Abstract
The objectives of the present study were to compare nine dominant plant species growing in mine tailings and nonmining areas in terms of biomass and Cd concentrations and to search for Cd accumulation and tolerance. Also, more detailed experiments were conducted on Athyrium wardii using a pot experiment to assure its Cd-accumulation ability and tolerance as a potential phytostabilizer of Cd-polluted soils. Nine dominant plant species growing on Pb/Zn mine tailings and their corresponding nonmining ecotypes were investigated for their potential to phytostabilize Cd. The performance of A. wardii exposed to high levels of Cd was investigated under controlled conditions. A field study revealed that the Cd concentrations in the roots of these plants ranged from 0.21 to 251.07 mg kg−1, and the highest concentrations were found in A. wardii, which reached a concentration of 69.78, 251.07, and 126.35 mg kg−1 during the early growth stage (May), vigorous growth stage (August), and late growth stage (October), respectively. The Cd concentrations of roots among the nine mining ecotypes were positively correlated with available content of Cd in the rhizosphere soils, whereas a negative correlation was observed in the nonmining ecotypes. A pot experiment showed that the mining ecotype of A. wardii had a higher biomass production and Cd retention capacity in roots than that of the nonmining ecotype. Due to the relatively high tolerance to Cd and the capacity of roots to retain this metal, A. wardii may be useful for the phytostabilization of soils contaminated by Cd.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There are a large number of sites worldwide that have been polluted with heavy metals and metalloids as a result of human activities. The mining industry has produced a significant legacy of polluted and degraded soils (González and González-Chávez 2006). Mining sites have encroached about 40,000 km2 of land in China, and this has led to an annual increase in mining wastelands of 330 km2; accordingly, these areas serve as a growing and persistent source of pollutants (Li and Jiang 2004). Moreover, little policy and/or legislation were issued in China to prevent dumping of mine tailings into bodies of uncontaminated areas, thus escalating the existing problem of abandoned mine tailings sites nationwide. Furthermore, mine tailings are characterized by high concentrations of heavy metals (Boularbah et al. 2006), low pH (Wong et al. 1998), no organic matter or macronutrients, and a high incidence of erosion agents (wind and water runoff) (Conesa et al. 2006). These unfavorable soil qualities result in slow colonization of plants and poor growth, which inhibit natural succession and rehabilitation efforts (Ho et al. 2008). Such effects may be particularly serious and pose a severe ecological and human health risk when mining activities are located in the vicinity of urban environments (Haque et al. 2008). Therefore, there is an urgent need for development of economical and efficient technologies for remediation of mine tailings, and these issues have attracted wide public attention (Arienzo et al. 2004; Tordoff et al. 2000; Yang et al. 2001).
Conventionally, technologies for the remediation of mine tailings have focused on physical and chemical stabilization (Mendez and Maier 2008). Physical stabilization entails covering mine waste with nonpolluted materials. These solutions are often temporary in nature because of the impermanence of the capping process (Johnson and Bradshaw 1977). Chemical stabilization is a temporary stabilization technique in which chemical amendments are used to prevent wind and water erosion. However, this technique is not favored due to its unproven durability as well as the need for regular inspections (Tordoff et al. 2000). In general, traditional remediation techniques are expensive and often impractical, owing to the large areas that these tailings usually cover and to their large volumes (Conesa et al. 2006).
Phytostabilization primarily focuses on sequestration of the metals within the roots and rhizosphere, but not into the aboveground plant tissues. This technique creates a vegetative cap for the long-term stabilization and containment of tailings (Mendez and Maier 2008). Plant canopies serve to reduce aeolian dispersion, while plant roots prevent water erosion, immobilize heavy metals by adsorption or accumulation, and provide a rhizosphere wherein metals precipitate and stabilize (Boularbah et al. 2006). In addition, plant roots reduce the metal fluxes in the soil due to the effect of transpiration by rendering them harmless (Eapen and D'souza 2005; Küpper et al. 2007). These processes decrease metal mobility and reduce the likelihood of metals entering the food chain (Wong 2003). Consequently, phytostabilization has great practical significance and flexibility in ecological restoration of tailings and remediation of soil polluted by heavy metals (Neuman and Ford 2006).
Phytostabilization is an in situ technology involving soil amendments and metal-tolerant plants to establish a ground cover that can then reduce the migration of metals to air, surface water, and ground water, enabling reduced soil toxicity (Mendez and Maier 2008). This technology has been found to be effective under certain conditions (Neuman and Ford 2006). Recently, there has been some research conducted to evaluate the use of plants for phytostabilization and revegetation of heavy metals polluted soils, such as Bidens humilis (Bech et al. 2002), Schinus molle L., Isocoma veneta, Euphorbia sp., Teloxys graveolens Willd., Dalea bicolor (González and González-Chávez 2006), Quercus ilex subsp. ballota (Domínguez et al. 2009), and Typha latifolia L. (Varun et al. 2011). However, there has been little research on the phytostabilization of Cd-polluted soils.
Ideally, plant candidates for phytostabilization should possess an extensive root system and a large amount of biomass in the presence of high concentrations of heavy metals while keeping the translocation of metals from roots to shoots as low as possible (Alvarenga et al. 2008). Moreover, they should be native to the area in which the mine tailings are found and have evolved survival mechanisms appropriate to the climate. Furthermore, the use of native plants avoids the introduction of nonnative and potentially invasive species that may result in decreasing regional plant diversity (Mendez and Maier 2008). Therefore, identification of suitable phytostabilization plant candidates and understanding their metal accumulation patterns are areas in which additional research is critically needed. It has been reported that comparison of different ecotypes in terms of their absorptive capacities can identify actual accumulators, and such studies are important for understanding microevolution mechanisms of tolerance and accumulation in plants (Castillo-Michel et al. 2009; He et al. 2008; Yang et al. 2001). Unfortunately, there is little information available regarding the use of metal-tolerant plants in China for phytostabilization.
Cd is a nonessential heavy metal that is highly toxic to plants, animals, and humans at very low concentrations (Mendez and Maier 2008; Santos et al. 2007; Wan et al. 2011). The objectives of the present study were to compare the nine dominant plant species growing in mine tailings and nonmining areas in terms of biomass and Cd concentration of roots and shoots at three growth stages to search for Cd accumulation and tolerance. In addition, more detailed experiments were conducted on Athyrium wardii (Hook.) Makino using a pot experiment to assure its Cd-accumulation ability and tolerance as a potential phytostabilizer of Cd-polluted soils.
Materials and methods
Field survey and sample collection
The study area was a Pb/Zn mine located in Sanhe, Yingjing County, Ya'an, Sichuan Province, China (102°31′ E, 29°47′ N) at an elevation of 1,358–1,445 m. Mine tailings have been deposited in sites throughout the study area without any protection system, which has resulted in their spreading to surrounding soils by natural processes (wind and water rainfall). In this study, these sites were investigated as mining ecotypes (ME). The climate is generally subtropical moist monsoon with an average temperature of 15.3 °C and an annual rainfall of more than 1,500 mm. The maximum temperature generally occurs in June and July, whereas December and January are normally the coldest months. Pb and Zn are the primary products mined in the district, and mining occurred from the 1950s to 2003. Corresponding nonmining ecotypes (NME) were collected from the Yucheng District, Ya'an (102°51′–103°12′ E, 29°40′–30°14′ N), Sichuan Province, which has climatic and topographic conditions similar to those of the mining area.
To examine the potential for effectively stabilizing Cd, an in-situ survey of mine tailings in Yingjing County, Ya'an, Sichuan Province, was conducted in 2007 and 2008. Thirty-two plant species belonging to 19 families were examined for biomass and heavy metals concentration (data not shown). Nine predominant plant species were found to be growing well, with a wide distribution in the area (Table 1). Generally, according to the changes of the number of leaves, morphology and biomass of shoots, and climatic and topographic conditions, we distinguished the three growth stages at early growth stage (May), vigorous stage (August), and late growth stage (October). Therefore, sampling was conducted at three growth stages for the nine dominant plant species in 2008. There were three replicates of each species. For each replicate, ten individual plants were collected at random from the sampling area and mixed to give a composite whole plant sample. Rhizosphere soil was collected by shaking the soil adhering to the plant roots into labeled plastic bags (Zhu et al. 2003).
Pot experiment
Seedlings from the ME and NME were obtained from the Sanhe Pb/Zn mine tailings and uncontaminated agriculture soils in Ya'an, respectively, in June 2010. The ferns were separated into similar size plant segments composed of five to six fronds. Healthy plants of similar size were selected and cultured for 2 weeks in quartz sands with 1:10 Hoagland's solution, which was continuously watered every 3 days (Zou et al. 2011).
The pot experiment of A. wardii was conducted in plastic boxes (400 × 300 × 140 mm) filled with 5 kg of sterilized soil, which was collected in Daxing, Ya'an, and then sieved (2 mm). Physicochemical properties of the original soils and the soil used in the experiment are shown in Table 2. The Cd treatments consisted of the addition of 5 mg Cd kg−1 (Cd5), 10 mg Cd kg−1 (Cd10), 25 mg Cd kg−1 (Cd25), and 50 mg Cd kg−1 (Cd50) to soil supplied as CdCl2·2.5H2O, with four replicates in each treatment. The control (CdK) consisted of the treatment without any Cd. To simulate the Cd-impacted soils, pot soils were homogenized for 4 weeks with the added Cd prior to the transplantation of seedlings. The pot experiment was conducted in a net house with transparent polythene sheets on the roof. The water-holding capacity of filled soil in plastic boxes was maintained at 70–80 % by applying deionized water every 3 days during the plant growth. After 4 weeks, the plants were harvested, and the soil around the plant roots was collected. The pots were then turned upside down, and soil around plant roots was carefully removed.
Plant and soil analysis
Prior to analysis, plant samples were carefully washed with tap water and thoroughly rinsed with distilled water to remove any soil particles attached to the plant surfaces. After washing, the samples were divided into aboveground (shoot) and belowground (roots plus rhizomes) parts and then oven dried at 70 °C to constant weight (> 48 h). The dried tissues were weighed and then ground with a stainless grinder (FW-100, Tianjin Taisite Instruments, Tianjin, China) to be able to pass through a 100-mesh sieve. The samples of rhizosphere soil were naturally air dried, ground into fine powder, and sieved through 2- and 0.15-mm nylon sieves.
The measurement of soil physicochemical properties was referred to Lu (1999). Soil available P was extracted with 0.5 M NaHCO3 (soil/solution, 1:20) and then measured colorimetrically at 700 nm according to the molybdenum blue method. Soil available N was determined by quantifying alkali-hydrolysable N. Soil available K was extracted with 1 M neutral CH3COONH4 and then measured by flame photometry. Organic matter of the soil was determined by potassium dichromate titrimetric method. Calcium carbonate was determined by acid–base titration. Soil available Cd were extracted with the extractant containing 0.005 M diethylenetriaminepentaacetic acid, 0.1 M triethanolamine, and 0.01 M CaCl2 and then measured by a flame atomic absorption spectrophotometer (Mk M6, Thermo elemental, USA) (soil/solution, 1:2). The pH value (soil/distilled water, 1:2.5) of the soil samples was measured with a pH meter.
A given amount (0.3 g) of plant sample was digested with 5 mL concentrated HNO3 and 1 mL HClO4 in closed Teflon vessels at 160 °C until the liquid was clear (8 h). A similar digestion procedure was then conducted as described above for soil digestion; 0.3 g soil sample (particle size of < 0.15 mm) was digested with 5 mL concentrated HNO3, 1 mL HClO4, and 1 mL HF at 180 °C (10 h). The digested material was subsequently washed into a 50-mL flask and made up to volume using deionized water (Huang et al. 2008). Finally, the concentrations of Cd in the plants and soils were determined by flame atomic absorption spectrophotometry (Mk M6, Thermo elemental, USA) (Zou et al. 2011). The instrument working conditions were wavelength 228.8 nm, slit 0.7 nm, atomization 1,550 °C, read time 3 s, and sample volume 10 μL (Szkoda and Żmudzki 2005). Analytical procedure control was synchronously performed 14 times by measuring the reference materials GBW08503b with Cd content of 0.15 mg kg−1 purchased from the National Center for Certificate Reference Materials, China. The relative standard deviation and average recovery were 0.134–1.56 and 97.33–106.59 %, respectively. Concentrations of Cd were expressed as milligram per kilogram dry weight after correction with residual moisture for both soil and plant samples at 105 °C.
Generally, the translocation factor value is described as the ratio of heavy metals in shoots to root to estimate the translocation efficiency (Yoon et al. 2006). Therefore, the bioconcentration factor of the root (BCF) and translocation factor (TF) were calculated as follows:
where, C root is the concentration of Cd in the roots, and C soil is the concentration of total Cd in the soils.
where, C shoot is the concentration of Cd in the shoots, and C root is the concentration of Cd in the roots.
Statistical analysis
Data were expressed as the means and standard deviations. The data were statistically analyzed by one-way analysis of variance using SPSS (version 13.0) for Windows. The least significant difference was used to test the biomass and Cd concentrations in plants and soils. A p < 0.05 was considered to indicate statistical significance. Correlation at p < 0.05 or p < 0.01 was determined based on Spearman's coefficients.
Results
Field survey
Cd concentration and pH in soils
The total content of Cd in rhizosphere soils varied greatly depending on the plant species (Table 3). The total content of Cd in mining tailings ranged from 7.29 to 55.79 mg kg−1, whereas in the nonmining areas, from 2.46 to 5.23 mg kg−1, respectively. The average content for ME plants was 18.5 mg kg−1, which was five times higher than those of the NME plants. However, the available content of Cd in the soil was only a small portion of the total, accounted for 7.29–10.23 %. The pH values differed among the plant species and ecotypes (Table 3). Both ME and NME soil samples had slightly acid to alkaline pH values.
Cd concentration in roots
The Cd concentrations of the roots were highly variable, depending on the plant species and ecotypes (Table 4). The average concentration of Cd in the roots of ME plants was 30.7 times higher than that of NME during all three growth stages. The root Cd concentrations of ME plants ranged from 0.38 to 69.78 mg kg−1 during the early growth stage. A. wardii had the highest concentration, which was 9.2–183.6 times higher than that of the other species. The concentration of Cd increased in most plant roots during the vigorous growth stage ranged from 0.75 to 251.07 mg kg−1, whereas it was reduced in the late growth stages. The root of ME A. wardii had the highest Cd concentration in all three growth stages, being 65.8, 251.1, and 82.6 times greater than those of corresponding NME, respectively (Table 4).
Cd concentration in shoots
A significant difference (p < 0.05) was observed in the Cd concentration of the shoots between ME and NME for all nine species (Table 5). The Cd concentrations in the shoots were dependent on plant species, and the variation tendency and effect of growth period were consistent with concentrations in roots.
The Cd concentration in the shoots of the ME plants during the early growth stage varied from 0.66 to 30.25 mg kg−1, with the maximum level being observed in the shoots of A. wardii. Specifically, the concentrations of Cd in A. wardii shoots were 28.0 times higher than those of its NME and 10.4–45.8 times higher than those of the other species. The concentration of Cd increased in most plant shoots during the vigorous growth stage, ranging from 1.40 to 28.55 mg kg−1, while it was reduced in the late growth stages. During the late growth stage, the maximum value was observed in the shoots of A. wardii.
Plant biomass
The biomass of the nine species investigated here varied among species (Table 6) and was affected by the high Cd concentrations. The shoot biomass of the ME plants was lower than that of the NME plants during all three growth stages. Conversely, the root biomass of the ME plants was generally higher than those of the NME plants. The root biomass of A. wardii was also the highest among the tested species over the entire growth period.
BCF and TF
The BCF and TF during different growth stages for the nine plant species are presented in Table 7. The BCF of A. wardii growing in mine tailings was greater than 1 during the three growth stages, and the highest value was 3.57. The TF of A. wardii and Equisetum arvense across the growth period was lower than 1. During the entire growth period, A. wardii had lower TF, which ranged between 0.11 and 0.43, especially in vigorous and late growth stages.
Pot experiment
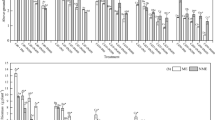
A pot experiment was conducted to test the Cd-accumulation ability and tolerance of the two ecotypes of A. wardii. A significant decrease (p < 0.05) in shoot biomass was observed as the soil Cd concentration increased (Fig. 1). The biomass of NME A. wardii declined by 25 % when compared with the control and was more greatly affected than that of ME A. wardii when the Cd concentration was at 5 mg Cd kg−1 of soil. The shoot biomass of the ME and NME A. wardii decreased by 62 and 52 % at 50 mg Cd kg−1 of soil, respectively. The average biomass in ME A. wardii was up to 1.8 times higher than that of NME A. wardii. However, increasing Cd concentrations in A. wardii were observed with increasing soil Cd levels. The concentration of ME A. wardii in the shoots and roots were 165 and 207 % higher than those of NME A. wardii, on average, under Cd stress conditions (Fig. 2).
Shoot biomass of the two ecotypes of A. wardii grown under different Cd additions for 4 weeks. Data are means ± SD of three individual replicates. ME mining ecotype, NME nonmining ecotype. Mean values labeled with different letters (lower case for ME and upper case for NME) in each series are significantly different (p < 0.05) at different Cd concentrations of soil. *p < 0.05; significantly different at different ecotypes
Cd concentrations in the shoot (a) and root (b) of the two ecotypes of A. wardii grown under different Cd additions for 4 weeks. ME mining ecotype, NME nonmining ecotype. Mean values labeled with different letters (lower case for ME and upper case for NME) in each series are significantly different (p < 0.05) at different Cd concentrations of soil. *p < 0.05; significantly different at different ecotypes
Discussion
The total content of Cd in rhizosphere soils of mine tailings varied greatly, depending on the plant species. Specifically, it varied from 7 to 56 mg kg−1, which was similar to those found at Zacatecas in Mexico, which ranged from 11 to 47 mg kg−1 (González and González-Chávez 2006). According to the National Soil Environmental Quality Standards of China (GB15618–1995), the total content of Cd in the soil of the Pb/Zn mine tailings studied here greatly exceeded the standard III, which is the critical value (1 mg kg−1) of the soil for ensuring the agricultural and forestry production and the normal growth of plants. Nevertheless, the nine dominant plant species observed in the area were still able to grow. These findings indicated that the plants found growing in the mine tailings had strong Cd adaptability. However, the total content of Cd in NME soils was slightly higher than 1 mg kg−1, which needs more supervision and precautions.
Plants can readily uptake the soil available Cd, in the forms of which include water-soluble, acid-soluble, chelate, and absorbed pools (Fangueiro et al. 2002). However, the available content of Cd in the soil was only a small portion of the total (Table 3); the rest of which was in the insoluble pools, and its solubility would be affected by soil pH. The solubility of virtually insoluble Cd compounds is mainly pH dependant. Therefore, soil pH is considered to be one of the most important chemical factors controlling the availability of Cd. In our study, both ME and NME soil samples had slightly acid to alkaline pH values. However, a negative correlation was observed between the pH and total/available content of Cd in rhizosphere soil (p > 0.05). The available content of Cd concentrations of ME soils had a significant relationship with the levels in plant shoots and roots (p < 0.01), whereas a negative correlation was observed for the NME soils in the field survey (Table 8). These findings indicated that the Cd concentrations in soils could influence the concentration in plants growing on Cd-contaminated areas. Moreover, the Cd concentration of plants could be affected by many factors, such as soil available content of Cd, soil pH, plant species, and even genotypes.
Phytoextraction and phytostabilization are currently the two main plant-based technologies used for remediation of heavy metals polluted soils, which is cost-effective and ecologically friendly (Alkorta and Garbisu 2001; Guala et al. 2011). Phytoextraction uses hyperaccumulators to take up heavy metals, to transfer them into the shoots, and to sequester them in nonmetabolically active tissues and organs in less harmful forms (Küpper et al. 2007). Hyperaccumulators have been defined as: (1) plants that can accumulate of >100 mg kg−1 Cd, >1,000 mg kg−1 Cu and Pb, and >10,000 mg kg−1 Zn in their shoot dry biomass; (2) the metal concentrations in shoots are greater than that in roots or shoot/root quotients of >1, and (3) extraction coefficients (metal concentration in plant shoot/total metal concentration in soil) of >1 (Baker and Brooks 1989; Baker et al. 1994; Rotkittikhun et al. 2006). Based on the results of this study, no tested plants were regarded as Cd hyperaccumulators according to the aforementioned criteria.
Alvarenga et al. (2008) pointed out that plant candidates for phytostabilization should possess an extensive root system and a large amount of biomass in the presence of high concentrations of heavy metals while keeping TF as low as possible. Meanwhile, Mendez and Maier (2008) pointed out that the BCF and TF are important for estimating the potential of a plant for phytostabilization purposes. Furthermore, those plants should be native to mining tailings, which avoids the introduction of nonnative and potentially invasive species that may result in decreasing regional plant diversity (Mendez and Maier 2008). However, there is no standard rule to determine if any plant is a candidate for phytostabilization (Jefferson 2004; Mendez et al. 2007; Santibáñez et al. 2008). Therefore, four indexes were considered in the present study: (1) Cd concentration of roots, (2) BCF, (3) TF, and (4) root biomass (the Cd accumulation is related to concentration and biomass and should therefore be excluded). The results of the field survey revealed that the concentrations of Cd in the roots of A. wardii were higher than those of other plants. The highest content for A. wardii was greater than 250 mg kg−1 during the vigorous growth period (Table 4), while the BCF was >1, and TF was <1, indicating that a large portion of Cd was absorbed into roots, and only a small portion was transported from the roots to the shoots. The root biomass of A. wardii was also highest among the tested species over the entire growth period (Table 6). In the field survey, A. wardii exhibits high BCF, low TF values, and high biomass and is thus a potential candidate for phytostabilization of mine tailings to minimize the migration of Cd into groundwater while reducing the risk of entry into the food chain. Therefore, we investigated the performance of A. wardii exposed to high levels of Cd under controlled conditions. Moreover, A. wardii showed the ability to accumulate Cd in roots (up to 190.31 mg kg−1) while translocating only a small amount to the shoots in the pot experiment (Fig. 2).
Domínguez et al. (2009) found that Q. ilex seedlings, a Cd phytostabilizer, had a high Cd retention capacity in fine roots (up to 7 g kg−1) and low rates of Cd translocation to the leaves due to the inert substrate and the soluble Cd salt after planting 6 months. To our best knowledge, these were the highest concentrations recorded for a tree. In the present pot experiment, the Cd uptake capability of A. wardii did not reach the level reported previously. However, Domínguez et al. (2010) reported that the survival of the Q. ilex saplings during the first several years after planting showed lowest survival rates (less than 30 %) and lowest relative growth rates when compared to other six Mediterranean woody species. Ferns have been confirmed to be plants tolerant to different heavy metals by virtue of their strong levels of tolerance and accumulation in polluted soils (Kachenko et al. 2007). Pteris vittata is a type of fern that is well known as an arsenic hyperaccumulator (Ma et al. 2001; Wang et al. 2002). The fern Athyrium yokoscense and its gametophytes were known as Pb hyperaccumulators (Yoshihara et al. 2005). A. wardii is a common perennial plant; specifically, a type of fern that grows in fascicles. In a previous study, A. wardii was found to be a candidate for phytostabilization of Pb-contaminated sites owing to its well-developed root system, high biomass and maintenance of a high concentration of Pb in the root tissues (above 15,000 mg kg−1) (Zou et al. 2011). In this study, we found that A. wardii exhibited a strong accumulative ability for Cd in roots (in field survey and pot experiment) and therefore might be useful for phytostabilization of Cd after further investigation of its accumulation mechanism. Furthermore, it is very important to note that A. wardii naturally established itself at this site.
This work is still in the preliminary stage, and further analyses are required to investigate the mechanisms of uptake and translocation of Cd by this plant, as well as to improve our understanding of the effects of Cd on intra- and extracellular levels. Additionally, we also plan to investigate the interaction mechanisms between Cd and Pb in A. wardii. A better understanding of metal detoxification in plants may eventually contribute to the development of techniques for the phytostabilization of soils contaminated with these two metals.
Conclusion
A. wardii demonstrated higher Cd accumulation in its roots than the other tested species while keeping the bioconcentration factor of the root, which was >1, and translocation factor, <1. Specifically, it immobilized large portions of the total plant Cd in roots in both the field survey and pot experiment. The mining ecotype of this plant had a significantly higher capability to accumulate Cd in the roots than the nonmining ecotype. Therefore, A. wardii is a potentially suitable species for phytostabilization with the purpose of improving the ecological as well as economic values of degraded areas.
References
Alkorta I, Garbisu C (2001) Phytoremediation of organic contaminants in soils. Bioresour Technol 79:273–276
Alvarenga P, Gonçalves AP, Fernandes RM, Varennes AD, Vallini G, Duarte E, Cunha-Queda AC (2008) Evaluation of composts and liming materials in the phytostabilization of a mine soil using perennial ryegrass. Sci Total Environ 406:43–56
Arienzo M, Adamo P, Cozzolino V (2004) The potential of Lolium perenne for revegetation of contaminated soil from a metallurgical site. Sci Total Environ 319:13–25
Baker AJM, Brooks RR (1989) Terrestrial higher plants which hyperaccumulate metallic elements—a review of their distribution, ecology and phytochemistry. Biorecovery 1:81–126
Baker AJM, McGrath SP, Sidoli CMD, Reeves RD (1994) The possibility of in situ heavy metal decontamination of polluted soils using crops of metal-accumulating plants. Resour Conserv Recycl 11:41–49
Bech J, Poschenrieder C, Barceló J, Lansac A (2002) Plants from mine spoils in the South American area as potential sources of germplasm for phytoremediation technologies. Acta Biotechnol 22:5–11
Boularbah A, Schwartz C, Bitton G, Aboudrar W, Ouhammou A, Morel JL (2006) Heavy metal contamination from mining sites in South Morocco: 2. Assessment of metal accumulation and toxicity in plants. Chemosphere 63:811–817
Castillo-Michel HA, Zuverza-Mena N, Parsons JG, Dokken KM, Duarte-Gardea M, Peralta-Videa JR, Gardea-Torresdey JL (2009) Accumulation, speciation, and coordination of arsenic in an inbred line and a wild type cultivar of the desert plant species Chilopsis linearis (Desert willow). Phytochemistry 70:540–545
Conesa HM, Faz Á, Arnaldos R (2006) Heavy metal accumulation and tolerance in plants from mine tailings of the semiarid Cartagena-La Unión mining district (SE Spain). Sci Total Environ 366:1–11
Domínguez MT, Madrid F, Marañón T, Murillo JM (2009) Cadmium availability in soil and retention in oak roots: potential for phytostabilization. Chemosphere 76:480–486
Domínguez MT, Madejón P, Marañón T, Murillo JM (2010) Afforestation of a trace-element polluted area in SW Spain: woody plant performance and trace element accumulation. Eur J Forest Res 129:47–59
Eapen S, D'souza SF (2005) Prospects of genetic engineering of plants for phytoremediation of toxic metals. Biotechnol Adv 23:97–114
Fangueiro D, Bermond A, Santos E, Carapuça H, Duarte A (2002) Heavy metal mobility assessment in sediments based on a kinetic approach of the EDTA extraction: search for optimal experimental conditions. Anal Chim Acta 459:245–256
González RC, González-Chávez MCA (2006) Metal accumulation in wild plants surrounding mining wastes. Environ Pollut 144:84–92
Guala SD, Vega FA, Covelo EF (2011) Development of a model to select plants with optimum metal phytoextraction potential. Environ Sci Pollut Res 18:997–1003
Haque N, Peralta-Videa JR, Jones GL, Gill TE, Gardea-Torresdey JL (2008) Screening the phytoremediation potential of desert broom (Baccharis sarothroides Gray) growing on mine tailings in Arizona, USA. Environ Pollut 153:362–368
He JY, Zhu C, Ren YF, Yan YP, Cheng C, Jiang DA, Sun ZX (2008) Uptake, subcellular distribution, and chemical forms of cadmium in wild-type and mutant rice. Pedosphere 18:371–377
Ho WM, Ang LH, Lee DK (2008) Assessment of Pb uptake, translocation and immobilization in kenaf (Hibiscus cannabinus L.) for phytoremediation of sand tailings. J Environ Sci 20:1341–1347
Huang H, Li T, Tian S, Gupta D, Zhang X, Yang X (2008) Role of EDTA in alleviating lead toxicity in accumulator species of Sedum alfredii H. Bioresour Technol 99:6088–6096
Jefferson LV (2004) Implications of plant density on the resulting community structure of mine site land. Restor Ecol 12:429–438
Johnson MS, Bradshaw AD (1977) Prevention of heavy metal pollution from mine wastes by vegetative stabilisation. Trans Inst Min Metall Sect A 86:47–55
Kachenko AG, Singh B, Bhatia NP (2007) Heavy metal tolerance in common fern species. Aust J Bot 55:63–73
Küpper H, Parameswaran A, Leitenmaier B, Trtílek M, Šetlík I (2007) Cadmium-induced inhibition of photosynthesis and long-term acclimation to cadmium stress in the hyperaccumulator Thlaspi caerulescens. New Phytol 175:655–674
Li YG, Jiang GM (2004) Ecological restoration of mining wasteland in both China and abroad: an over review. Acta Ecol Sin 24:95–100 (in Chinese)
Lu RK (1999) Analysis of soil agrochemistry. Chinese Agricultural Science and Technology, Beijing (in Chinese)
Ma LQ, Komar KM, Tu C, Zhang W, Cai Y, Kennelley ED (2001) A fern that hyperaccumulates arsenic. Nature 409:579
Mendez MO, Maier RM (2008) Phytostabilization of mine tailings in arid and semiarid environments—an emerging remediation technology. Environ Health Perspect 116:278–283
Mendez MO, Glenn EP, Maier RM (2007) Phytostabilization potential of quailbush for mine tailings: growth, metal accumulation, and microbial community changes. J Environ Qual 36:245–253
Neuman DR, Ford KL (2006) Phytostabilization as a remediation alternative at mining sites. Technical Note 420. BLM/ST/ST-06/003 + 3720. Bureau of Land Management, Denver.
Rotkittikhun P, Kruatrachue M, Chaiyarat R, Ngernsansaruay C, Pokethitiyook P, Paijitprapaporn A, Baker AJM (2006) Uptake and accumulation of lead by plants from the Bo Ngam lead mine area in Thailand. Environ Pollut 144:681–688
Santibáñez C, Verdugo C, Ginocchio R (2008) Phytostabilization of copper mine tailings with biosolids: implications for metal uptake and productivity of Lolium perenne. Sci Total Environ 395:1–10
Santos FSD, Magalhães MOL, Mazur N, Sobrinho NMBDA (2007) Chemical amendment and phytostabilization of an industrial residue contaminated with Zn and Cd. Sci Agr 64:506–512
Szkoda J, Żmudzki J (2005) Determination of lead and cadmium in biological material by graphite furnace atomic absorption spectrometry method. Bull Vet Inst Pulawy 49:89–92
Tordoff GM, Baker AJM, Willis AJ (2000) Current approaches to the revegetation and reclamation of metalliferous mine wastes. Chemosphere 41:219–228
Varun M, D’Souza R, Pratas J, Paul MS (2011) Evaluation of phytostabilization, a green technology to remove heavy metals from industrial sludge using Typha latifolia L. Soc Appl Biotech 1:137–145
Wan G, Najeeb U, Jilani G, Naeem MS, Zhou W (2011) Calcium invigorates the cadmium-stressed Brassica napus L. plants by strengthening their photosynthetic system. Environ Sci Pollut Res 18:1478–1486
Wang J, Zhao FJ, Meharg AA, Raab A, Feldmann J, McGrath SP (2002) Mechanisms of arsenic hyperaccumulation in Pteris vittata. Uptake kinetics, interactions with phosphate, and arsenic speciation. Plant Physiol 130:1552–1561
Wong M (2003) Ecological restoration of mine degraded soils, with emphasis on metal contaminated soils. Chemosphere 50:775–780
Wong JWC, Ip CM, Wong MH (1998) Acid-forming capacity of lead-zinc mine tailings and its implications for mine rehabilitation. Environ Geochem Health 20:149–155
Yang XE, Long XX, Ni WZ, Ni SF (2001) Zinc tolerance and hyperaccumulation in a new ecotype of Sedum alfredii Hance. Acta Phytoecol Sin 25:665–672 (in Chinese)
Yoon J, Cao X, Zhou Q, Ma LQ (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ 368:456–464
Yoshihara T, Tsunokawa K, Miyano Y, Arashima Y, Hodoshima H, Shoji K, Shimada H, Goto F (2005) Induction of callus from a metal hypertolerant fern, Athyrium yokoscense, and evaluation of its cadmium tolerance and accumulation capacity. Plant Cell Rep 23:579–585
Zhu LX, Zhang JE, Liu WG (2003) Review of studies on interactions between root exudates and rhizopheric microorganisms. Ecol Environ 12:102–105 (in Chinese)
Zou T, Li T, Zhang X, Yu H, Luo H (2011) Lead accumulation and tolerance characteristics of Athyrium wardii (Hook.) as a potential phytostabilizer. J Hazard Mater 186:683–689
Acknowledgments
This work was financially supported by the Natural Science Foundation of China (40901138). The authors are thankful to Dr. C. Hu (Sichuan Agricultural University) for his help with plant determinations and to Margaret Cargill for her critical comments regarding the language and construction of the manuscript. We also thank Deyong Wu, Lin Ji, Xia Huang, Hongjiang Zhang, Xin Guo, Minying Liu, Xiaona Lv, Xuemei Yi, Jingzhou Sha, and Dan He of Sichuan Agricultural University for supporting the investigation and research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Zhang, S., Li, T., Huang, H. et al. Cd accumulation and phytostabilization potential of dominant plants surrounding mining tailings. Environ Sci Pollut Res 19, 3879–3888 (2012). https://doi.org/10.1007/s11356-012-1060-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-1060-4