0.00), Cu (P = 0.00), Pb (P = 0.00) and Zn (P = 0.00) in heart; Fe (P = 0.00), Mn (P = 0.00) in kidney; Cd (P = 0.00), Co (P = 0.00), Cu (P = 0.00), Fe (P = 0.00), Mn (P = 0.00), Ni (P = 0.00) in muscle; Co (P = 0.00), Ni (P = 0.00), Pb (0.02) in liver; Cd, (P = 0.00) Co (P = 0.00), Cr (P = 0.00), Cu (P = 0.00), Mn (P = 0.00), Ni (0.01), Pb (0.03), Zn (0.05) in Plastron were differed significantly. Higher metal concentrations of Cd, Co, Cu, Mn, Pb, Fe, and Zn were measured in kidney, liver, and heart in comparison to those concentrations measured in carapace and plastron. Fe showed the highest concentration in all tissues. The mean concentrations of Fe, Zn, and Pb in kidneys of Ganges soft-shell turtles from the Baloki barrage were relatively higher (267.45, 53.06, and 2.32 μg/g) than the Rasul barrage. The general trend of metals in kidneys was: Fe > Zn > Pb > Cu > Co > Mn > Cd > Cr > Ni.
Heart samples collected from Baloki barrage exhibited high mean concentrations of Cd, Cr, Mn and Ni whereas mean concentration of Co, Cu, Fe, Pb and Zn were higher in Rasul barrage. The mean concentration of metals in heart was in order: Fe > Zn > Cu > Pb > Ni > Co > Mn > Cr > Cd. Mean concentration of metals such as Cd, Cr, Cu, Fe, Mn, Pb and Zn were higher in muscle at Baloki, and Co and Ni at Rasul barrage. The trend of metal accumulation in muscle followed the order: Fe > Zn > Pb > Mn > Co > Cu > Ni > Cr > Cd. Liver samples from Baloki showed higher concentrations of Cd, Co, Cu, Fe, Mn, Ni, and Zn while Pb was higher in liver samples from Rasul barrage. The metal accumulation trend in liver was in following order: Fe > Zn > Cu > Mn > Co > Pb > Ni > Cr > Cd. The metals were significantly different in plastrons samples collected from Baloki. The pattern of metal accumulation in plastrons followed the order: Fe > Zn > Ni > Cu > Mn > Pb > Cr > Co > Cd.
Concentrations of Cd and Cr in carapace were higher than those in plastron, heart, liver, muscle, and kidney (Table 1). However, mean values of Fe, Co, Pb, and Zn were comparatively lower in carapace. The pattern of metal levels in carapace was in the following order: Fe > Zn > Cu > Pb > Mn > Cr > Ni > Co > Cd. The mean concentration of Fe and Mn were significantly different in kidneys and Cu, Pb, and Zn in heart between two sites. However, the mean concentrations of Cd, Co, Cu, Fe, Mn, and Pb were significantly different in muscles between two sites while, the mean concentrations of Co, Ni, and Pb were significantly different in liver between both sites. Significant differences between Baloki and Rasul barrages were observed for Cd, Co, Cr, Cu, Mn, Ni, Pb, and Zn in carapace.
Discussion
The results indicated that most of the metals were significantly different between the sites and organs (Table 1). These differences in sites and body organ may be due to the sources of heavy-metal pollution in both barrages and accumulation period in the Ganges soft-shell turtles. Both sites receive untreated, industrial, and agriculture waste from both point sources and nonpoint sources of pollution. The Baloki barrage receives domestic and industrial waste from the Lahore city, Sialkot city, Faisalabad while Rasul barrage receives from the Jehlum city (Qadir and Malik 2011; Farooq et al. 2011; Hashmi et al. 2013). However, in the vicinity of both barrages, agriculture activities were also recorded. The level of metal accumulation along with other factors such as age, gender nutritional status, seasonal and annual variations, geographic variation and trophic level may also affect metal levels, toxicity, and elimination in various organisms (Gardner et al. 2006). However, specific organs have metal accumulation preferential which causes the metal variability in the organisms. For example, Cd accumulate preferably in kidney (Law 1996) Mn, Pb, and Zn accumulate in calcareous tissues (bone and carapace) (Anan et al. 2009). Food is probably the main source of exposure to heavy metals in aquatic organisms. As turtles feed mainly on variety of aquatic plants and other herbivorous organisms like fish, mollusks, insects, and amphibians etc. to meet their feeding needs (Caurant et al. 1999).
Chrome, which is an essential element required in trace quantities for normal glucose metabolism (Mertz 1969). Cr concentration in liver (0.39 μg/g), kidney (1.0 μg/g), and muscle (2.18 μg/g) in the present study is lower (Tables 2, 3, and 4) than to those reported for green turtles from the south China coast (Lam et al. 2004). Cr exposure generally affects the liver and kidneys of the aquatic organisms (Gardner et al. 2006). Toxic Cr concentration may cause kidney and liver damage leading to death (D’Ilio et al. 2011).
Copper concentrations in the liver of Ganges turtles measured in present study were similar to those found in green turtles (Chelonia mydas) and hawksbill turtles (Eretmochelys imbricata) from Yaeyama Islands, Okinawa, Japan (Anan et al. 2009). However, Cu concentrations in liver reported in present study, were lower to those found in Loggerhead marine turtles from Canary Islands, Spain (Fernandez-Turiel et al. 2003). Cu concentrations in kidney recorded in the present study were comparable to those found in Green turtles (8.2 μg/g) in Adriatic Sea, Italy (Storelli et al. 2008) while lower concentrations of Cu in kidneys were reported in Loggerhead turtles from Yaeyama Islands, Okinawa, Japan (Sakai et al. 2000a). Cu Concentrations in muscles were similar to those found in green turtles from south China coast (Lam et al. 2004) while lower metal concentrations as compared to the present study were found in Loggerhead turtles from eastern Mediterranean Sea, Italy (Storelli et al. 2005).
Zinc concentrations in liver found in the present study were similar to those of green turtles from south-eastern Queensland, Australia (39.7 μg/g) (Gordon et al. 1998). However, high concentrations in liver were reported from South China coast in green turtles (Lam et al. 2004). The differences in the concentration of Zn from other regions may be due to the level of contamination, environment, and metabolism of turtle species involved (Gardner et al. 2006).
Cadmium concentrations reported in liver (0.22 μg/g) from Baloki barrage were similar to leatherback turtle from UK (Davenport and Wrench 1990). Cd concentrations recorded in the present study were very low as compared to those found in green turtles from the South China coast (Lam et al. 2004). The concentration of Cd (2.48 μg/g) reported in kidney of green turtles by Lam et al. (2004) from south China coast was similar to our study. High Cd concentrations (142 μg/g) were found by Anan et al. (2009) in green turtles from Japan as compared to present study.
Cd is stored in kidney and in liver (O’Shea 1999) and distribution of metals may also influence by the duration and concentration of exposure. Studies of freshwater turtles have demonstrated that after a single injection (dose), the highest concentration of Cd is found initially in liver, which is a major site of short-term storage (Thomas et al. 1994; Rie et al. 2001). However, tissue Cd concentrations found in free-ranging reptiles generally indicates a shift from the liver to kidney. During long-term exposure, Cd is redistributed from the liver via the blood and transported as a metallothionein-complex to the kidneys, where it is absorbed and concentrated (Linder and Grillitsch 2000).
Concentrations of Cd were found in muscle of Loggerhead turtle from southwestern Mediterranean, Spain (García-Fernández et al. 2009) were similar to the present study. Higher Cd concentrations were found in green turtles from Baja California peninsula, Mexico (Gardner et al. 2006). Mining, agricultural runoff, and wastes from metal smelting are primary sources of Cd contamination. Cd is a non-essential element not metabolically regulated by vertebrates and, as such, is toxic to humans and other animals (Linder and Grillitsch 2000). Food digestion rather than inhalation is the most likely uptake route of Cd in vertebrates. After ingestion, Cd is transported to the gut and is excreted in the feces. The kidney is the principal storage site for Cd, followed by the liver and other tissues (muscle, skin, and bone) in humans and marine vertebrates (Gardner et al. 2006). Cd has a long biological half-life and accumulates with age by binding with metallothionein within the liver (D’Ilio et al. 2011). Cd environmental concentration such as 1 μg/g may not only affect gonadal developmental processes of freshwater turtles (Trachemys scripta) during post-natal and embryonic stages but also may disrupt reproductive processes later in life (Kitana and Callard 2008). In this study the authors studied the effect of environmentally relevant dose of cadmium on germ cell number and oocyte apoptosis in lab-reared T. scripta, a closely related freshwater turtle species. They found that Cd dose at 1 μg/g reduced the germ cell numbers in T. scripta but oocytes apoptosis increased with Cd concentrations. Furthermore, they also found that the effects of Cd on turtle gonadal development was extended up to 3 months post hatch. In contrast, our study showed the higher environmental levels of Cd in kidney from the Baloki barrage (2.12 μg/g) and Rasul barrage (1.28 μg/g), suggesting Cd may pose threats to reproductive success of freshwater turtles.
Lead is non-essential metal and poses toxic effects to various organisms at elevated concentrations. Comparison of Pb concentration in liver samples revealed that it was significantly different at both sites. Pb concentrations in turtle liver samples from Rasul barrage were higher (2.33 μg/g) than the Baloki barrage (1.93 μg/g). Pb concentrations reported in liver in the present study were similar to Olive Ridley turtles in Mexico (Frías-Espericueta et al. 2006). However, as compared to the present study, higher Pb concentrations (2.75 μg/g, dry wt.; 2.94 μg/g, wet wt. respectively) in liver were found for Loggerhead turtles from southwestern Mediterranean, Spain (García-Fernández et al. 2009). The concentrations of Pb were greater in kidneys at Baloki barrage (2.32 μg/g) as compared to the Rasul barrage (1.93 μg/g). Comparison of Pb with previous studies revealed that it was higher in the present study than reported in Loggerhead marine turtles from Canary Islands, Spain (Torrent et al. 2004).
At Baloki barrage, A. gangeticus showed higher Pb concentrations (1.23 μg/g) in muscle as compared to those measured at Rasul barrage (1.03 μg/g). The concentrations of Pb were higher in the present study as compared to the Loggerhead turtles from southwestern Mediterranean, Spain (García-Fernández et al. 2009) South China coast (Lam et al. 2004). Significantly high Pb concentrations were observed in heart tissue of Ganges soft-shell turtle between both sites.
High Co concentrations in liver were reported in the present study as compared to hawksbill turtles (E. imbricata) and green turtles (C. mydas) from Yaeyama Islands, Japan (Anan et al. 2009). Co concentrations in liver at Rasul barrage was within range reported by Lam et al. (2004) in Green turtles (0.58 μg/g) as compared to the present study. Higher Co concentrations in kidney (11.39 μg/g) were reported by Lam et al. (2004) in green turtles from the South China coast as compared to the current study. While lower concentrations (2.2 μg/g) in green turtles’ kidney were reported by Anan et al. (2009) from Japan as compared to the current study. The Co concentrations were measured higher as compared to those of muscle of green turtles from South China coast (Lam et al. 2004).
These concentrations were low as compared to those reported in green turtles from South China coast (Lam et al. 2004). The present study showed high concentration of Ni in turtle’s kidneys as compared those found by Lam et al. (Lam et al. 2004) in green turtles from South China coast and by Sakai et al. (2000a) in Loggerhead marine turtles from Japan. Similar concentrations of Ni in muscle (1.07 μg/g) were measured in green turtles from south China coast (Lam et al. 2004) while lower concentrations of Ni were observed in Loggerhead turtles from Yaeyama Islands, Okinawa, Japan (Kszos et al. 1992).
Iron is the fourth most abundant element in the Earth’s crust and occurs in most rocks and soils. The highest concentration of Fe (1,170 μg/g) was reported by Alonso Aguire et al. (Alonso Aguirre et al. 1994) in liver of green turtles from Hawaiian Islands than the present study. Comparatively, low concentrations of Fe in liver were observed in Loggerhead turtles from Yaeyama Islands, Okinawa, Japan (Sakai et al. 2000a). The concentrations in turtle kidney found in the present study were high as compared to those reported in Loggerhead turtles in Japan (Sakai et al. 2000a). Low concentrations in comparison with present study were found in muscles of Loggerhead turtles in Japan (Sakai et al. 2000a). Lam et al. (2004) reported high concentrations of Fe in livers of green turtles from South China as compared to those found in the present study.
Manganese is essential as a cofactor for some enzymes. Comparatively high concentrations of Mn were observed in green turtle kidney from South China (Lam et al. 2004) while low concentrations were observed in Loggerhead turtles from Japan (Sakai et al. 2000a). Low concentrations of Mn were reported in green turtles and Loggerhead turtles from South China and Japan, respectively (Lam et al. 2004).
Conclusion
The A. gangeticus samples (liver, kidney, heart, muscle, plastron, and carapace) collected from Baloki barrage showed higher metal concentrations i.e., of Cd, Co, Cr, Cu, Mn, Ni, and Pb than the turtle samples collected from Rasul barrage. Among tissues and organs, the kidney revealed high concentrations of Fe, Zn, and Pb from Baloki. Heart samples collected from Baloki barrage showed higher concentrations of Cd, Cr, Mn, and Ni as compared to those found in Rasul barrage. Cd, Cr, Cu, Fe, Mn, Pb, and Zn were higher in muscle tissues of A. gangeticus from Baloki while Co and Ni were high from Rasul barrage. High concentrations of Cd, Co, Cu, Fe, Mn, Ni, and Zn were found in livers of A. gangeticus at Baloki, while Pb was found in greater concentration in Rasul barrage samples. Among the external tissues plastron from Baloki showed a high concentration of Fe, Zn, Ni and Cu. Carapace tissue of A. gangeticus showed greatest differences between Baloki and Rasul barrage in Cd, Co, Cr, Cu, Mn, Ni, Pb, and Zn. Based on the metals accumulation in different tissues, it was found that the Baloki barrage was more polluted than the Rasul barrage. However, there is more need to investigate the environmental contamination impact on the freshwater turtle population for better conservation of freshwater turtles.
References
Alonso Aguirre A, Balazs GH, Zimmerman B, Galey FD (1994) Organic contaminants and trace metals in the tissues of green turtles (Chelonia mydas) afflicted with fibropapillomas in the Hawaiian Islands. Mar Pollut Bull 28(2):109–114
Anan Y, Kunito T, Watanabe I, Sakai H, Tanabe S (2009) Trace element accumulation in hawksbill turtles (Eretmochelys imbricata) and green turtles (Chelonia mydas) from Yaeyama Islands, Japan. Environ Toxicol Chem 20(12):2802–2814
Bonin F, Devaux B, Dupré A (2006) Turtles of the world. Johns Hopkins University Press, Baltimore. ISBN 0801884969
Caurant F, Bustamante P, Bordes M, Miramand P (1999) Bioaccumulation of cadmium, copper and zinc in some tissues of three species of marine turtles stranded along the French Atlantic coasts. Mar Pollut Bull 38(12):1085–1091
D’Ilio S, Mattei D, Blasi M, Alimonti A, Bogialli S (2011) The occurrence of chemical elements and POPs in loggerhead turtles (Caretta caretta): an overview. Mar Pollut Bull 62(8):1606–1615
Davenport J, Wrench J (1990) Metal levels in a leatherback turtle. Mar Pollut Bull 21:40–41
District Census Report (2012) Population census organization. Statistics Division. Ministry of Economic Affairs and Statistics, Government of Pakistan.
Farooq S, Eqani SA-M-A-S, Malik RN, Katsoyiannis A, Zhang G, Zhang Y, Li J, Xiang L, Jones KC, Shinwari ZK (2011) Occurrence, finger printing and ecological risk assessment of polycyclic aromatic hydrocarbons (PAHs) in the Chenab River, Pakistan. J Environ Monit 13(11):3207–3215
Fernandez-Turiel J, Gimeno D, Rodriguez J, Carnicero M, Valero F (2003) Spatial and seasonal variations of water quality in a Mediterranean catchment: the Llobregat River (NE Spain). Environ Geochem Heal 25(4):453–474
Franson J (1996) Interpretation of tissue lead residues in birds other than waterfowl. In: Beyer WN, Heinz GH, Redmon-Norwood AW (eds) Environmental contaminants in wildlife: interpreting tissue concentrations, SETAC special publications series., pp 265–279
Frías-Espericueta M, Osuna-López J, Ruiz-Telles A, Quintero-Alvarez J, López-López G, Izaguirre-Fierro G, Voltolina D (2006) Heavy metals in the tissues of the sea turtle Lepidochelys olivacea from a nesting site of the northwest coast of Mexico. Bull Environ Contam Toxicol 77(2):179–185
García-Fernández AJ, Gómez-Ramírez P, Martínez-López E, Hernández-García A, María-Mojica P, Romero D, Jiménez P, Castillo JJ, Bellido JJ (2009) Heavy metals in tissues from loggerhead turtles (Caretta caretta) from the southwestern Mediterranean (Spain). Ecotoxicol Environ Saf 72(2):557–563
Gardner SC, Fitzgerald SL, Vargas BA, Rodríguez LM (2006) Heavy metal accumulation in four species of sea turtles from the Baja California peninsula, Mexico. BioMetals 19(1):91–99
Godley BJ, Thompson DR, Furness RW (1999) Do heavy metal concentrations pose a threat to marine turtles from the Mediterranean Sea? Mar Pollut Bull 38(6):497–502
Gordon A, Pople A, Ng J (1998) Trace metal concentrations in livers and kidneys of sea turtles from south-eastern Queensland, Australia. Mar Freshw Res 49(5):409–414
Hashmi MZ, Malik RN, Shahbaz M (2013) Heavy metals in eggshells of cattle egret (Bubulcus ibis) and little egret (Egretta garzetta) from the Punjab province, Pakistan. Ecotoxicol Environ Saf 89:158–165. doi:10.1016/j.ecoenv.2012.11.029, Epub 2012 Dec 20
Kitana N, Callard IP (2008) Effect of cadmium on gonadal development in freshwater turtle (Trachemys scripta, Chrysemys picta) embryos. J Environ Sci Health A 43(3):262–271
Kszos LA, Stewart AJ, Taylor PA (1992) An evaluation of nickel toxicity to Ceriodaphnia dubia and Daphnia magna in a contaminated stream and in laboratory tests. Environ Toxicol Chem 11(7):1001–1012
Lam JCW, Tanabe S, Chan SKF, Yuen EKW, Lam MHW, Lam PKS (2004) Trace element residues in tissues of green turtles (Chelonia mydas) from South China Waters. Mar Pollut Bull 48(1):174–182
Law R (1996) Metals in marine mammals. In: Nelson Beyer W, Heinz GH, Redmon-Norwood AW (eds) Environmental contaminants in wildlife: interpreting tissue concentrations. CRC Press, Boca Raton, p 357
Linder G, Grillitsch B (2000) Ecotoxicology of metals. Ecotoxicology of amphibians and reptiles setac. Pensacola, FL, USA, pp 325–459
Mertz W (1969) Chromium occurrence and function in biological systems. Physiol Rev 49:163–239
O’Shea TJ (1999) Environmental contaminants and marine mammals. In: Reynolds JEI, Rommel SA (eds) Biology of marine mammals. Smithsonian Institution Press, Washington, pp 485–564
Páez-Osuna F, Calderón-Campuzano M, Soto-Jiménez M, Ruelas-Inzunza J (2010) Lead in blood and eggs of the sea turtle, Lepidochelys olivacea, from the Eastern Pacific: concentration, isotopic composition and maternal transfer. Mar Pollut Bull 60(3):433–439
Páez-Osuna F, Calderón-Campuzano M, Soto-Jiménez M, Ruelas-Inzunza J (2011) Mercury in blood and eggs of the sea turtle Lepidochelys olivacea from a nesting colony in Oaxaca, Mexico. Mar Pollut Bull 62(6):1320–1323
Qadir A, Malik RN (2011) Heavy metals in eight edible fish species from two polluted tributaries (Aik and Palkhu) of the River Chenab, Pakistan. Biol Trace Elem Res 143(3):1524–1540
Rie M, Lendas K, Callard I (2001) Cadmium: tissue distribution and binding protein induction in the painted turtle, Chrysemys picta. Comp Biochem Physiol C Toxicol Pharmacol 130(1):41–51
Sakai H, Ichihashi H, Suganuma H, Tatsukawa R (1995) Heavy-metal monitoring in sea-turtles using eggs. Mar Pollut Bull 30(5):347–353. doi:10.1016/0025-326x(94)00185-c
Sakai H, Saeki K, Ichihashi H, Kamezaki N, Tanabe S, Tatsukawa R (2000a) Growth-related changes in heavy metal accumulation in green turtle (Chelonia mydas) from Yaeyama Islands, Okinawa, Japan. Arch Environ Contam Toxicol 39(3):378–385
Sakai H, Saeki K, Ichihashi H, Suganuma H, Tanabe S, Tatsukawa R (2000b) Species-specific distribution of heavy metals in tissues and organs of loggerhead turtle (Caretta caretta) and green turtle (Chelonia mydas) from Japanese coastal waters. Mar Pollut Bull 40(8):701–709. doi:10.1016/s0025-326x(00)00008-4
Storelli M, Barone G, Storelli A, Marcotrigiano G (2008) Total and subcellular distribution of trace elements (Cd, Cu and Zn) in the liver and kidney of green turtles (Chelonia mydas) from the Mediterranean Sea. Chemosphere 70(5):908–913
Storelli M, Storelli A, D’Addabbo R, Marano C, Bruno R, Marcotrigiano G (2005) Trace elements in loggerhead turtles (Caretta caretta) from the eastern Mediterranean Sea: overview and evaluation. Environ Pollut 135(1):163–170
Thomas P, Baer K, White R (1994) Isolation and partial characterization of metallothionein in the liver of the red-eared turtle (Trachemys scripta) following intraperitoneal administration of cadmium. Comparative Biochemistry and Physiology Part C. Pharmacol Toxicol Endocrinol 107(2):221–226
Torrent A, Gonzalez-DInaz O, Monagas P, Orós J (2004) Tissue distribution of metals in loggerhead turtles (Caretta caretta) stranded in the Canary Islands, Spain. Mar Pollut Bull 49(9):854–860
Acknowledgments
The authors are grateful to Pakistan Wetlands Program (PWP) for providing transport during sampling and the first author especially acknowledges the support of Mr Richard Garstang toward this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vera Slaveykova
Rights and permissions
About this article
Cite this article
Malik, R.N., Ghaffar, B. & Hashmi, M.Z. Trace metals in Ganges soft-shell turtle (Aspideretes gangeticus) from two barrage: Baloki and Rasul, Pakistan. Environ Sci Pollut Res 20, 8263–8273 (2013). https://doi.org/10.1007/s11356-013-1805-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1805-8