Abstract
Athyrium wardii is one of the dominant plant species flourishing on the Pb–Zn mine tailings in Sichuan Province, China. A greenhouse pot experiment was conducted to evaluate the chemical forms, subcellular distribution, and thiol compounds in A. wardii under different Pb treatments. The results showed that plants of the mining ecotype (ME) of A. wardii were more tolerant to Pb than those of the non-mining ecotype (NME) in spite of accumulation of higher Pb concentrations. The Pb concentrations in shoots and roots of the ME were 3.2∼8.6 times and 3.0∼24.6 times higher than those of the NME, respectively. The ME was more efficient in Pb uptake than the NME. Moreover, 27.8∼39.0 % of the total Pb in ME was sodium chloride (NaCl) extractable and 38.0∼48.5 % was acetic acid (HAc) extractable, whereas only a minority of total Pb was in ethanol and H2O extractable. In subcellular level, 77.4∼88.8 % of total Pb was stored in the cell walls of ME and 9.0∼18.9 % in soluble fractions. Increasing Pb concentrations enhanced sequestration of Pb into the cell walls and soluble fractions of ME tissues to protect organelles against Pb. Synthesis of non-protein thiols (NP-SH) and phytochelatins (PCs) in roots of ME significantly enhanced in response to Pb stress, and significant increases in glutathione (GSH) were observed in shoots of ME. Higher levels of NP-SH, GSH, and PCs were observed in roots of the ME comparing with NME, especially under high Pb treatments. The results indicated that Pb was localized mainly in cell wall and soluble fraction of ME plants with low biological activity by cell wall deposition and vacuolar compartmentalization, which might be the important adapted Pb detoxification mechanisms of ME.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pb is one of the most abundant and ubiquitously distributed toxic metals that originate from continuous exploitation of mineral resources, electronic wastes, and the disposal of sewage sludge (Alvarenga et al. 2008; Sharma and Dubey 2005). Pb mining tailing was an important source of Pb pollution, which brought many potential environment problems and had been a serious threat to human health and food safety (Nouri et al. 2011; Machado-Estrada et al. 2013). Many studies had demonstrated that phytostabilization was a suitable remediation strategy for such sites, which mainly used native plants creating a vegetative cap to reduce eolian dispersion and soil erosion and to sequester metals within the roots or rhizosphere (Mendez and Maier 2008; Sarah et al. 2012). This strategy has great practical materiality and flexibility in the ecological restoration of mining tailings and in the remediation of soil polluted by heavy metals (Claudia et al. 2008). Tolerant plants that can accumulate metals in their root tissues are encouraged to be used for the phytostabilization of soils contaminated with high levels of metals (Zou et al. 2012). However, the detoxification mechanisms of potential phytostabilizer for Pb stabilization and tolerance are still poorly understood.
In general, metal detoxification and tolerance in plants can be achieved by different strategies, including subcellular compartmentalization, chelation by metal-binding compounds, and synthesis of stress metabolites (Florence et al. 2013; Liana et al. 2012; Vogel-Mikuš et al. 2010). The avoidance of metal buildup at sensitive sites within cells is considered as the prime importance for the detoxification and survival of plants (Mishra et al. 2006a). Some evidence showed that chemical forms and subcellular distribution of heavy metals were associated with metal accumulation and detoxification in plants (Wang et al. 2008; Weng et al. 2012). Chemical forms of heavy metals were closely related to their biotoxicity and migration capability (Zhang et al. 2014a, b). Heavy metal taken up by plants existed in different chemical forms. Heavy metals in the protoplasm are not in the form of metal ions but combined with ligands and formatted compounds with low biological activity to reduce the toxicity (He et al. 2013; Tian et al. 2010). Metal compartmentalized in the cell wall or vacuoles could sequester metal into a limited space, thus reducing the concentration in cytosol and decreasing the interference with organelles (Bhargava et al. 2012). As the first barrier protecting the protoplast from metal toxicity, plant cell walls can bind and restrict the transportation of metal ions across the cytomembrane (Fu et al. 2011). In case of Sedum alfredii, large fractions of Pb were retained in the cell walls during transportation in plants (Tian et al. 2010).
Of the various detoxification pathways in plants, complexation of Pb by particular ligands and sequestration of these complexes in vacuoles are important for dealing with intracellular metal stress (Bhargava et al. 2012; Mishra et al. 2009; Zeng et al. 2009). An increased synthesis of thiol compounds in response to heavy metal has been observed in Bacopa monnieri and Najas indica (Mishra et al. 2006b; Ragini et al. 2010). The formation of glutathione (GSH)–metal and phytochelatin (PC)–metal complexes has been well demonstrated in literatures (Mishra et al. 2008; Yadav 2010). Nevertheless, not all studies have supported the role of these compounds in metal tolerance and detoxification. Gupta et al. (2010) found that the detoxification of Pb in S. alfredii is not related to PCs but mainly to GSH. According to Zeng et al. (2009), the synthesis of PCs was not important for Cd detoxification in the shoot of Arabis paniculata. Thus, the function of these compounds on metal detoxification is unclear.
Athyrium wardii (Hook.) is a common perennial plant, specifically a type of fern that grows in fascicles. It was found to be a promising material for Pb and Cd phytostabilization with a fast growth rate and large amount of biomass (Zhang et al. 2012; Zou et al. 2012). Zou et al. (2011) have provided evidences that the mining ecotype had a greater tolerance to Pb than the non-mining ecotype owing to the stronger activity of the antioxidant enzymes. Zhang et al. (2014a) found that Cd integrated with undissolved Cd–phosphate complexes in the cell wall or compartmentalization in the vacuole might be responsible for the adaptation of the mining ecotypes of A. wardii to Cd stress. However, little information was available on detoxification strategies in A. wardii under Pb stress. Therefore, the objective of this study is to analyze the change in chemical forms, subcellular distribution, and thiol compounds under different Pb treatments and their roles in Pb detoxification and accumulation in A. wardii.
Material and methods
Plant material
Seedlings for the mining ecotype (ME) were obtained from the Sanhe Pb–Zn mine tailings, Yingjing County, Ya’an, Sichuan Province, China (102° 31′ E, 29° 47′ N), where the average concentration of total Pb was about 7.62 %. Seedlings for non-mining ecotype (NME) were from uncontaminated agricultural soils in Yucheng District, Ya’an, Sichuan Province, China (103° 12′ E, 29° 40′ N) in April of 2013 (Fig. 1). The seedlings were separated into similar size plant segments composed of four∼five fronds and cultured in quartz sand for 2 weeks with 1/10 Hoagland nutrient solution (every 3 days) and deionized water during the growing season when necessary. After 2 weeks, healthy and uniform seedlings were chosen for the pot experiment at the rate of two seedlings per pot.
Pb treatment condition
A pot experiment was set up and designed as five Pb treatments (0, 200, 400, 600 and 800 mg Pb kg−1 soil) supplying as Pb(C2H3O2)2·3H2O with five replicates. The control was the treatment without Pb application. The pot experiment of A. wardii was conducted in 10-L plastic pots filled with 5 kg air-dried soil which consisted of 3 kg calcareous alluvial soil and 2 kg humus. The calcareous alluvial soil was collected from an uncontaminated farmland in Dujiangyan, Chengdu, Sichuan Province, China (Fig. 1). We set up five quadrats as an S-shaped sampling method on the farmland site. Each quadrat measured 1 m × 1 m. The soil sample was obtained from 0∼20 cm surface layer of each quadrat, mixed thoroughly, air-dried. and sieved through a 2-mm mesh screen. The humus was collected at a flower market in Dujiangyan. Pot soil was homogenized for 4 weeks with the additive Pb before the transplantation of seedlings to simulate the Pb-impacted soil. The basic characteristics of the soil after homogenization are presented in Table 1.
The pot experiment was conducted in a greenhouse with natural light, day/night temperature of 30/25 °C, and day/night humidity of 70/90 %. To maintain the suitable water-holding capacity of filled soil in pots, 400∼500 mL deionized water was applied every 3 days during the plant growth. After 40 days exposure to Pb, plants were harvested and separated into shoots and roots (including entire belowground parts). The roots were soaked in 20 mmol L−1 Na2–EDTA for 15 min to remove Pb adhering to the root surface, and the whole plants were washed with deionized water for the study of various parameters (Huang et al. 2008).
Analysis of plant and soil
Dry plant samples (100 mg) were powdered and digested in HNO3:HClO4 (5:1, v/v) at 170 °C in closed Teflon vessels until the tissues were pellucid. Digested materials were then cooled to room temperature and diluted with deionized water (Zou et al. 2011); 1 g soil was extracted with 20 mL extractant containing 5 mmol L−1 diethylene triamine pentaacetic acid (DTPA), 0.1 mol L−1 triethanolamine, and 0.01 mol L−1 CaCl2; shaken at 180 rpm min−1 for 2 h at room temperature; and extracted supernatant fluid after filtration for the determination of soil available Pb. All chemicals used were of guarantee reagent grade. The concentrations of Pb in the plants and soils were determined by atomic absorption spectrometry (AA6300, Shimadzu, Japan). The instrument working conditions were wavelength 283.3 nm, slit 0.7 nm, atomization 2000 °C, read time 3 s, sample volume 10 μL, and modifier volume 20 μL (Zou et al. 2011). Working standard solutions were prepared by dilution of standard solutions. Moreover, analytical procedure control was synchronously performed 10 times by measuring the reference materials GBW10015 with Pb content of 11.1 mg kg−1 purchased from the National Center for Certificate Reference Materials, China. Each batch included reagent blank and reference materials. The mean recoveries of Pb concentration in reference materials were 92.74 %.
Extraction of Pb in different chemical forms
Six chemical forms of Pb were differentiated between Pb in ethanol extractable (F1), H2O extractable (F2), NaCl extractable (F3), HAc extractable (F4), HCl extractable (F5), and residual fractions (F6) in plant tissues by the method of Wang et al. (2008). The sequential extraction procedure and the corresponding chemical forms are showed in Table 2. A 0.3 g fresh and frozen plant sample was first powdered in 20 mL extraction solution with a mortar and a pestle, transported in a 25-mL centrifuge tube, and shook for 22 h at 25 °C. After being shaken, the samples were centrifuged at 4000 rpm min−1 for 15 min, and the supernatant was collected and put in a 100-mL conical flask. The sedimentation was then re-extracted twice with the same 20 mL extraction solution by shaking for 2 h at 25 °C, centrifuging at 4000 rpm min−1 for 15 min and collecting the supernatants. All the three supernatants in conical flasks were evaporated, digested with HNO3:HClO4 (5:1, v/v), and then diluted with deionized water for the determination of corresponding Pb form. Then, the sedimentations were extracted step-by-step using a sequence of designated extractants in the order of 80 % ethanol, deionized water, 1 mol L−1 NaCl, 2 % CH3COOH, and 0.6 mol L−1 HCl. After extracted by five extraction solutions, the residue in centrifuge tube was digested with HNO3:HClO4 (5:1, v/v) and then diluted with deionized water. Pb concentration in the digests associated with different chemical forms was determined by atomic absorption spectrometry.
Extraction of Pb subcellular distribution
The fresh samples were quickly frozen in liquid nitrogen and stored at −75 °C. A heavy metal extraction procedure was carried out to determine what proportion of the metal was bound to according to the method described by Zhang et al. (2014a). Pb subcellular distribution of shoots and roots in both ecotypes of A. wardii was divided into cell wall, organelle fraction, and soluble fraction, respectively. The fractions, except for the soluble fraction, were evaporated separately and digested with HNO3:HClO4 (5:1, v/v) until the liquid was clear and then diluted with deionized water. Pb concentration after digesting was determined by atomic absorption spectrometry (AA6300, Shimadzu, Japan).
Determination of non-protein thiols and GSH
The determination of non-protein thiols (NP-SH) was measured according to the method of Ellman (1959) using GSH as standard. NP-SH concentration was measured in the supernatant by reaction with Ellman reagent, and absorbance was recorded at 412 nm. Level of GSH (reduced) was determined fluorometrically following Hissin and Hilf (1976).
Statistical analysis and calculation
Data were statistically analyzed by one-way ANOVA test between treatments and ecotypes using a statistical package, SPSS version 13.0. Least significant difference (LSD) was used for multiple comparisons between treatments and ecotypes. A level of p < 0.05 was considered to indicate statistical significance. PCs were calculated as NP-SH content subtracted from GSH content (Chandra et al. 2008).
Results
Plant growth and Pb concentration
As shown in Fig. 2, a significant decrease (p < 0.05) was observed in shoot and root biomass of both ecotypes of A. wardii with increasing Pb treatments. The shoot biomass of ME declined by 26.5 % at 800 mg Pb kg−1 and was less greatly affected than that of NME which declined by 44.4 % compared to the plants in control. The root biomass of ME and NME declined by 24.0 and 48.0 % at 800 mg Pb kg−1 compared to plants in control, respectively. The shoot and root biomass of ME were up to 1.2∼1.7 times and 1.5∼2.4 times higher than those of NME.
Effects of Pb treatments on shoot (a) and root (b) biomasses of two ecotypes of A. wardii. ME mining ecotype, NME non-mining ecotype, DW dry weight. Each value was the mean of five independent determinations. Error bars represent SD. Different letters represent significant difference (p < 0.05) between Pb treatments within each ecotype. An asterisk indicates a significant difference (p < 0.05) between ecotypes within each Pb treatment
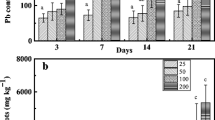
Increasing Pb accumulations in both ecotypes of A. wardii was observed with increasing soil Pb levels (Fig. 3). The highest Pb concentration was 4048 mg kg−1 (DW) in shoots of ME and 51,412 mg kg−1 (DW) in roots of ME grown in 800 mg Pb kg−1 soil, which were 21.6 times and 7.5 times higher than that grown in the control treatment, respectively. The Pb concentrations in shoots and roots of ME were 3.2∼8.6 times and 3.0∼24.6 times higher than those of NME, respectively. It is observed that the ME had a greater ability to endure high Pb stress and uptake more Pb from Pb-contaminated soils.
Effects of Pb treatments on Pb concentration in shoot (a) and root (b) of two ecotypes of A. wardii. ME mining ecotype, NME non-mining ecotype, DW dry weight. Each value was the mean of five independent determinations. Error bars represent SD. Different letters represent significant difference (p < 0.05) between Pb treatments within each ecotype An asterisk indicates a significant difference (p < 0.05) between ecotypes within each Pb treatment
Pb accumulation
Increasing Pb accumulations in both ecotypes of A. wardii was observed with increasing soil Pb levels (Fig. 4). The highest Pb accumulation was 8.66 mg plant−1 DW in shoots of ME and 175.45 mg plant−1 DW in roots of ME grown in 800 mg Pb kg−1 soil, which was 15.92 times and 5.73 times higher than that grown in the control. The Pb accumulations in shoots and roots of ME were 3.9∼13.7 times and 6.8∼36.0 times higher than those of NME, respectively. It was observed that the ME had a greater ability to endure high Pb stress and can uptake more Pb from Pb-contaminated soils.
Effects of Pb treatments on Pb accumulation in shoot (a) and root (b) of two ecotypes of A. wardii. ME mining ecotype, NME non-mining ecotype, DW dry weight. Each value was the mean of five independent determinations. Error bars represent SD. Different letters represent significant difference (p < 0.05) between Pb treatments within each ecotype. An asterisk indicates a significant difference (p < 0.05) between ecotypes within each Pb treatment
Pb chemical forms
The Pb concentrations of different chemical forms in both ecotypes of A. wadii significantly increased with increasing Pb treatments (Tables 3 and 4). Pb concentrations of each chemical form in roots of ME were significantly higher than those of NME (p < 0.05), while no significant differences showed on residual form in shoots of both ecotypes at 200∼400 mg Pb kg−1 soil. In shoots and roots of ME, the highest concentration of Pb was found in HAc extractable, followed by NaCl extractable, and the concentrations of Pb in H2O extractable and ethanol extractable were relatively lower. However, in shoots and roots of NME, the highest concentration of Pb was found in NaCl extractable following by HAc extractable.
The proportions of HAc extractable and NaCl extractable in shoots of ME represented 72.6∼85.1 % of the total amount, while the forms in H2O extractable and ethanol extractable occupied 6.5∼11.5 %. In shoots of NME, proportions of H2O extractable and ethanol extractable represented 14.0∼21.9 %, which were higher than those of ME. The proportions of HAc extractable and NaCl extractable in shoots of ME increased with increasing Pb treatments in soil, and the proportions of H2O extractable and ethanol extractable decreased, whereas an opposite trend appeared in shoots of NME (Fig. 5a). In roots of ME, HAc extractable and NaCl extractable occupied 67.5∼76.1 % of total Pb, and 7.5∼15.6 % was HCl extractable. The proportion of Pb in H2O extractable and ethanol extractable occupied 10.6∼14.8 % in roots of ME, which was higher than that of NME. The proportions of HAc extractable and NaCl extractable in roots of NME represented 78.8∼87.1 % of the total amount, while the forms in H2O extractable and ethanol extractable occupied 6.9∼12.7 % (Fig. 5b).
Change in Pb chemical forms in shoot (a) and root (b) of two ecotypes of A. wardii as affected by different Pb treatments. ME mining ecotype, NME non-mining ecotype. F1, F2, F3, F4, F5, and F6 represent Pb forms in ethanol extractable, H2O extractable, NaCl extractable, HAc extractable, HCl extractable, and residual fractions, respectively
Pb subcellular distribution
The order of Pb concentrations in both ecotypes of A.wadii tissues was cell wall > soluble fraction > cell organelle (Table 5). Pb concentrations of all subcellular fractions in ME significantly increased with increasing Pb treatments (p < 0.05). Pb concentrations of cell wall in shoots and roots of ME were significantly higher than those of NME (p < 0.05), while no significant differences showed on Pb concentrations of soluble fraction and cell organelle in shoots of both ecotypes at 200 mg Pb kg−1 soil and on Pb concentrations of organelle fraction in roots of both ecotypes at 800 mg Pb kg−1 soil.
As shown in Fig. 6, 78.1∼83.6 % of total Pb in shoots of ME was found in the cell wall, and 12.4∼18.9 % was presented in soluble fraction, while only a small portion was found in organelle fraction. In roots of ME, 77.4∼88.8 and 9.0∼18.4 % of total Pb was in cell wall and soluble fraction, respectively. The same trend appeared in shoots and roots of NME, where most of Pb was found in cell wall and soluble fraction of NME. With increasing Pb treatments, the proportion of Pb in soluble fraction increased followed by a decrease in cell wall. The proportion of Pb in organelle fraction remained fairly constant in shoots and roots of ME, while increased in NME with increasing Pb supply in soil.
Thiol compounds
As shown in Table 6, NP-SH contents in shoots and roots of ME increased steadily in response to increasing Pb treatments (Table 3). The maximum NP-SH contents in shoots and roots of ME were 1.68 and 1.75 times higher than in NME, respectively. GSH contents in shoot of ME showed a significant increase (p < 0.05) with increasing Pb treatments. The maximum in shoot of ME was 1.03 nmol g−1 FW at 800 mg Pb kg−1, which enhanced 1.6 times comparing with CK and was significantly higher than that of NME (p < 0.05). The GSH contents in roots of ME were significantly higher than those of NME. PC contents in shoots of ME had no significant change as compared to CK. At 800 mg Pb kg−1, PC content in shoots of ME was 2.6 times higher than that of NME. Meanwhile, a steady increase of PC content in roots of ME was found with increase in Pb treatments.
Discussion
Plant growth and Pb accumulation
It has been reported that plants always accumulate higher concentrations of Pb in roots and lower in shoots due to long-distance translocation from roots to shoots (Estrella-Gómez et al. 2009; Weng et al. 2012). This Pb distribution system may indicate roots serving as a partial barrier to prevent Pb transporting to the shoots (Sharma and Dubey 2005). In our study, Pb concentration in shoots of ME had reached 4048 mg kg−1 DW at 800 mg Pb kg−1, which was 7.3 times higher than the result of Zou et al. (2011), who found that Pb concentration in shoots of ME was as high as 556 mg kg−1 DW at 800 mg Pb kg−1. This may be relative to a higher level of Pb availability in soil in our study. To supply adequate nutrients, we added 2 kg humus in each pot. Humus supplied large amount of organic matter to soils that can serve as chelates and increase metal availability to plants (Antoniadis et al. 2008). It had been reported that organic matter content was positively correlated with extractable Cr contents in soils and Cr concentrations in rice tissues(Zeng et al. 2011), which was in line with our study. At 800 mg Pb kg−1, Pb accumulation in root of ME was 6.8 times higher than that of NME and Pb uptake in shoot of ME was much higher as compared to the NME (Fig. 4), which suggested that ME plants had extraordinary capacity to take up and transport Pb from roots to shoots as compared to the NME. We also found that after 40 days of treatment with Pb, approximately 95 % of the total Pb uptake was retained in the roots. Justin et al. (2011) indicated that Cu was highly concentrated in the roots of Acacia mangium when grown in heavy metal-contaminated soils. Waranusantigul et al. (2011) found that Eucalyptus camaldulensis, one of the ideal candidates for rehabilitation of heavy metal-contaminated areas, accumulated Pb dominantly in roots rather than shoots. Other reports also confirmed that roots are the most important part for Pisum sativum and Sorghum halepense plants to accumulate Pb (Małecka et al. 2008; Salazar and Pignata 2014). This indicated that plant roots played an important role in heavy metal accumulation and storage and prevented metal transporting to shoot to endure high metal stress.
Pb chemical forms
Chemical forms of heavy metals were closely related to their toxicity and migration in plants (Wu et al. 2011; Zhang et al. 2014b). For instance, ethanol-extractable and water-soluble Pb had the highest activity, followed by NaCl extractable, while HAc and HCl extractable showed the weakest migration activity (Xu et al. 2012). For plants containing high concentration of metal that showed low toxicity, metal ions should be in chemical forms that cause low phytotoxicity (Fu et al. 2011).
In our study, the HAc and NaCl extractable were dominant in the shoots and roots of ME under various Pb treatments, while only minority of total Pb was in ethanol and H2O extractable (Fig. 5), which suggests that larger proportion of Pb in low phytotoxicity was responsible for the lower toxicity of Pb in ME. NaCl extractable was found to bind to proteins and pectic acids, and 2 % HAc extracted insoluble Pb–phosphate complexes (Wang et al. 2008). Pb integrated to these chemical forms in cell walls or vacuoles might be crucial for Pb detoxification and accumulation in ME under Pb stress. This result was in line with the study of He et al. (2013), who found that NaCl and HAc extractable was the dominating form of Pb in shoots of Lolium perenne. Previous studies in maize and Kandelia obovata also showed that a majority of Cd associated with pectates and proteins, and Cd–phosphate complexes led to the detoxification in plants (Wang et al. 2008; Weng et al. 2012). Moreover, Pb proportions of ethanol and H2O extractable in roots of ME were higher than those of NME. Pb extracted by ethanol and H2O had higher ability to migrate, which were mainly combined with nitrate ions, chlorides, organic acids, and dihydric phosphates (He et al. 2013; Zhang et al. 2014a). These forms in roots of ME suggested a higher capacity transferring Pb to the aboveground. This might be contributed to the higher Pb accumulation in shoots of ME.
Pb Subcellular distribution
The compartmentalization of metals at cellular and subcellular levels is extremely important for the detoxification and accumulation of metals in plants (Zhou et al. 2010). Cell walls were considered as the first barrier to protect the protoplast from heavy metal poisoning. The cell wall usually includes protein and polyoses, which have a lot of potential ligands such as hydroxyl, carboxyl, amino group, aldehyde group, phosphate, and thiol (Wang et al. 2008; Xin et al. 2013). These ligands can participate in a variety of reactions leading to sequestration of the metals in a cellular compartment with low metabolic activity and prevent Pb from entering into the cell interior (Lux et al. 2011; Weng et al. 2012).
In this study, 77.4∼88.8 % of Pb was stored in the cell walls in shoots and roots of the ME (Fig. 6). Wang et al. (2008) found that immobilization of Pb in the cell walls was important to maintain normal physiological activities in ramie. Małecka et al. (2008) found that binding of Pb into the cell walls is one of the defense mechanisms of P. sativum, which plants developed against the toxic effect of heavy metals. Similar results had been observed in Phytolacca americana and Boehmeria nivea (Fu et al. 2011; Zhu et al. 2013). It suggested that, as the primary subcellular fraction for Pb storage, binding Pb in the cell walls is both an effective and important mechanism for Pb detoxification in ME. A larger portion of Pb in the cell walls was contributed to lower Pb accumulations in organelles and solution fractions implying a higher capacity for Pb biologically detoxify in ME.
However, the capability of the cell walls to bind Pb is not unlimited. When cell wall binding sites reached saturation, most intracellular metal ions were transported to vacuoles so that metal compartmentation was achieved (Krämer 2000). In the present study, 9.0∼18.9 % of total Pb was found in soluble fraction including cell vacuoles in shoots and roots of ME. Plant vacuoles principally composed of sulfur-rich peptides and organic acids, which can reduce toxicity via complexation of metal with organic ligands (Vogel-Mikuš et al. 2010). Sequestration of heavy metal into vacuoles could further prevent metal ions from interfering with organelle function (Lux et al. 2011; Xin et al. 2013). Fu et al. (2011) found that 53.7∼68.3 % of Cd was stored in the soluble fraction, which is the major storage site of Cd in pokeweed. As reported by Ma et al. (2005), 91 % of Zn in the protoplast was found in the vacuoles of Thlaspi caerulescens leaves, which indicated that vacuoles were involved in metal tolerance mechanisms. It was suggested that sequestration of Pb into vacuoles could further reduce Pb toxicity in the cytoplasm and further avoid Pb buildup at organelle fraction in ME. In root of pea, Pb was localized mainly in the cell wall, vacuoles, or cell voids; however, Pb was also localized in plant cell organelles under higher Pb stress (Małecka et al. 2008). In our study, the proportion of Pb in organelle fraction increased in shoots and roots of NME, while remained fairly constant in ME with increasing Pb supply in soil. Moreover, the biomass of NME significantly decreased at 200 mg Pb kg−1 soil, and the biomass of ME declined at 400∼600 mg Pb kg−1 soil. These results indicated that the ME being more tolerant to Pb might be attributed to cell wall deposition and vacuolar compartmentalization, which avoided Pb buildup at organelle fraction and protected organelles against Pb.
Thiol compounds
It has been well documented that one of the important strategies for the heavy metal detoxification in plants is the complexation with strong ligands such as NP-SH and PCs (Isaure et al. 2006; Vogel-Mikuš et al. 2010). The plant exhibiting high content of NP-SH under metal stress indicated its ability to tolerate cellular metal load (Chandra et al. 2008). In our study, NP-SH content in shoots and roots of ME showed a significant increase in response to Pb and was higher than that of NME, particularly in root (Table 6). It was suggested that ME had a greater ability to tolerate cellular Pb load. Moreover, the content of NP-SH increased at lower Pb stress but decreased at higher Pb stress both in shoots and roots of NME plants, which was not observed in ME plants. The synthesis of NP-SH could be attributed to increased activity of enzymes of sulfate reduction pathway and assimilate into GSH, PCs, and cysteine and possibly also reflects a defense reaction to enhanced production of reactive oxygen species (ROS) (Michael et al. 2006; Mishra et al. 2006a). However, some physiological functions of plants might be damaged under high Pb stress, which impacted the synthesis of NP-SH. This observation was in agreement with the previous studies in Ceratophyllum demersum and N. indica (Mishra et al. 2009; Ragini et al. 2010).
As the most important low molecular weight peptides in plants, GSH took part in a plethora of cellular detoxification processes (Rouhier et al. 2008; Yadav 2010). Besides its function in defense against ROS and protecting the membrane, GSH acts as a powerful detoxifier of heavy metals by complexation and it can serve as a precursor of PCs (Amalia et al. 2011; Weng et al. 2012). It had been reported that a significant increase in the GSH concentration was also found in roots and shoots of Salvinia minima and in roots of Melilotus alba and Melilotus officinalis in short- and long-term Pb treatments, respectively (Estrella-Gómez et al. 2012; Fernández et al. 2012). In our study, GSH content in shoots of ME conspicuously enhanced following increasing Pb treatments and was notably higher than that of NME at 800 mg Pb kg−1. GSH content in root of ME was 1.3 times higher than that of NME, although no significant change was observed in roots of both ecotypes of A. wardii in response to Pb. The increasing GSH content may be relative to that Pb-stimulated GSH biosynthesis gene transcription and the synthesis of glutathione synthetase, glutathione reductase, and other enzymes (Maserti et al. 2005). Depletion of GSH in the shoots of NME might be thus a consequence of its participation in oxidative stress quenching, but this assumption needs further thorough investigations. The higher GSH content might play an important role in protecting shoots of ME from the detrimental effects of Pb by counteracting the effect of free radicals and lead to the Pb detoxification. The change of GSH in roots being not in line with the shoots of ME might be the result of the fact that GSH was metabolized as a precursor for PCs ensuring the production of this tripeptide as the main mechanism of Pb detoxification operating in roots (Gupta et al. 2010). Estrella-Gómez et al. (2012) found that GSH and PCs participated in a complex and coordinated mechanism of Pb detoxification in S. minima, PCs being relatively more important in roots while GSH appears to be more important in leaves. It suggested that GSH and PCs play different roles in the Pb detoxification in different tissues of plants. In our study, GSH might be relatively more crucial in shoots of ME. A higher level of GSH in root of ME than NME provides a better condition for the synthesis of PCs.
PC binds to Pb ions leading to sequestration of Pb in plants and thus serves as an important component of the detoxification mechanism in plants (Gupta et al. 2013). Estrella-Gómez et al. (2009) found that the accumulation of PCs in S. minima was a direct response to Pb accumulation. PC contents in roots of ME enhanced following the increasing Pb treatments and were markedly higher than that of NME at 600 and 800 mg Pb kg−1 (Table 6). Formation of PC–metal complex in response to heavy metal was also well documented in literature (Yadav 2010). PCs formed complexes with metal ions in the cytoplasm, thus reducing metal toxicity in this metabolically active cellular compartment; subsequently, such complexes were transferred into the vacuole and vacuolar compartmentalization was achieved (Zhang et al. 2014b; Yadav 2010). The presence of PC–Pb complexes was shown in vivo in, e.g., M. alba and M. officinalis (Fernández et al. 2012) or vetiver grass (Andra et al. 2010), suggesting their role in Pb tolerance. According to Wójcik and Tukiendorf (2014), Pb treatment induced synthesis of PCs only in the roots of Dianthus carthusianorum, with significantly higher concentrations thereof detected in the NME. In this study, the PC content in roots of ME significantly higher than NME at high Pb conditions indicated that the ME might possess a superior capacity to formation of PC–Pb complexes for the cleanup and alleviation of Pb toxicity in roots. Similar to the present finding, synthesis of PCs in response to heavy metal stress had been well documented in K. obovata and Vetiver grass (Andra et al. 2010; Weng et al. 2012). These PC–Pb complexes migrating into vacuoles might contribute to the compartmentalization and the higher Pb concentration in soluble fraction of ME tissues. However, the determination method of PCs used in this study is rough. It cannot distinguish between PCs, γ-EC, and cysteine precisely. Hence, further thorough investigations by a quantity HPLC analysis of thiol compounds were needed to confirm the exact role of theses thiols in elevated Pb tolerance.
Conclusions
In the present research, there was a notable difference in growth inhibition for Pb among both ecotypes of A. wardii. The ME was more efficient in Pb accumulation than the NME and had a greater Pb accumulation in roots than in shoots. The majority of Pb in the ME was extracted by HAc and NaCl, suggesting that larger proportion of Pb was in low phytotoxicity. Larger percentage of Pb was bound to the cell wall fraction and soluble fraction at the subcellular level of the ME. Furthermore, the ME was able to induce more thiol compound synthesis to further avoid Pb buildup at organelle fraction under Pb stress.
These characteristics indicated that cell wall deposition and vacuolar compartmentalization might be the important adapted Pb detoxification mechanisms of the ME, which ensured it adapt well to growing in extremely Pb-polluted habitats as a good candidate for phytostabilization.
References
Alvarenga P, Goncalves AP, Fernandes RM, De Varennes A, Vallini G, Duarte E, Cunha-Queda AC (2008) Evaluation of composts and liming materials in the phytostabilization of a mine soil using perennial ryegrass. Sci Total Environ 406:43–56
Amalia MM, Ernesto FT, Fernando RC, Tania LVS (2011) Lead bioaccumulation in Acacia farnesiana and its effect on lipid peroxidation and glutathione production. Plant Soil 339:377–389
Andra SS, Datta R, Sarkar D, Makris KC, Mullens CP, Sahi SV, Stephan BH (2010) Synthesis of phytochelatins in vetiver grass upon lead exposure in the presence of phosphorus. Plant Soil 326:171–185
Antoniadis V, Robinson JS, Alloway BJ (2008) Effects of short-term pH fluctuations on cadmium, nickel, lead, and zinc availability to ryegrass in a sewage sludge-amended field. Chemosphere 71:759–764
Bhargava A, Carmona FF, Bhargava M, Srivastava S (2012) Approaches for enhanced phytoextraction of heavy metals. J Environ Manage 105:103–120
Chandra SS, Pranav KC, Virendra M (2008) The role of phytochelatins and antioxidants in tolerance to Cd accumulation in Brassica juncea L. Ecotox Environ Safe 71:76–85
Claudia S, Cesar V, Rosanna G (2008) Phytostabilization of copper mine tailings with biosolids: implications for metal uptake and productivity of Lolium perenne. Sci Total Environ 395:1–10
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Estrella-Gómez NE, Mendoza-Cozatl DG, Moreno SR, González MD, Zapata PO, Martínez HA, Santamaría JM (2009) The Pb-hyperaccumulator aquatic fern Salvinia minima Baker, responds to Pb2+ by increasing phytochelatins via changes in SmPCS expression and in phytochelatin synthase activity. Aquat Toxicol 91:320–328
Estrella-Gómez NE, Sauri-Duch E, Zapata-Pérez O, Santamaría JM (2012) Glutathione plays a role in protecting leaves of Salvinia minima from Pb2+ damage associated with changes in the expression of SmGS genes and increased activity of GS. Environ Exp Bot 75:188–194
Fernández R, Bertrand A, García JI, Tamés RS, González A (2012) Lead accumulation and synthesis of non-protein thiolic peptides in selected clones of Melilotus alba and Melilotus officinalis. Environ Exp Bot 78:18–24
Florence A, Mouna F, Patricia M, Anaïs B, Laurent L, Mohamed EM, Abdelkarim FM, Patrick D, Abdelaziz S (2013) Lead tolerance and accumulation in Hirschfeldia incana, a Mediterranean Brassicaceae from metalliferous mine spoils. PLoS ONE 8:1–10
Fu XP, Dou CM, Chen YX, Chen XC, Shi JY, Yu MG, Xu J (2011) Subcellular distribution and chemical forms of cadmium in Phytolacca americana L. J Hazard Mater 186:103–107
Gupta DK, Huang HG, Yang XE, Razafindrabe BHN, Inouheb M (2010) The detoxification of lead in Sedum alfredii H. is not related to phytochelatins but the glutathione. J Hazard Mater 177:437–444
Gupta DK, Huang HG, Corpas FJ (2013) Lead tolerance in plants: strategies for phytoremediation. Environ Sci Pollut Res 20:2150–2161
Huang HG, Li TX, Tian SK, Gupta DK, Zhang XZ, Yang XE (2008) Role of EDTA in alleviating lead toxicity in accumulator species of Sedum alfredii H. Bioresource Technol 99:6088–6096
He SY, Wua QL, He ZL (2013) Effect of DA-6 and EDTA alone or in combination on uptake, subcellular distribution and chemical form of Pb in Lolium perenne. Chemosphere 93:2782–2788
Hissin PJ, Hilf R (1976) A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 74:214–226
Isaure MP, Fayard B, Sarret G, Pairis S, Bourguignon J (2006) Localization and chemical forms of cadmium in plant samples by combining analytical electron microscopy and X-ray spectromicroscopy. Spectrochim Acta B 61:1242–1252
Justin V, Majid NM, Islam MM, Abdu A (2011) Assessment of heavy metal uptake and translocation in Acacia mangium for phytoremediation of cadmium-contaminated soil. J Food Agric Environ 9:588–592
Krämer U (2000) Cadmium for all meals—plants with an unusual appetite. New Phytol 145:1–3
Liana VR, Fernando TN, Júlia GF, Denise C, Luciane AT, Fabiane GA, Valderi LD, Vera MM, Maria RCS (2012) Effects of lead on the growth, lead accumulation and physiological responses of Pluchea sagittalis. Ecotoxicology 21:111–123
Lux A, Vaculík M, Martinka M, Lišková D, Kulkarni MG, Stirk WA, Van Staden J (2011) Cadmium induces hypodermal periderm formation in the roots of the monocotyledonous medicinal plant Merwilla plumbea. Ann Bot 107:285–292
Ma JF, Daisei U, Zhao FJ, Steve PM (2005) Subcellular localisation of Cd and Zn in the leaves of a Cd-hyperaccumulating ecotype of Thlaspi caerulescens. Planta 220:731–736
Maserti BE, Ferrillo V, Avdis O, Nesti U, Garbo AD, Catsiki A, Maestrini PL (2005) Relationship of non-protein thiol pools and accumulated Cd or Hg in the marine macrophyte Posidonia oceanica (L.) Delile. Aquat Toxicol 75:288–292
Mendez MO, Maier RM (2008) Phytostabilization of mine tailings in arid and semiarid environments-an emerging remediation technology. Environ Health Perspect 116:278–283
Michael R, Krauss GJ, Gregor G, Dirk W (2006) Sulphate assimilation under Cd2+ stress in Physcomitrella patens—combined transcript, enzyme and metabolite profiling. Plant Cell Environ 29:1801–1811
Machado-Estrada B, Calderón J, Moreno-Sánchez R, Rodríguez-Zavala JS (2013) Accumulation of arsenic, lead, copper, and zinc, and synthesis of phytochelatins by indigenous plants of a mining impacted area. Environ Sci Pollut Res 20:3946–3955
Małecka A, Piechalak A, Morkunas I, Tomaszewska B (2008) Accumulation of lead in root cells of Pisum sativum. Acta Physiol Plant 30:629–637
Mishra S, Srivastava S, Tripathi RD, Kumar R, Seth CS, Gupta DK (2006a) Lead detoxification by coontail (Ceratophyllum demersum L.) involves induction of phytochelatins and antioxidant system in response to its accumulation. Chemosphere 65:1027–1039
Mishra S, Srivastava S, Tripathi RD, Govindarajan R, Kuriakose SV, Prasad MNV (2006b) Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L. Plant Physiol Bioch 44:25–37
Mishra S, Srivastava S, Tripathi RD, Trivedi PK (2008) Thiol metabolism and antioxidant systems complement each other during arsenate detoxification in Ceratophyllum demersum L. Aquat Toxicol 86:205–215
Mishra S, Tripathi RD, Srivastava S, Dwivedi SJ, Trivedi PK, Dhankher OP, Khare A (2009) Thiol metabolism play significant role during cadmium detoxification by Ceratophyllum demersum L. Bioresource Technol 100:2155–2161
Nouri J, Lorestani B, Yousefi N, Khorasani N, Hasani AH, Seif F, Cheraghi M (2011) Phytoremediation potential of native plants grown in the vicinity of Ahangaran lead–zinc mine (Hamedan, Iran). Environ Earth Sci 62:639–644
Ragini S, Tripathi RD, Sanjay D, Amit K, Trivedi PK, Chakrabarty D (2010) Lead bioaccumulation potential of an aquatic macrophyte Najas indica are related to antioxidant system. Bioresource Technol 101:3025–3032
Rouhier N, Lemaire SD, Jacquot JP (2008) The role of glutathione in photosynthetic organisms: emerging functions for glutaredoxins and glutathionylation. Annu Rev Plant Biol 59:143–66
Salazar MJ, Pignata ML (2014) Lead accumulation in plants grown in polluted soils. Screening of native species for phytoremediation. J Geochem Explor 137:29–36
Sarah CRS, Sara ALA, Lucas AS, Marlene AS (2012) Lead tolerance and phytoremediation potential of Brazilian leguminous tree species at the seedling stage. J Environ Manag 110:299–307
Sharma P, Dubey RS (2005) Lead toxicity in plants. Braz J Plant Physiol 17:35–52
Tian SK, Lu LL, Yang XE, Samuel MW, Du YH, Patrick HB (2010) Spatial imaging and speciation of lead in the accumulator plant Sedum alfredii by microscopically focused synchrotron X-ray investigation. Environ Sci Technol 44:5920–5926
Vogel-Mikuš K, Arčon I, Kodre A (2010) Complexation of cadmium in seeds and vegetative tissues of the cadmium hyperaccumulator Thlaspi praecox as studied by X-ray absorption spectroscopy. Plant Soil 331:439–451
Waranusantigul P, Lee H, Kruatrachue M, Pokethitiyook P, Auesukaree C (2011) Isolation and characterization of lead-tolerant Ochrobactrum intermedium and its role in enhancing lead accumulation by Eucalyptus camaldulensis. Chemosphere 85:584–590
Wang X, Liu YG, Zeng GM, Chai LY, Song XC, Min ZY, Xiao X (2008) Subcellular distribution and chemical forms of cadmium in Bechmeria nivea (L.) Gaud. Environ Exp Bot 62:389–395
Weng BS, Xie XY, Weiss DJ, Liu JC, Lu HL, Yan CL (2012) Kandelia obovata (S., L.) Yong tolerance mechanisms to cadmium: subcellular distribution, chemical forms and thiol pools. Mar Pollut Bull 64:2453–2460
Wu Q, Lu YF, Shi JZ, Liang SX, Shi JS, Liu J (2011) Chemical form of metals in traditional medicines underlines potential toxicity in cell cultures. J Ethnopharmacol 134:839–843
Wójcik M, Tukiendorf A (2014) Accumulation and tolerance of lead in two contrasting ecotypes of Dianthus carthusianorum. Phytochemistry 100:60–65
Xin JL, Huang BF, Yang ZY, Yuan JG, Zhang YD (2013) Comparison of cadmium subcellular distribution in different organs of two water spinach (Ipomoea aquatica Forsk.) cultivars. Plant Soil 373:431–444
Xu QS, Min HL, Cai SJ, Fu YY, Sha S, Xie KB, Du KH (2012) Subcellular distribution and toxicity of cadmium in Potamogeton crispus L. Chemosphere 89:114–120
Yadav SK (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot 76:167–179
Zeng XW, Mab LQ, Qiu RL, Tang YT (2009) Responses of non-protein thiols to Cd exposure in Cd hyperaccumulator Arabis paniculata Franch. Environ Exp Bot 66:242–248
Zeng FR, Shafaqat A, Zhang HT, Ouyang YN, Qiu BO, Wu FB, Zhang GP (2011) The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ Pollut 159:84–91
Zhang SJ, Li TX, Huang HG, Zou TJ, Zhang XZ, Yu HY, Zheng ZC, Wang YD (2012) Cd accumulation and phytostabilization potential of dominant plants surrounding mining tailings. Environ Sci Pollut Res 19:3879–3888
Zhang SJ, Li TX, Huang HG, Zou TJ, Zhang XZ, Yu HY, Zheng ZC, Wang YD, Zou TJ, Hao XQ, Pu Y (2014a) Phytoremediation of cadmium using plant species of Athyrium wardii (Hook.). Int J Environ Sci Technol 11:757–764
Zhang W, Lin KF, Zhou J, Zhang W, Liu LL, Zhang QQ (2014b) Cadmium accumulation, sub-cellular distribution and chemical forms in rice seedling in the presence of sulfur. Environ Toxicol Phar 37:348–353
Zhou YQ, Huang SZ, Yu SL, Gu JG, Zhao JZ, Han YL, Fu JJ (2010) The physiological response and sub-cellular localization of lead and cadmium in Iris pseudacorus L. Ecotoxicology 19:69–76
Zhu QH, Huang DY, Liu SL, Luo ZC, Rao ZX, Cao XL, Ren XF (2013) Accumulation and subcellular distribution of cadmium in ramie (Boehmeria nivea L. Gaud.) planted on elevated soil cadmium contents. Plant Soil Environ 59:57–61
Zou TJ, Li TX, Zhang XZ, Yu HY, Huang HG (2012) Lead accumulation and phytostabilization potential of dominant plant species growing in a lead–zinc mine tailing. Environ Earth Sci 65:621–630
Zou TJ, Li TX, Zhang XZ, Yu HY, Luo HB (2011) Lead accumulation and tolerance characteristics of Athyrium wardii (Hook.) as a potential phytostabilizer. J Hazard Mater 186:683–689
Acknowledgments
This study was carried out with support from the Sichuan Science and Technology Support Program (2013NZ0029), the Sichuan Science and Technology Support Program (2014NZ0008), and the Project of Sichuan Education Department (14ZB0017). The authors also wish to thank Craig Stapleton and Shujin Zhang for their critical comments regarding the language and construction of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Zhao, L., Li, T., Yu, H. et al. Changes in chemical forms, subcellular distribution, and thiol compounds involved in Pb accumulation and detoxification in Athyrium wardii (Hook.). Environ Sci Pollut Res 22, 12676–12688 (2015). https://doi.org/10.1007/s11356-015-4464-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4464-0