Abstract
Screening out plants that are hyper-tolerant to certain heavy metals plays a fundamental role in remediation of mine tailing. In this study, nine dominant plant species growing on lead–zinc mine tailing and their corresponding non-mining ecotypes were investigated for their potential phytostabilization of lead. Lead concentration in roots of these plants was higher than in shoots, and the highest concentrations of lead were found in Athyrium wardii: 15542 and 10720 mg kg−1 in the early growth stage (May) and vigorous growth stage (August) respectively, which were 426 and 455 times higher than those of the non-mining ecotypes. Because of poor lead translocation ability, lead accumulation in roots reached as high as 42 mg per plant. Available lead in the rhizosphere soils of A. wardii was 310 mg kg−1, which was 17 times higher than that of the non-rhizosphere soil. Lead concentrations of roots for the nine mining ecotypes were positively correlated with available lead in the rhizosphere soils, whereas a negative correlation was observed in the non-mining ecotypes. These results suggest that A. wardii was the most promising candidate among the tested species for lead accumulation in roots, and it could be used for phytostabilization in lead polluted soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mining sites have encroached about 40,000 km2 of land in China, and the annual increase of mining wasteland of 330 km2 serves as a growing and persistent source of pollutant (Li and Jiang 2004). The resulting environmental problems are becoming increasingly serious and pose significant threats to human health and food safety (Sahi et al. 2002; Li 2005). Therefore, development of economical and efficient technologies for remediation of polluted soil is an urgent need, which has attracted wide public attention (Tordoff et al. 2000; Yang et al. 2001; Shu et al. 2002; Arienzo et al. 2004).
Conventionally, applying biosolids in combination with alkaline residues, such as sludge and manure, has been considered to be effective in reducing the concentration of heavy metals in soil solution and stabilizing them in the rhizosphere by raising soil pH (Wong 2003; Walker et al. 2004). However, this measure cannot be widely used as it causes secondary pollution. At present, a commonly adopted approach to treat lead contaminated soil is to use chemical compounds containing phosphorus to immobilize soil lead (Melamed et al. 2003; Cao et al. 2004; Chaturvedi et al. 2007; Kumpiene et al. 2008). However, long-term application of some phosphorus compounds may cause a decrease in soil pH and an increase in solubility of other heavy metals (McGowen et al. 2001). Furthermore, phosphorus becomes another important pollutant. Due to leaching and runoff, high levels of phosphorus are leading to non-point pollution of lakes, rivers, and other water bodies, which will cause eutrophication in the aquatic environment (Correll 1998).
An alternative and widely used approach with great advantages over traditional methods is phytoremediation of soil heavy metals, which is cost-effective and eco-friendly (Mulligan et al. 2001; Lasat 2002). There are two main plant-based technologies for remediation of soil pollution: phytoextraction and phytostabilization (Lin and Mendelssohn 1998; Alkorta and Garbisu 2001). Previous studies have mostly focused on phytoextraction, which uses hyperaccumulators to take up heavy metals, transfer them into the shoots, and sequester them in non-metabolically active tissues and organs in less harmful forms (Küpper et al. 2007). A hyperaccumulator has been defined as (1) a plant that can accumulate cadmium > 100 mg/kg, copper, lead > 1,000 mg/kg and zinc > 10,000 mg/kg in their shoot dry matter, (2) in accumulator plants, the metal concentrations in shoots are invariably greater than those in roots or shoot/root quotient > 1, and (3) extraction coefficient > 1 (Salt et al. 1995; Brooks et al. 1998; Rotkittikhun et al. 2006).
However, even though heavy metals can be absorbed by hyperaccumulators, these plants are not suitable for every kind of mining site. Mine tailings as unreclaimed mining sites generally remain unvegetated for tens to hundreds of years, and exposed tailings can spread over tens of hectares via aeolian dispersion and water erosion, which can pose particular threats to the health of people and plants (Cobb et al. 2000; González and González-Chávez 2006). Therefore, phytoextraction is not suitable for such sites. Phytostabilization, on the other hand, primarily focuses on sequestration of the metals within the roots and rhizosphere (Mendez and Maier 2008). This remediation strategy creates a vegetative cap for the long-term stabilization and containment of tailings. Plant canopies serve to reduce aeolian dispersion, while plant roots prevent water erosion, immobilize heavy metals by adsorption or accumulation, and provide a rhizosphere wherein metals precipitate and stabilize (Boularbah et al. 2006; Santibáñez et al. 2008). Consequently, phytostabilization has great practical significance and flexibility in ecological restoration of tailings and remediation of soil polluted by heavy metals.
To be suitable for phytostabilization, plants should possess an extensive root system and a large amount of biomass in the presence of high concentrations of heavy metals, and keep the translocation of metals from roots to shoots as low as possible (Alvarenga et al. 2008). Therefore, with reference to the standard for hyperaccumulators, the bioconcentration factor of root (BCFR) is an additional evaluation index for phytostabilization. Although analyzing the total content of heavy metal in the soil can give some information about the enrichment of heavy metal in soil, it can neither demonstrate translocation ability and bio-availability nor provide a sufficient standard for evaluating the effects on the organism (Liu et al. 2002). However, plants are directly affected by the available content of heavy metal, when metals enter the soil, so the ability of tolerance and accumulation in root could be well reflected by the bioconcentration factor of root.
During the process of ecological adaptation, different populations of the same species are affected over the long term by different environmental conditions that will generate differentiation in ecology among the populations, namely different ecotypes (Yang et al. 2001; Islam et al. 2007). Populations with high tolerance to heavy metal toxicity are usually distributed in mines and tailings. To date, most screening investigations on plant selection for accumulators of heavy metals have been carried out in mining areas with different plant species, but have seldom involved different ecotypes of the same varieties. Yang et al. (2001) reported that Sedum alfredii Hance. growing in a zinc-lead mining area and non-mining areas in southeast China gradually evolved into different ecotypes, which showed significant differences in absorption and accumulation of Zn. Therefore, comparison of different ecotypes in terms of their absorptive capacities can identify real accumulators and is important for understanding microevolution mechanisms of tolerance and accumulation in plants.
In spite of the potential usefulness of phytostabililization for use on tailings of heavy metal mines, documentation of this remediation strategy is rare in the published literature and understanding of its requirements is limited, especially in terms of screening suitable plant species to stabilize lead in mine tailings. In order to examine the potential for effectively stabilizing lead, an on-site survey of lead tailings in Yingjing County, Ya’an, Sichuan Province was conducted in 2007 and 2008. Thirty-two plant species belonging to 19 families were examined for biomass and lead concentration (data not shown). Nine predominant plant species were found growing well and with a wide distribution in this area (Table 1). The objectives of the present study were to compare these nine plant species growing in tailing and non-mining areas in terms of biomass and lead concentration of roots at three growth stages, to evaluate their lead accumulation potentials and identify candidate species for application in phytostabilization of lead polluted soils.
Materials and methods
Site description
Mining ecotypes of the 9 previously identified plant species were collected at the Sanhe lead–zinc mine, which is located in Yingjing County, Ya’an, Sichuan Province (29°47′E, 102°31′N) (Fig. 1), at an elevation of 1358–1445 m. The area has a subtropical moist monsoon climate with an average temperature of 15.3°C and annual rainfall of over 1500 mm. This mine was in operation from the 1950 s to 2003, and the average content of lead in the mine tailings is about 7.62%.
Corresponding non-mining ecotypes were collected from Yucheng District, Ya’an (102°51′–103°12′E, 29°40′–30°14′N), Sichuan Province, which has similar climatic and topographic conditions to the mining area.
Plant sampling and soil collection
Three sampling times were chosen for the nine plant species: early growth stage (May), vigorous growth stage (August) and late growth stage (October). There were three replicates of each species, pictured in Fig. 2. For each replicate, five or more individual plants were randomly collected within the sampling area and mixed to give a composite whole plant sample. Rhizosphere soil was collected by shaking the soil adhering to the plant roots into labeled plastic bags (Zhu et al. 2003). Non-rhizosphere soil was collected from the surface soil (0–20 cm depth) immediately surrounding the plants.
Sample preparation and analytical methods
Whole plants were thoroughly washed in running tap water and then three times with distilled water. The plants were divided into shoot and root parts and oven-dried at 70°C for 48 h, after which the oven-dried samples were ground with a stainless grinder (FW-100, China) to be able to pass through a 100 mesh sieve (0.15 mm). The samples of rhizosphere and non-rhizosphere soil were naturally air-dried, ground into fine powder and sieved through 2 and 0.15 mm nylon sieves.
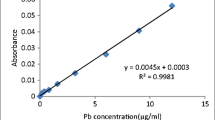
The plant samples were digested with HNO3 + HClO4 (5:1, v:v) in closed Teflon vessels until the liquid was clear. The digested material was washed into a 50 ml flask and made up to the volume using de-ionized water. To obtain the total lead content, soil samples (0.15 mm) were digested in the same way with HNO3 + HClO4 + HF (5:1:1, v:v:v) and available lead of soil (2 mm) was extracted with diethylenetriaminepenta acid (DTPA) (Bao 1999). The concentrations of lead in plants and soil were then measured by a flame atomic absorption spectrophotometer (Mk M6, Thermo elemental, USA). Soil pH (1:2.5 soil to water, w:w) was measured with a conventional pH meter after soil and water were equilibrated for 30 min.
Bioconcentration factor of root
Bioconcentration factor of root was calculated as follows: lead concentration of root/available content of lead in rhizosphere soil. Bioconcentration factor of root can be used to evaluate the ability of root to accumulate lead.
Translocation factor
Translocation factor was calculated as follows: lead concentration in plant shoot/lead concentration in plant root.
Statistical analyses
Lead concentrations in shoot and root samples are presented for the three individual replicates. Data on concentrations of lead in plants and soils on different plant species were analyzed using one-way ANOVA followed by the LSD in SPSS statistical software package (Version 11.0) (Deng et al. 2007; Xiao et al. 2009).
Results
Lead concentration in roots
Large differences existed in the lead concentration of roots between the two ecotypes, and average concentration of lead in the roots of mining ecotypes was much higher than that of non-mining ecotypes in all three growth stages (Table 1). For most of the tested plants, root lead concentrations were higher in the early and vigorous growth stages than in the late stage.
The root lead concentrations of mining ecotypes were 1.4–426.0 times higher than those of their corresponding non-mining ecotypes in the early growth stage (Table 1). More than 500 mg kg−1 of lead was found in the roots of the mining ecotypes of A. wardii, Equisetum arvense L. and Pseudocyclosorus subochthodes. A. wardii had the highest concentration, which was 21 times higher than that of E. arvense L. and 251 times higher than that of the lowest species, Anemone vitifolia. The highest difference between the mining and non-mining ecotypes was also found in A. wardii, which was 22–785 times higher than that of the other species. The concentration of lead in most plant roots was reduced in the late growth stage, ranging from 41.0–4132.2 mg kg−1. The maximum was still found in the roots of A. wardii; the lead concentration of the mining ecotype was 235 times that of its non-mining ecotype in the late growth stage.
Lead concentration in shoots
A significant difference (p < 0.05) was found in lead concentration of the shoots between mining and non-mining ecotypes for all the species (Table 2). Lead concentrations in shoots was dependent on different plant species, the variation tendency and effect of growth period were consistent with concentrations in the roots.
Lead concentration in the shoots of the mining ecotypes in the early growth stage varied from 40.9 to 656.8 mg kg−1, with the maximum level being in the shoots of A. wardii, which was 26 times that of its non-mining ecotype and 2–16 times those of the other species. In the late growth stage, lead concentration in the shoots of the plant species decreased to various degrees; the maximum value was also found in the shoots of A. wardii. Over the total growth period, none of the plant species accumulated lead above 1000 mg kg−1 in the shoots, the criterion for a hyperaccumulator.
Bioconcentration factor of root and translocation factor
The bioconcentration factor of root (BCFR) and translocation factor (TF) in different growth stages for the plant species are presented in Table 3. The BCFR for lead of A. wardii and E. arvense L. growing in mine tailings was greater than 1 during the whole growth period, and the highest BCFR was found in A. wardii (BCFR = 50.2); the rest of the plants had scores over 1 in only one or two growth periods.
A low translocation factor is one of the important characteristics of plants suitable for phytostabilization. The translocation factors of the mining ecotypes of the plant species in different growth stages were less than 1, except for A. vitifolia in the late growth stage. During the whole growth period, A. wardii and E. arvense L. had very low translocation factors. In general, A. wardii and E. arvense L. have strong potential for phytostabilization due to their higher BCFR and lower TF than the other plant species.
Lead accumulation
Large differences were found in the lead concentrations and biomass of the species, regarding the amounts of lead accumulation (Table 4). In the early growth stage, the highest lead accumulation among the mining ecotypes was found for A. wardii, for which the lead accumulation in root accounted for 97.9% of the whole plant, whereas the root accumulations of the remaining plant species were close to each other, 0.01–0.51 mg plant−1. In the vigorous stage, lead accumulations of plants were enhanced to different degrees. The highest increment of accumulation in root was found in A. wardii and this was much higher than the ones for the other plants. In the late growth stage, 19–98% of lead uptake by the plants was accumulated in the roots. Though lead accumulation of plants showed downtrend in late growth stage, accumulation in root of A. wardii was still the highest, while accumulations of other plant species were less than 1 mg plant−1.
Discussion
Variation between different plant species in lead accumulation
There were correlations between the lead concentrations in plants and soil. The total lead content in the rhizosphere soils was significantly higher than that of the non-rhizosphere soils (Table 5). The mine tailing was rich in lead but with uneven distribution, 263–9978 mg kg−1 in the study area. Previous studies have reported that soil lead concentrations of lead–zinc mines or mine tailings in China, the USA, and Australia were in the range of 3000–13,000 mg kg−1, levels much exceeded by the Bo Ngam lead mine in Thailand, which contained extremely high lead concentrations (6000–100,000 mg kg−1) (Wenzel and Jockwer 1999; Zu et al. 2004; Li 2005; Rotkittikhun et al. 2006; Yoon et al. 2006). According to the soil quality standard of the United Kingdom (ICRCL59/83), the initial concentration of lead in soil for courtyard and place of sideline production is 500 mg kg−1. However, the total lead content in the soil from the lead–zinc mine tailings for the studies here greatly exceeded the standard, and nevertheless allowed to grow nine dominant plant species. Therefore, the plants growing in these mine tailings demonstrated strong lead adaptability. The average content for the mining ecotypes was 1845 mg kg−1, which was 26 times more than that of the non-mining ecotypes. The highest variation in the available content of lead between rhizosphere and non-rhizosphere soil was found for species A. wardii, Seneci scandens and Pilea sinofasciata (Table 5).
The order of lead concentration in soils was similar to the order in the plant roots and shoots; this result meant that the lead concentrations in soils could have had an effect on the concentrations in plants. Only the available content of lead in soil of mining ecotypes was positively correlated to those plants, whereas a negative correlation was found in the non-mining ecotypes (Table 6). The lead concentrations had a significant relationship with levels in plant shoots and roots (p < 0.01 and p < 0.05, respectively) of the two ecotypes. These results indicated the lead concentrations of plant were affected by many factors; lead concentration of shoots may be mainly due to translocation from root to the shoot.
Plant uptake of lead is affected by the characteristics of the substrates where the plants are rooted, such as pH value, organic C, phosphorus and SO4 contents, carbonates and cation exchange capacity. Plants can readily uptake the available lead, in the forms of which include water-soluble, acid-soluble, chelate and absorbed pools (Fangueiro et al. 2002). However, the available content of lead in the soil was only a small portion of the total (Table 5); the rest of it was in the insoluble pools, and its solubility would be affected by soil pH. The solubility of virtually insoluble lead compounds is mainly pH-dependant. As the pH decreases, H+ reacts with solid phases then the concentration of lead in soil solution increases, especially those of carbonates (Yang et al. 2005). Therefore, water-soluble lead content will increase to promote the migration of lead in soil. On the contrary, increased soil pH decreases the solubility and mobility of lead, which eventually reduces plant uptake of lead. In this investigation, the pH of the rhizosphere soils for the mining ecotypes was lower than that of the non-rhizosphere soils (Table 5). This demonstrated that the low-molecular-weight organic acids secreted by the plant roots modified the pH of the rhizosphere and hence the chemical forms of the lead, increased its bio-availability to promote its absorption. Different plant species can excrete different secretions to activate available lead in the rhizosphere soil, and the mechanisms for absorbing lead are not identical. It has been reported that the activating impact of organic and amino acids on Pb, Zn, Cu and Cd in contaminated soils is strong, and that citric, tartic and oxalic acids are the strongest acids involved in activating lead polluted soil (Yang et al. 2000).
Phytoremediation includes both phytoextraction (removal of metals from soil through hyperaccumulators) and phytostabilization (accumulation of metals into root tissue or precipitation in the root zone) (Cunningham et al. 1995; Salt et al. 1995). If the general standard for hyperaccumulators is used as a criterion, no sample in the present study could be regarded as a hyperaccumulator. However, for effective restoration of mine wasteland, identification and characterization of native plant species on mine lands is required to develop phytostabilization technologies (Wong 2003). The biomass of the nine plant species studied here was affected by the high lead concentrations. The shoot biomass of the mining ecotypes was lower than that of the non-mining ecotypes in all the three growth stages. On the other hand, the roots of the mining ecotypes in general grew more stronger than those of the non-mining ecotypes (Table 7). Plants living in mine areas have adopted to long-term heavy metal stress. This process will change the ability of accumulation in such plants and create a difference from non-mining ecotypes (Yang et al. 2001). Storage in roots is most beneficial for phytostabilization of metal contaminants that are least available. In South China, pioneer herbal species Cynodon dactylon, Imperata cyclindrica var. major, Sesbania rostrata, Paspalum notatum, and Vetiveria zizanioides (Wong 2003) have been proven to be suitable for phytoextraction of Pb/Zn mine. In this study, the lead concentrations of root remained higher than the concentrations of shoot during the entire growth period. The highest variations of lead concentration between the mining and non-mining ecotypes were found in A. wardii. The tolerance and accumulation of the root should be tested in planned pot experiments, in order to understand the relevant physiological mechanisms.
Selection of plant for phytostabilization of lead
It is important to choose the right phytoremediation strategy for each polluted area. Plants in which the transport of metals is restricted and nearly constant metal concentrations are maintained in the shoot over a wide range of soil concentrations are suitable for phytostabilization for reclamation and vegetative restoration of mine tailings. Although the standards for identifying hyperaccumulators for phytoextraction are well known, up to date no standard for phytostabilization has been scientifically defined. In the present study, four indexes were considered: (1) lead concentration of roots, (2) bioconcentration factor of root, (3) translocation factor, and (4) root biomass (lead accumulation is related to concentration and biomass, therefore it should be excluded). The concentrations of lead in the roots of A. wardii were higher than those of the other plants. The highest content for A. wardii was above 15,000 mg kg−1 in the early growth period (Table 1). When the translocation factor > 1 and lead accumulation of root were considered, A. wardii showed the highest value among the mining ecotypes. The bioconcentration factor of root of A. wardii was the lowest, meaning that a small part of absorbed lead is transported from root to shoot. The root biomass of A. wardii was also the highest among the other species over the entire growth period (Table 7).
A. wardii is a common perennial plant, a kind of fern which grows in fascicles. It has advantageous features for phytostabilization, such as a well-developed root system, high biomass and maintenance of a high concentration of lead in the root tissues. Although ferns have been confirmed to be plants tolerant to different heavy metals by virtue of their strong levels of tolerance and accumulation in contaminated soils (Ma et al. 2001; Wei et al. 2002), very little information exists on the phytoremediation of lead for fern species. Lead removal was reported by ferns such as Azolla pinnata (Benaroya et al. 2004) and Pteris vittata (Ma et al. 2001), but no research has been published on the use of ferns for phytostabilization of lead. A field survey of a fern named Petridium revolutum growing on a Cu mine tailing found that the Cu concentration of plant shoot was in the range of 30–567 mg kg−1, while the content reached 7554 mg kg−1 in soil (Zheng et al. 2006). Thus, this kind of fern has remarkable tolerance and could be used in the phytostabilization of Cu polluted soils. In the present study, the fern A. wardii showed strong tolerance ability to lead and its capability of accumulating lead was stable across the screening period.
Conclusion
A. wardii demonstrated higher lead accumulation in its root than the other tested species, all of which were previously reported to grow strongly in lead contaminated sites. The lowest ability to transfer lead to shoot was also found in this species. Its mining ecotype had a significantly higher capability of accumulating lead in root than the non-mining ecotype. Therefore it has the potential for phytostabilization of lead polluted soils, and further experiments are planned to confirm this potential under laboratory and field trial conditions.
References
Alkorta I, Garbisu C (2001) Phytoremediation of organic contaminants in soils. Bioresour Technol 79:273–276
Alvarenga P, Gonçalves AP, Fernandes RM, de Varennes A, Vallini G, Duarte E, Cunha-Queda AC (2008) Evaluation of composts and liming materials in the phytostabilization of a mine soil using perennial ryegrass. Sci Total Environ 406:43–56
Arienzo M, Adamo P, Cozzolino V (2004) The potential of Lolium perenne for revegetation of contaminated soils from a metallurgical site. Sci Total Environ 319:13–25
Bao SD (1999) Soil and agriculture chemistry analysis. Chinese Agriculture Press, Beijing, pp 373–375 380–382 (in Chinese)
Benaroya RO, Tzin V, Tel-Or E, Zamski E (2004) Lead accumulation in the aquatic fern Azolla filiculoides. Plant Physiol Biochem 42:639–645
Boularbah A, Schwartz C, Bitton G, Aboudrar W, Ouhammon A, Morel JL (2006) Heavy metal contamination from mining sites in South Morocco: assessment of metal accumulation and toxicity in plants. Chemosphere 63:811–817
Brooks RR, Chambers MF, Nicks LJ, Robinson BH (1998) Phytomining. Trends Plant Sci 3:359–362
Cao X, Ma LQ, Rhue DR, Appel CS (2004) Mechanisms of lead, copper and zinc retention by phosphate rock. Environ Pollut 131:435–444
Chaturvedi PK, Seth CS, Misra V (2007) Selectivity sequences and sorption capacities of phosphatic clay and humus rich soil towards the heavy metals present in zinc mine tailing. J Hazard Mater 147:698–705
Cobb GP, Sands K, Waters M, Wixson BG, Dorward-King E (2000) Accumulation of metals by vegetables grown in mine wastes. Environ Toxicol Chem 19:600–607
Correll DL (1998) The role of phosphorus in the eutrophication of receiving water: a review. J Environ Qual 27:261–266
Cunningham SD, Berti WR, Huang JW (1995) Phytoremediation of contaminated soils. Trends Biotechnol 13:393–397
Deng DM, Shu WS, Zhang J, Zou HL, Lin Z, Ye ZH, Wong MH (2007) Zinc and cadmium accumulation and tolerance in populations of Sedum alfredii. Environ Pollut 147:381–386
Fangueiro D, Bermond A, Santos E, Carapuça H, Duarte A (2002) Heavy metal mobility assessment in sediments based on a kinetic approach of the EDTA extraction: search for optimal experimental conditions. Anal Chim Acta 459:245–256
González RC, González-Chávez MCA (2006) Metal accumulation in wild plants surrounding mining wastes. Environ Pollut 144:84–92
Islam E, Yang X, Li T, Liu D, Jin X, Meng F (2007) Effect of Pb toxicity on root morphology, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J Hazard Mater 147:806–816
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—a review. Waste Manage 28:215–225
Küpper H, Parameswaran A, Leitenmaier B, Trtílek M, Šetlík I (2007) Cadmium-induced inhibition of photosynthesis and long-term acclimation to cadmium stress in the hyperaccumulator Thlaspi caerulescens. New Phytol 175:655–674
Lasat MM (2002) Phytoextraction of toxic metals: a review of biological mechanism. J Environ Qual 31:109–120
Li MS (2005) Ecological restoration of mineland with particular reference to the metalliferous mine wasteland in China: a review of research and practice. Sci Total Environ 357:38–53
Li YG, Jiang GM (2004) Ecological restoration of mining wasteland in both China and abroad: an over review. Acta Ecol Sinica 24(1):95–100 (in Chinese)
Lin Q, Mendelssohn IA (1998) The combined effects of phytoremediation and biostimulation in enhancing habitat restoration and oil degradation of petroleum contaminated wetlands. Ecol Eng l0:263–274
Liu X, Liu S, Tang Z (2002) The relationship between Cd and Pb forms and their availability to rape in major soils of Hebei Province. Acta Ecol Sinica 22(10):1689–1694 (in Chinese)
Ma LQ, Komar KM, Tu C, Zhang W, Cai Y, Kennelley ED (2001) A fern that hyperaccumulates arsenic. Nature 409:579
McGowen SL, Basta NT, Brown GO (2001) Use of diammonium phosphate to reduce heavy metal solubility and transport in smelter-contaminated soil. J Environ Qual 30:493–500
Melamed R, Cao X, Chen M, Ma LQ (2003) Field assessment of lead immobilization in a contaminated soil after phosphate application. Sci Total Environ 305:117–127
Mendez MO, Maier RM (2008) Phytostabilization of mine tailings in arid and semiarid environments-an emerging remediation technology. Environ Health Perspect 116:278–283
Mulligan CN, Yong RN, Gibbs BF (2001) Remediation technologies for metal-contaminated soils and groundwater: an evaluation. Eng Geol 60:193–207
Rotkittikhun P, Kruatrachue M, Chaiyarat R, Ngernsansaruay C, Pokethitiyook P, Paijitprapaporn A, Baker AJM (2006) Uptake and accumulation of lead by plants from the Bo Ngam lead mine area in Thailand. Environ Pollut 144:681–688
Sahi SV, Bryant NL, Sharma NC, Singh SR (2002) Characterization of a lead hyperaccumulator shrub, Sesbania drummondii. Environ Sci Technol 36:4676–4680
Salt DE, Blaylock M, Kumar N, Dushenkov V, Ensley B, Chet I, Raskin I (1995) Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Nature Biotechnol 13:468–474
Santibáñez C, Verdugo C, Ginocchio R (2008) Phytostabilization of copper mine tailings with biosolids: Implications for metal uptake and productivity of Lolium perenne. Sci Total Environ 395:1–10
Shu W, Zhang Z, Lan C (2002) Strategies for restoration of mining wastelands in China (I). Ecol Sci 19(2):24–30 (in Chinese)
Tordoff GM, Baker AJM, Willis AJ (2000) Current approaches to the revegetation and reclamation of metalliferous mine wastes. Chemosphere 41:219–228
Walker DJ, Clemente R, Bernal MP (2004) Contrasting effects of manure and compost on soil pH, heavy metal availability and growth of Chenopodium album L. in soil contaminated by pyritic mine waste. Chemosphere 57:215–224
Wei CY, Chen TB, Huang ZC, Zhang XQ (2002) Cretan brake (Pteris cretica L.): an arsenic-accumulating plant. Acta Ecol Sinica 22(5):777–778 (in Chinese)
Wenzel WW, Jockwer F (1999) Accumulation of heavy metals in plants grown on mineralised soils of the Austrian Alps. Environ Pollut 104:145–155
Wong MH (2003) Ecological restoration of degraded soils with emphasis on metal contaminated soils. Chemosphere 50:775–780
Xiao GL, Li TX, Zhang XZ, Yu HY, Huang HG, Gupta DK (2009) Uptake and accumulation of phosphorus by dominant plant species growing in a phosphorus mining area. J Hazard Mater 171:542–550
Yang RB, Zeng QR, Zhou XH, Tie BQ, Liu SY (2000) The activated impact of plant root exudates on heavy metals in soils contaminated by tailing of Lead–Zinc ore. Agro-environmental Prot 19(3):152–155 (in Chinese)
Yang X, Long X, Ni W, Ni S (2001) Zinc tolerance and hyperaccumulation in a new ecotype of sedum Alfredii Hance. Acta Phytoecol Sinica 25(6):665–672 (in Chinese)
Yang JY, Yang XE, He ZL (2005) Resource and bio-availability of lead in soil. Chin J Soil Sci 36(5):667–772 (in Chinese)
Yoon J, Cao X, Zhou Q (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ 368:456–464
Zheng JM, Lou LP, Wang SH, Tang SR (2006) Petridium revolutum, a promising plant for phytoremediation of Cu-polluted soil. Chin J Appl Ecol 17(3):507–511 (in Chinese)
Zhu LX, Zhang JE, Liu WG (2003) Review of studies on interactions between root exudates and rhizopheric microorganisms. Ecol Environ 12(1):102–105 (in Chinese)
Zu Y, Li Y, Schvartz C, Langlade L, Liu F (2004) Accumulation of Pb, Cd, Cu and Zn in plants and hyperaccumulator choice in Lanping lead–zinc mine area, China. Environ Int 30:567–576
Acknowledgments
This work was financially supported by the Sichuan Youth Science & Technology Foundation (06ZQ026–020), Youth Foundation of the Sichuan Education Bureau (2006B009), key project from Sichuan Education Bureau (2006A008), and Open Research Fund Program from Ministry of Education Key Laboratory of Polluted Environmental Remediation and Ecological Health, Zhejiang University, Huajiachi Campus, Hangzhou 310029, China. The authors are thankful to Dr. C. Hu (Sichuan Agriculture University) for his help in plant determinations, and to Margaret Cargill and Dr. P. Huang for critical comments on the language and construction of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zou, T., Li, T., Zhang, X. et al. Lead accumulation and phytostabilization potential of dominant plant species growing in a lead–zinc mine tailing. Environ Earth Sci 65, 621–630 (2012). https://doi.org/10.1007/s12665-011-1109-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-011-1109-6