Abstract
Athyrium wardii (Hook.) is a promising herbaceous plant species for phytostabilization of cadmium (Cd)-contaminated sites with large biomass and fast growth rate. However, little information is available on its tolerance mechanisms toward Cd. To further understand the mechanisms involved in Cd migration, accumulation and detoxification, the present study investigated subcellular distribution and chemical forms of Cd in the mining ecotypes and corresponding non-mining ecotypes of A. wardii via greenhouse pot experiment. Subcellular fractionation of Cd-containing tissues demonstrated that the majority of the element was mainly located in soluble fraction in cell walls. This indicated that both the vacuoles and cell walls might be evolved the Cd tolerance mechanisms to protect metabolically active cellular compartments from toxic Cd concentrations. Meanwhile, Cd taken up by the plant existed in different chemical forms. Results showed that the majority of Cd in plant was in undissolved Cd–phosphate complexes (extracted by 2 % CH3COOH), followed by water-soluble Cd–organic acid complexes, Cd(H2PO4)2, pectates and protein form (extracted by deionized water and 1 M NaCl), whereas only small amount of Cd in roots was in inorganic form (extracted by 80 % ethanol), which suggests low capacity to be transported to aboveground tissues. It could be suggested that Cd integrated with undissolved Cd–phosphate complexes in cell wall or compartmentalization in vacuole might be responsible for the adaptation of the mining ecotypes of A. wardii to Cd stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The accumulation of heavy metals in the soil, food chain and drinking water is becoming a major environmental problem, mainly due to human activities, such as application of heavy metal-containing pesticides and fertilizers, irrigation with sewage sludge, application of municipal wastes, mining, refining and through natural rock mineralization processes (He et al. 2008; Mahvi et al. 2008; Ling et al. 2011; Wu et al. 2011; Dede et al. 2012). Cadmium (Cd) is a non-essential heavy metal that is highly toxic to plants, animals and humans even at very low concentrations and is released to the environment as a consequence of industrial and agricultural activities (Domínguez et al. 2009; Feng et al. 2009; Sanità di Toppi and Gabbrielli 1999). An important cause of Cd toxicity is its chemical similarity with essential elements, in particular Zn, but also Ca and Fe, deregulating the homeostasis of the latter elements or causing their displacement from proteins (Verbruggen et al. 2009). Moreover, excess Cd can profoundly interfere with a series of physiological processes in plants, such as enzyme activity, respiration, photosynthesis and nutrient assimilation (Ashraf et al. 2011; Harada et al. 2002; Vig et al. 2003; Zhang et al. 2010). To avoid Cd toxicity in Cd-contaminated areas, plants have evolved intra- and extracellular mechanisms for metal detoxification including binding and precipitation in the cell wall and/or compartmentalization in vacuoles via metal–organic acid complex (Krämer et al. 2000; Clemens 2001; Hall 2002). Although numerous studies on the interaction between Cd and plants have been conducted, many mechanisms of Cd toxicity in plants remain unclear (He et al. 2008).
There is some evidence that determination of Cd distribution and chemical speciation in plants is essential for understanding the mechanisms involved in Cd migration, accumulation and detoxification (He et al. 2008; Wang et al. 2008). However, the ability to take up, transport and accumulate Cd differs greatly among plant species and even among genotypes (He et al. 2008; Krämer et al. 2000). Wang et al. (2008) observed that in case of Bechmeria nivea (L.) Gaud. (ramie), a promising species for Cd phytoextraction with large biomass and fast growth rate, most of Cd was present in the cell wall and soluble fraction, and similar subcellular distribution pattern has been reported in Phytolacca americana L. (pokeweed) (Fu et al. 2011) and Cd-resistant Hordeum vulgare L. (barely) (Wu et al. 2005). Additionally, chemical speciation of Cd is closely related to its biological function, and different chemical forms of Cd extracted by different extracting agents have distinct toxicity degree and migration of Cd (Fu et al. 2011). However, previous studies have not yet provided consistent results. For instance, Zhou et al. (2008) found that the greatest amount of Cd in Sedum jinianum was in the form of pectate- and protein-integrated Cd as well as water-soluble Cd–organic acid complexes and Cd(H2PO4)2.
Athyrium wardii (Hook.) is a common perennial plant; specifically, a type of fern that grows in fascicles. It was found to have a high potential in accumulating high concentrations of lead (Pb) and Cd in roots at the contaminated sites in China, which may be useful for the phytostabilization of soils contaminated by these two metals (Zou et al. 2011; Zhang et al. 2012). However, there is little information available regarding the Cd uptake and distribution patterns in response to Cd stress in this plant species. Therefore, the objectives of the present study were to investigate the characteristics of Cd subcellular distribution and chemical forms in the mining ecotypes and corresponding non-mining ecotypes of A. wardii and their implication in Cd tolerance. The experiment was carried out in the climatic conditions of Ya’an, PR China from March 15, 2010, to October 10, 2010.
Materials and methods
Plant materials and Cd treatments
Seeds for the mining ecotype (ME) were obtained from the Sanhe Pb/Zn mine tailings, Yingjing County, Ya’an, Sichuan Province, China (102°31′E, 29°47′N) at an elevation of 1,358–1,445 m, in March 2010. The climate is generally subtropical moist monsoon with an average temperature of 15.3 °C and an annual rainfall of more than 1,500 mm. Corresponding non-mining ecotypes (NME) seeds were collected from the Yucheng District, Ya’an (102°51′–103°12′ E, 29°40′–30°14′ N), Sichuan Province, which has climatic and topographic conditions similar to those of the mining area. The ferns were separated into similar size plant segments composed of 5–6 fronds. Healthy plants of similar size were selected and cultured for 2 weeks in quartz sand with 1/10 Hoagland’s solution, which was watered every 3 days (Zou et al. 2011).
The pot experiment of A. wardii was conducted in plastic boxes (400 × 300 × 140 mm) filled with 5 kg of sterilized soil (sterilized by 0.5 % potassium permanganate solution for 1 h) (Guo et al. 2011). A soil sample of Ochric Aquic Cambosol (1–10 cm) was collected in Daxing, Ya’an, and then, approximately 150 kg soils sieved through a sieve with pore size of 2 mm, which was air-dried until using for the pot experiment. The physicochemical properties of the soil include 57.8 % sand, 16.0 % clay, 26.2 % silt, 2.14 % organic matter, 1.18 g kg−1 total N, 90.9 mg kg−1 available N, 44.3 mg kg−1 Olsen P, 67.6 mg kg−1 available K, a pH of 6.53, and a Cd concentration of 0.35 mg kg−1. The Cd treatments consisted of the addition of 25 and 50 mg Cd kg−1 soil and supplied as CdCl2·2.5H2O. The control consisted of the treatment without any Cd. There were 4 replicates (pots) in each treatment and 24 pots in total, which were arranged in a randomized block design and interchanged the positions of the pots every 3 days so as to get uniform environment (light, air, temperature and humidity) for all plants. To simulate the Cd-impacted soils, pot soils were homogenized for 4 weeks with the added Cd prior to the transplantation of seedlings. Five seedlings were grown in each pot. The pot experiment was conducted in a greenhouse with natural light, air, humidity and temperature. The water-holding capacity of filled soil in plastic boxes was maintained at 60–65 % by applying deionized water every 3 days before and during the plant growth. At the end of the experiment (after 4-week exposure to Cd), the plants were harvested and separated into roots, stems (petioles) and leaves (pinnates), and immediately frozen in liquid N2 and kept frozen until use.
Tissue fractionation
Frozen materials were homogenized thoroughly and then put into pre-cold extraction buffer containing 50 mM Tris–HCl (pH 7.5), 250 mM sucrose and 1.0 mM C4H10O2S2, respectively. Cells were separated into three fractions: cell wall, soluble fraction and organelle-containing fraction using differential centrifugation technique as suggested with some modifications by Weigel and Jäger (1980). The homogenate was centrifuged at 4,000 r min−1 for 15 min (model ALLEGRA-64R, Beckman Coulter, Inc., USA) and repeated twice, and the precipitation was designated as ‘cell wall fraction’ mainly consisting of cell walls and cell wall debris. The resulting supernatant solution was further centrifuged at 16,000 r min−1 for 30 min. The resultant deposition and supernatant solution were referred to as ‘organelle-containing fraction’ and ‘soluble fraction,’ respectively. All steps were performed at 4 °C. Cell wall and organelle-containing fractions were digested with concentrated HNO3:HClO4 (5:1, v/v) until the liquid was clear approximately 0.5 h. The digested material was subsequently washed into a 10-ml flask and made up to volume using deionized water. Cd concentrations in different fractions were then determined by atomic absorption spectrometry (AA6300, Shimadzu, Japan). The instrument working conditions were wavelength 228.8 nm, slit 0.7 nm, atomization 1,550 °C, read time 3 s and sample volume 10 μL (Szkoda and Żmudzki 2005).
Extraction of chemical forms
Cd associated with different chemical forms was successively extracted by designated solutions in the following order (Yang et al. 1995):
-
F1: 80 % ethanol, extraction of aminophenol-bound Cd and inorganic-bound Cd such as nitrate/nitrite, chloride.
-
F2: Deionized water, extraction of water-soluble Cd–organic acid complexes and Cd(H2PO4)2.
-
F3: 1 M NaCl (sodium chloride), extraction of pectate- and protein-integrated Cd.
-
F4: 2 % CH3COOH (acetic acid, HAC), extraction of undissolved cadmium phosphate including CdHPO4, Cd3(PO4)2 and other Cd–phosphate complexes.
-
F5: 0.6 M HCl (hydrochloric acid), extraction of Cd oxalate.
-
F6: Cd in residual form (no extraction solution).
Frozen tissues were homogenized in extraction solution with a mortar and a pestle, diluted at the ratio of 1:100 (w/v) and shaken for 22 h at 25 °C. After that, the homogenate was centrifuged at 4,000 r min−1 for 10 min, and first supernatant solution was obtained in a conical flask. The sedimentation was re-suspended twice in extraction solution and shaken for 2 h at 25 °C, and centrifuged at 4,000 r min−1 for 10 min. Then, the supernatants of three suspension and centrifuge steps were pooled for each of the five extraction solutions. Each of the pooled supernatant solutions was then evaporated to constant weight (no liquid) and digested with HNO3:HClO4 (5:1, v/v) until the liquid was clear approximately 0.5 h. For determination of Cd in residual form, the plant material was digested as described above using HNO3:HClO4 (5:1, v/v) in the last step of the sequential extraction. Cd concentrations associated with different chemical forms were determined as described previously by atomic absorption spectrometry (AA6300, Shimadzu, Japan).
Statistical analysis
Data were expressed as the means and standard deviations (SDs). The data were statistically analyzed by one-way analysis of variance using SPSS (version 13.0) for Windows. The least significant difference (LSD) was used to test Cd concentration in different fractions and ecotypes. A p < 0.05 was considered to indicate statistical significance (Xiao et al. 2009).
Results and discussion
Subcellular distribution of cadmium
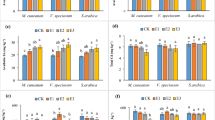
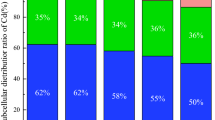
Subcellular distribution of Cd in the roots, stems and leaves of the two ecotypes of A. wardii without Cd supply in the medium (control) was investigated (Table 1). The results showed that only cell wall fraction in the roots of ME A. wardii was detectable, which was 0.11 mg kg−1. Therefore, the proportion in the two ecotypes of A. wardii was not calculated. Additionally, subcellular distribution of Cd and its proportions in A. wardii under Cd stress are investigated, as shown in Figs. 1 and 2, respectively. Generally, increase in Cd supply from 25 to 50 mg Cd kg−1 soil markedly increased Cd concentrations in all subcellular fractions in both the ME and NME of A. wardii, whereas, with increase in Cd supply, the Cd concentration remained constant in organelle fraction in the roots and leaves of ME and in cell wall fraction in the roots of NME (Fig. 1). Meanwhile, the majority of Cd was associated with cell wall and soluble fraction, and a minor part of this element was present in organelle fraction. Moreover, with increase in Cd supply in the medium, relative accumulation of Cd in soluble fractions was generally increased, whereas decreased in cell wall fractions. However, the proportion of Cd in soluble fraction in root of ME decreased (11.05 %) and relative accumulation of Cd in cell wall increased (23.13 %) with increasing Cd supply in the medium (Fig. 2).
Subcellular distribution of Cd in the roots (a), stems (b) and leaves (c) of the two ecotypes of A. wardii. T1, T2: Mining ecotype and non-mining ecotype of A. wardii in 25 mg Cd kg−1 soil, respectively; T3, T4: Mining ecotype and non-mining ecotype of A. wardii in 50 mg Cd kg−1 soil, respectively. Data are mean ± SD of three individual replicates. Mean values labeled with different letters (lower case for ME and upper case for NME) for same ecotype, and Cd level indicates a significant difference (p < 0.05). * Significantly different (p < 0.05) at different ecotypes for same Cd supply in the medium
Based on the results, Cd concentrations in tissues of the two ecotypes decreased following the order of roots > stems > leaves due to its long-distance translocation from roots to stems and leaves. Similar trend has also been observed in other plants (Gussarsson 1994; Wang et al. 2008). The previous studies have proved that metal-tolerant plants always accumulate higher concentration of toxic metal in roots and lower in shoots as compared with the non-tolerant ones (Baker 1984). Therefore, higher Cd concentrations in roots than those in other tissues could be considered as an important tolerance mechanism of A. wardii. Similar results have been reported in Cd-treated Lactuca sp. (lettuces) (Ramos et al. 2002).
Cd analysis at the subcellular level of tissue in plants demonstrated that large proportion of Cd (24.02–53.54 %) was bound to the cell wall fraction, which seems to function as the first barrier protecting the protoplast from Cd toxicity. Similarly, Wang and co-workers in 2008 revealed that 48.2–61.9 % of the total Cd was bound to the cell walls in ramie. Plant cell walls are principally composed of polyose (including cellulose, hemicellulose and pectin) and protein, providing carboxyl, hydroxyl, amino and aldehyde groups. Therefore, they can bind Cd ions and restrict their transportation across cytomembrane (Fu et al. 2011). Besides, 19.61–43.29 % of the total Cd was stored in the soluble fraction, which consisted mostly of vacuoles and acted as the subdominant site of preferential sink for Cd in all test tissues. Moreover, this strategy could further decrease the amount of Cd interfering with organelles and therefore believe to play an important role in metal tolerance. Sulfur-rich peptides and organic acids are essential for the transportation and storage of Cd in the vacuoles, which can decrease free Cd ions activity and thus reduce toxicity via complexation of Cd with organo-ligands within the storage sites (Bhatia et al. 2005; Sanità di Toppi and Gabbrielli 1999). This result was in line with the result of Wang et al. (2008), who found about 30.2–38.1 % Cd being stored in soluble fraction in ramie.
In addition, it is noted that the percentage of Cd associated with organelles was higher in leaves than in roots and stems for all treatments (Fig. 2), which could be considered as the referential accumulation of Cd in chloroplasts (Ramos et al. 2002). These results are to some extent in line with those found by Wang et al. (2008) in ramie. Moreover, the percentage of Cd in organelles of the ME leaves was higher than NME, indicating the chloroplasts of ME of A. wardii might play an important role in Cd accumulation and tolerance.
Meanwhile, the results showed that in the roots of ME of A. wardii, following the increase in Cd concentration, the proportion of Cd in the cell wall fraction increased, whereas in NME, the cell fraction decreased with increasing Cd stress, indicating that limitation of root cell walls of ME to subsequently translocate Cd to shoots. These results were in line with those presented for Cd-treated pokeweed (Fu et al. 2011) and soybean plants (Cataldo et al. 1981). However, Cd subcellular distribution pattern presented here is not entirely consistent with other internal detoxification mechanisms exhibited by some plants. As reported by He et al. (2008), Cd was mainly bound to the cell wall of the roots, leaf sheaths and leaves. Ramos et al. (2002) also found that Cd was mainly associated with cell wall fractions in lettuce leaves, which was fairly independent of Cd level in hydroponic solution. However, according to Vázquez et al. (1992), most of Cd was associated with vacuoles of bean roots and no Cd was detected in cell walls. These conflicting results may be attributed to the variable levels of Cd tolerance of the different plants or genotypes studied, which display specific characteristic in metal uptake, translocation and compartmentalization.
Chemical forms of cadmium
Different chemical forms of Cd in tissues can be characterized using different extracting agents. Chemical forms of Cd in the roots, stems and leaves of the two ecotypes of A. wardii without Cd supply in the medium (control) were investigated (Table 2). The results showed that only acetic acid (HAC)-extractable Cd in the roots of ME A. wardii was detectable, which was 0.09 mg kg−1. Hence, the proportion in the two ecotypes of A. wardii was not calculated. In addition, the Cd concentrations bound to different chemical forms in the two ecotypes of A. wardii increased in a concentration-dependent manner following Cd exposure (Figs. 3, 4). Generally, for both ME and NME of A. wardii, the amount of Cd forms extracted by 2 % HAC was predominant for all treatment levels, whereas extraction of ethanol had the lowest Cd. For sequential extraction of Cd in case of roots, the order of Cd extraction is F4 > F3 > F2 > F5 > F6 > F1, whereas in stems it is F4 > F2 > F3 > F6 > F5 > F1 and in leaves it is F4 > F2 = F3 > F6 > F5 > F1, where F1 = ethanol-extractable Cd, F2 = deionized water-extractable Cd, F3 = sodium chloride (NaCl)-extractable Cd, F4 = acetic acid (HAC)-extractable Cd, F5 = hydrochloric acid (HCl)-extractable Cd, F6 = residual Cd.
Different chemical forms of Cd in the roots (a), stems (b) and leaves (c) of the two ecotypes of A. wardii. T1, T2: Mining ecotype and non-mining ecotype of A. wardii in 25 mg Cd kg−1 soil, respectively; T3, T4: Mining ecotype and non-mining ecotype of A. wardii in 50 mg Cd kg−1 soil, respectively. F1: ethanol-extractable Cd, F2: deionized water-extractable Cd, F3: sodium chloride (NaCl)-extractable Cd, F4: acetic acid (HAC)-extractable Cd, F5: hydrochloric acid (HCl)-extractable Cd, F6: residual Cd. Data are mean ± SD of three individual replicates. Mean values labeled with different letters (lower case for ME and upper case for NME) for same ecotype, and Cd level indicates a significant difference (p < 0.05). * Significantly different (p < 0.05) at different ecotypes for same Cd supply in the medium
Proportion of Cd in different fractions of the two ecotypes of A. wardii. T1, T2: Mining ecotype and non-mining ecotype in 25 mg Cd kg−1 soil, respectively; T3, T4: Mining ecotype and non-mining ecotype in 50 mg Cd kg−1 soil, respectively. F1: ethanol-extractable Cd, F2: deionized water-extractable Cd, F3: sodium chloride (NaCl)-extractable Cd, F4: acetic acid (HAC)-extractable Cd, F5: hydrochloric acid (HCl)-extractable Cd, F6: residual Cd
As the chemical speciation of heavy metals is closely related to their biological function, the toxicity degree and migration of Cd are dependent on its chemical forms extracted by different designated extraction solutions (Yang et al. 1995; Xu et al. 2012). For instance, inorganic and organic water-soluble Cd (extracted by 80 % ethanol and deionized water, respectively), with higher capacity to migrate, is more deleterious to plant cells than the undissolved cadmium phosphate (extracted by 2 % HAC) and cadmium oxalate (extracted by 0.6 M HCl). Meanwhile, the plants that contained high concentration of Cd and yet showed little or no toxicity, Cd should be in a chemical form(s) that causes low or no phytotoxicity (Fu et al. 2011). In this study, Cd existed in different chemical forms among different plant tissues and genotypes. The majority of Cd was integrated with Cd–phosphate complexes (extracted by 2 % HAC). Therefore, it may be assumed that larger percentages of 2 % HAC-extractable Cd is responsible for the adaptation of A. wardii to Cd stress. Moreover, water-soluble Cd–organic acid complexes and Cd(H2PO4)2 (extracted by deionized water) and pectates and protein form (extracted by 1 M NaCl) were generally higher than the other chemical forms except HAC-extractable Cd for both ME and NME A. wardii. Hence, these chemical forms in vacuoles might be another responsible for the adaptation of the A. wardii to Cd stress.
However, only 1.87–5.69 % of total Cd in the roots of A. wardii was in inorganic form (extracted by 80 % ethanol), suggesting low capacity to be transported to aboveground tissues. Therefore, Cd was hypothesized to be chelated by some specific polar material, such as hydroxyl or carboxyl, to form a non-toxic complex. It may also be assumed that lower percentages of 80 % ethanol-extractable Cd in roots and larger percentages of 2 % HAC-extractable Cd in tissues are responsible for the adaptation of A. wardii to high Cd accumulation and stress, which stands for the view point that compartmentalization in vacuoles and sequestration of Cd in cell wall may be crucial for the detoxification of Cd and thus tolerance to metal stress.
Furthermore, genotypic difference may exist in heavy metal tolerance and detoxification. Previous studies found that the ME of A. wardii has higher Pb concentration and more bioactivities of antioxidant enzymes as compared with NME (Zou et al. 2011); especially, a Cd-sensitive barley genotype was found to have higher Cd concentration in inorganic and water-soluble forms, while lower in pectate-/protein-integrated Cd in comparison with the other three Cd-resistant genotypes (Wu et al. 2005). Conversely, Cd accumulation seems to be a constitutive trait of pokeweed (Fu et al. 2011). In this study, the proportion of Cd in the cell wall fraction in roots of ME was higher than that of NME in high Cd concentration (Fig. 1), whereas with increasing Cd supply in the medium, undissolved cadmium phosphate (extracted by 2 % HAC) increased significantly in roots of ME of A. wardii tissues (Fig. 3). These results indicated that ME may have evolved some mechanisms to accumulate and tolerate Cd in long-term evolution, which enables the ME of A. wardii as a promising plant species for phytostabilization of Cd-contaminated soils. However, more detailed experiments are needed to well document the mechanisms of Cd tolerance and detoxification.
Conclusion
This study showed that large proportion of Cd was bound to the cell wall fraction or stored in the soluble fraction at the subcellular level of A. wardii tissues. Besides, the majority of Cd in the plant was undissolved Cd–phosphate complexes, followed by water-soluble Cd–organic acid complexes, Cd(H2PO4)2, pectates and protein form, whereas only minority of total Cd in the roots was in inorganic form, which suggests low capacity to be transported to aboveground tissues. From this study, both the vacuoles and the cell walls might be involved in the Cd tolerance mechanisms to protect metabolically active cellular compartments from toxic Cd concentrations. Moreover, the mining ecotypes of A. wardii may have evolved some mechanisms to accumulate and tolerate Cd in the roots of this plant, which enables it as a promising plant for phytostabilization of Cd-contaminated soils.
References
Ashraf MA, Maah MJ, Yusoff I (2011) Heavy metals accumulation in plants growing in ex tin mining catchment. Int J Environ Sci Tech 8(2):401–416
Baker AJM (1984) Environmentally-induced cadmium tolerance in the grass Holcus lanatus L. Chemosphere 13(4):585–589
Bhatia NP, Walsh KB, Baker AJM (2005) Detection and quantification of ligands involved in nickel detoxification in a herbaceous Ni hyperaccumulator Stackhousia tryonii Bailey. J Exp Bot 56(415):1343–1349
Cataldo DA, Garland TR, Wildung RE (1981) Cadmium distribution and chemical fate in soybean plants. Plant Physiol 68:835–839
Clemens S (2001) Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212(4):475–486
Dede G, Ozdemir S, Dede OH (2012) Effect of soil amendments on phytoextraction potential of Brassica juncea growing on sewage sludge. Int J Environ Sci Tech 9(3):559–564
Domínguez MT, Madrid F, Marañón T, Murillo JM (2009) Cadmium availability in soil and retention in oak roots: potential for phytostabilization. Chemosphere 76(4):480–486
Feng XD, Dang Z, Huang WL, Yang C (2009) Chemical speciation of fine particle bound trace metals. Int J Environ Sci Tech 6(3):337–346
Fu X, Dou C, Chen Y, Chen X, Shi J, Yu M, Xu J (2011) Subcellular distribution and chemical forms of cadmium in Phytolacca americana L. J Hazard Mater 186(1):103–107
Guo SX, Han TT, Liu RJ (2011) Effects of arbuscular mycorrhizal (AM) fungi Glomus mosseae on characteristics of leaf development of Paeonia suffruticosa under salt stress. Afr J Microbiol Res 5(6):714–719
Gussarsson M (1994) Cadmium-induced alterations in nutrient composition and growth of Betula pendula seedlings: the significance of fine roots as a primary target for cadmium toxicity. J Plant Nutr 17(2):2151–2163
Hall JL (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53(366):1–11
Harada E, Yamaguchi Y, Koizumi N, Hiroshi S (2002) Cadmium stress induces production of thiol compounds and transcripts for enzymes involved in sulfur assimilation pathways in Arabidopsis. J Plant Physiol 159(4):445–448
He JY, Zhu C, Ren YF, Yan YP, Cheng C, Jiang DA, Sun ZX (2008) Uptake, subcellular distribution, and chemical forms of cadmium in wild-type and mutant rice. Pedosphere 18(3):371–377
Krämer U, Pickering IJ, Prince RC, Raskin I, Salt DE (2000) Subcellular localization and speciation of nickel in hyperaccumulator and non-accumulator Thlaspi species. Plant Physiol 122(4):1343–1354
Ling T, Jun R, Fangke Y (2011) Effect of cadmium supply levels to cadmium accumulation by Salix. Int J Environ Sci Tech 8(3):493–500
Mahvi AH, Gholami F, Nazmara S (2008) Cadmium biosorption from wastewater by Ulmus leaves and their ash. Eur J Sci Res 23(2):197–203
Ramos I, Esteban E, Lucena JJ, Gárate A (2002) Cadmium uptake and subcellular distribution in plants of Lactuca sp. Cd-Mn interaction. Plant Sci 162(5):761–767
Sanità di Toppi L, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41(2):105–130
Szkoda J, Żmudzki J (2005) Determination of lead and cadmium in biological material by graphite furnace atomic absorption spectrometry method. Bull Vet Inst Pulawy 49:89–92
Vázquez MD, Poschenrieder CH, Barceló J (1992) Ultrastructural effects and localization of low cadmium concentrations in bean roots. New Phytol 120(2):215–226
Verbruggen N, Hermans C, Schat H (2009) Mechanisms to cope with arsenic or cadmium excess in plants. Curr Opin Plant Biol 12(3):364–372
Vig K, Megharaj M, Sethunathan N, Naidu R (2003) Bioavailability and toxicity of cadmium to microorganisms and their activities in soil: a review. Adv Environ Res 8(1):121–135
Wang X, Liu Y, Zeng G, Chai L, Song X, Min Z, Xiao X (2008) Subcellular distribution and chemical forms of cadmium in Bechmeria nivea (L.) Gaud. Environ Exp Bot 62(3):389–395
Weigel HJ, Jäger HJ (1980) Subcellular distribution and chemical form of cadmium in bean plants. Plant Physiol 65:480–482
Wu FB, Dong J, Qian QQ, Zhang GP (2005) Subcellular distribution and chemical form of Cd and Cd-Zn interaction in different barley genotypes. Chemosphere 60(10):1437–1446
Wu Q, Lu YF, Shi JZ, Liang SX, Shi JS, Liu J (2011) Chemical form of metals in traditional medicines underlines potential toxicity in cell cultures. J Ethnopharmacol 134(3):839–843
Xiao G, Li T, Zhang X, Yu H, Huang H, Gupta DK (2009) Uptake and accumulation of phosphorus by dominant plant species growing in a phosphorus mining area. J Hazard Mater 171(1–3):542–550
Xu QS, Min HL, Cai SJ, Fu YY, Sha S, Xie KB, Du KH (2012) Subcellular distribution and toxicity of cadmium in Potamogeton crispus L. Chemosphere 89(1):114–120
Yang JR, He JQ, Zhang GX, Mao XQ (1995) Tolerance mechanism of crops to Cd pollution. Chinese J Appl Ecol 6(1):87–91 (in Chinese)
Zhang X, Zhang S, Xu X, Li T, Gong G, Jia Y, Li Y, Deng L (2010) Tolerance and accumulation characteristics of cadmium in Amaranthus hybridus L. J Hazard Mater 180(1–3):303–308
Zhang SJ, Li TX, Huang HG, Zou TJ, Zhang XZ, Yu HY, Zheng ZC, Wang YD (2012) Cd accumulation and phytostabilization potential of dominant plants surrounding mining tailings. Environ Sci Pollut Res 19(9):3879–3888
Zhou SB, Xu LS, Wu LH, Luo YM, Li N, Cui LQ (2008) Subcellular distribution and chemical forms of Cd and Zn in Sedum jinianum. Chinese J Appl Ecol 19(11):2515–2520 (in Chinese)
Zou T, Li T, Zhang X, Yu H, Luo H (2011) Lead accumulation and tolerance characteristics of Athyrium wardii (Hook.) as a potential phytostabilizer. J Hazard Mater 186(1):683–689
Acknowledgments
This work was financially supported by the Natural Science Foundation of China (40901138). The authors are thankful to Margaret Cargill (The University of Adelaide) for critical comments regarding the language and construction of the manuscript. The authors also thank Deyong Wu, Lin Ji, Xia Huang, Hongjiang Zhang, Minying Liu, Xiaona Lv, Xuemei Yi, Jingzhou Sha, Dan He, Xiaoli Fu, Shuang Yang and Hui Liu of Sichuan Agricultural University for supporting the investigation and research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, S.J., Li, T.X., Huang, H.G. et al. Phytoremediation of cadmium using plant species of Athyrium wardii (Hook.). Int. J. Environ. Sci. Technol. 11, 757–764 (2014). https://doi.org/10.1007/s13762-013-0384-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-013-0384-z