Abstract
Biomass of three species of macrophytes, Pistia stratoides (water lettuce), Eichhornia crassipes (water hyacinth), and Phragmites australis (common reed) as well as their combination, were added into baffled subsurface-flow constructed wetlands (BSFCWs) as carbon source for the treatment of nitrate-laden wastewater. Nitrogen removal performance and responses of substrate enzyme activities (nitrate reductase, dehydrogenase, CM-cellulase, β-glucosidase, urease, and protease) were investigated and assessed in the present study. Nitrogen removal was significantly improved by all of the biomass (averaging 0.27 g TN·m−2 day−1 for control and 2.15–2.80 g TN·m−2 day−1 for the experimental systems), with best performance achieved by P. stratiotes. Dissolved oxygen and oxidation-reduction potential values in the biomass-added systems were significantly lower, beneficial for nitrate reduction. Effluent organic carbon content was low due to the low amount of biomass added, but quick decline of nitrate removal efficiency and nitrite accumulation were observed. All of the enzyme activities were notably enhanced by the biomass addition, especially in the initial phase after addition, which might be significant for decomposition and utilization of the macrophyte biomass for denitrification and the enhancement of nitrogen removal of the BSFCWs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nitrate pollution is widespread and usually accounts for a large proportion in nitrogen loads to surface and underground water bodies, due to the receptions of nitrate-laden wastewater such as agricultural runoff, secondary treated nitrified effluent from sewage plants, and greenhouse wastewater (Leverenz et al. 2010; Díaz et al. 2012; Gruyer et al. 2013; Wang and Chu 2016). High levels of nitrate play an important role in the eutrophication of surface waters and deterioration of underground waters. Thus, effective nitrate elimination from nitrate-contaminated wastewater/runoff before discharge is necessary for the protection and remediation of receiving water bodies.
Heterotrophic denitrification, a microbial-mediated pathway converting nitrate ultimately to nitrogen gas through a sequence of enzymatic reactions using labile organic carbon as electron and energy donor under a reducing environment, is regarded as a practical approach for nitrate removal, particularly on a large scale, due to its low cost, high efficiency, simple operation, and little secondary pollution (Van Rijn et al. 2006; Schipper et al. 2010). As one of the biological technologies for wastewater treatment, constructed wetland (CW) is suitable for nitrate reduction since the presence of large amount of anoxic and anaerobic microzones within its matrices is beneficial for denitrification (Wen et al. 2010; Chang et al. 2013). Besides, it has many other advantages such as cost-effective operation and maintenance, and high ecological and aesthetic values (Lin et al. 2002; Kadlec and Wallace 2009). However, organic carbon in nitrate-laden wastewater is usually deficient and less biodegradable, inhibiting the denitrification process greatly (Wen et al. 2010; Leverenz et al. 2010; Zhang et al. 2016a).
Introducing an external carbon source has been recognized as an efficient way to enhance nitrate removal capacity of a CW (Lin et al. 2002; Leverenz et al. 2010; Hang et al. 2016). Various natural cellulosic materials, presented in large amount with low/no cost, high renewal capacity, and convenient availability, are highly preferred in practical application and have been successfully employed to improve nitrate reduction (Cameron and Schipper 2010; Warneke et al. 2011; Wang and Chu 2016; Li et al. 2017). Moderate and sustained nitrogen removal performance and low adverse effects (e.g., high releases of carbon, color, and N2O) can be achieved by using some materials with high carbon content and relatively slow and stable release, such as woodchips (Warneke et al. 2011; Healy et al. 2012; Li et al. 2017). However, easy availability of organic materials is more significant in practice. Pistia stratiotes L. (Water lettuce), Eichhornia crassipes (water hyacinth), and Phragmites australis (common reed), three common macrophytes distributed widely in shallow eutrophic water bodies, are substantially presented in the ponds, marshes, and wetlands nearby Dianchi Lake, a large eutrophic lake in Kunming City, Yunnan province, China. Some ecological risk has been caused by the quick propagation and excessive distribution of P. stratiotes and E.crassipes at the water surface. Biomass of these macrophytes is expected to be feasible carbon source candidates for CW when treating nitrate-laden runoff into the lake although their performance was rarely reported. Moreover, the deterioration of effluent water quality caused by excess organic carbon release from the macrophytes may be partly counteracted from the view of algae inhibition, as the organic compounds (such as polyphenols, linoleic acid, alkaloid) leached by macrophytes can inhibit the growth of algae, named allelopathy (Zhang et al. 2016a, b).
Enzyme plays a highly significant role in pollutant removal and transformations and its activity can indicate and affect the operating status and performance of a CW (Weaver et al. 2012; Yi et al. 2016). Introduction of external solid organic material may influence the enzyme activities of a CW, partly reflecting mechanisms of the carbon source, but little information on it was available.
In this study, five laboratory-scale baffled subsurface-flow constructed wetland (BSFCW) microcosms were established to treat nitrate-laden wastewater, with dry biomass of P. stratiotes, E. crassipes, P. australis, and their combination added as external carbon sources. The macrophytes were added in a relatively low amount and replenished when nitrate removal was unsatisfactory to reduce effluent organic pollution and color problem, which is troublesome for the application of natural organic materials (Healy et al. 2012; Chang et al. 2016). Nitrogen removal and substrate enzyme (including nitrate reductase, dehydrogenase, CM-cellulase, β-glucosidase, urease, and protease) activities of the BSFCW systems were investigated and assessed. The purpose of the study was to investigate the effects of the macrophyte biomass addition on the nitrogen removal performance and substrate enzyme activities of BSFCWs treating nitrate-laden wastewater.
2 Methods and Materials
2.1 Experimental Setup of the BSFCW Microcosms
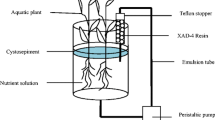
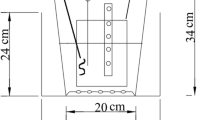
The laboratory-scale BSFCW microcosms comprised of five tanks made of toughened glass sheets located at the Yunnan University campus, Kunming, China, each measuring 1.0 m in length, 0.3 m in width, and 0.7 m in height. Details of the BSFCW microcosm were illustrated in Fig. 1. Each CW was lengthwise separated by five vertically installed toughened glass sheets to form six cells and force a sequential down- and up-flow water flow inside the CW microcosm (Fig. 1). In general, wastewater flows vertically within each cell while horizontally across the CW. It is expected that water pathway within the CW can be prolonged; thereby, pollutants removal performances can be enhanced (Tee et al. 2012). The inlet and outlet cells, each with a dimension of 0.05 × 0.3 × 0.7 m, were filled with cobbles in diameter of 15–25 mm to a height of 0.4 m for even inflow distribution and outflow collection. The second and third cells, both measuring 0.3 × 0.3 × 0.7 m and filling cobbles in diameter of 3–6 mm to a height of 0.6 m, were set as treatment zone with organic materials added. Macrophyte biomass was added into the perforated polyvinylchloride (PVC) pipes (diameter 150 mm) vertically installed in the two cells (Fig. 1). Dissolved organic carbon can transfer through the holes on the wall of the pipes but bulks of organic materials cannot; thus, clogging and collapse of wetland media may be avoided and the replacement of solid carbon is convenient. Another two cells were set as post-treatment zone for further purification of the wastewater, filling cobbles in diameter of 3–6 mm to a height of 0.1 m in the fourth (0.1 × 0.3 × 0.7 m) and 0.5 m in the fifth cell (0.2 × 0.3 × 0.7 m), respectively. A perforated PVC pipe in diameter of 20 mm was installed vertically at the center of each cell except the inlet and outlet cells for water quality monitoring (named sampling points A, B, C, and D, Fig. 1). Sides of the BHFCWs were covered with black-out plastic sheets to prevent algal growth within the microcosms from sunlight.
Canna indica was employed as wetland plant with seedlings transplanted into the treatment zone and fifth cell at a density of 20 plants/m2. The formal experiment began when the plants grew well and the CWs exhibited a limited but stable nitrogen removal after an adaptation period of several months with the wastewater fed and refreshed once a week.
2.2 Nitrate-Laden Wastewater and Operations of the BSFCWs
Synthetic nitrate-laden wastewater was used in this study by dissolving NaNO3 into tap water to form a NO3−-N concentration of 63.5 ± 1.2 mg L−1 according to the characteristics of various nitrate-contaminated wastewaters (Park et al. 2009; Leverenz et al. 2010; Wang and Chu 2016; Zhang et al. 2016a, b). KH2PO4 and K2HPO4 were applied to give a PO43−-P concentration of 5.0 ± 0.2 mg L−1. No organic substance was added and other trace nutrients were supplied by adding 1 mL micronutrient solution to 1 L wastewater. The composition of the micronutrient solution was as follows (per L): CaCl2·2H2O 4.0 g, MgSO4·7H2O 1.0 g, FeSO4·7H2O 1.0 g, ZnSO4·7H2O 0.4 g, MnCl2·4H2O 0.5 g, CoCl2·6H2O 0.5 g, CuSO4·5H2O 0.1 g, Na2MoO4·2H2O 0.1 g, and H3BO3 0.1 g (Wen et al. 2010; Li et al. 2013, 2017). All the chemical reagents used in the experiment were analytical grade.
The wastewater was dosed into the inlet cell of each BSFCW in a continuous mode via a multi-path peristaltic pump (BT-300CA, Jieheng, Chongqing, China) with the hydraulic loading rate (HLR) set at 0.1 m3 m−2 day−1 according to our previous trial. The theoretical hydraulic retention time (HRT) was approximately 2.5 days and water temperature during the experiment was approximately 18 ± 2 °C.
2.3 Organic Carbon Addition and Algal Inhibition Effect of BSFCW Effluent
Aboveground parts of P. stratiotes, E.crassipes, and P. australis were collected in the marsh near Dianchi Lake. The biomass was washed thoroughly to remove other debris, cut into around 5 cm in length, and dried in an air oven at 60 °C until a constant weight reached. P. stratiotes, E. crassipes, and P. australis as well as their combination (w:w:w = 1:1:1) was added into BSFCWs 1, 2, 3, and 4, respectively. CW 0 without organic carbon addition was set as the control system. A relatively low amount of 90 g (dry weight) biomass was added at first, as deterioration of effluent quality due to excessive release of colored organic matter would be caused by a high amount of plant biomass addition (Cameron and Schipper 2010; Zhang et al. 2014; Chang et al. 2016). The formal experiment commenced immediately after the biomass addition. Afterwards, another 120 and 200 g biomass were successively supplemented due to the unsatisfactory nitrate removal performance. Agitation of the organic materials in the pipes was also conducted in the last phase of the experiment to facilitate carbon diffusion in the microcosms.
Excess organic matter released from the macrophyte biomass may have an inhibition effect on algal growth, mitigating its adverse effect of secondary organic pollution. The outflow of the BHFCWs was collected on December 5 (1 day after the second fresh biomass addition) and its inhibition effect on the growth of Microcystis aeruginosa and Aphanizomenon flos-aquae was determined. The algae were provided by the Freshwater Algae Culture Collection of the Institute of Hydrobiology, the Chinese Academy of Sciences, and cultured according to the previous study (Zhang et al. 2016a, b).
2.4 Sampling and Analysis
Inflow and outflow from each BSFCW system were collected every 1–2 days, and NO3−-N, total nitrogen (TN), NO2−-N, NH4+-N, and color degree were spectrophotometrically measured according to the standard methods (SEPA 2002). Total organic carbon (TOC) concentration was analyzed by a TOC Analyzer (GE Sievers InnovOx, USA). Dissolved oxygen (DO) concentration and oxidation-reduction potential (ORP) along the length of the BSFCWs were determined in situ by putting a DO meter (INESA, China) and an ORP meter (INESA, China) into the center (30-cm depth) of the perforated PVC pipes established for water sampling.
With respect to the evaluation of inhibition effect of organic matter released by macrophyte biomass on algal growth, 10 mL outflow collected on December 5 was added into conical flasks (250 mL) containing 50 mL algae in exponential growth phase. The optical density (OD) values were determined using a spectrophotometer every day. Triplicate assays were conducted and inhibition rate was calculated according to the decrease of OD values.
As for the determination of substrate enzyme activities of the BSFCWs, three mixed substrate samples were collected, named substrate S1 (0–5-cm depth in the treatment zone), substrate S2 (30–35-cm depth in the treatment zone), and substrate S3 (0–5-cm depth in the fifth cell). Three sampling campaigns were carried out, which were on 10 days before 120 g fresh macrophyte biomass was added, 3 and 10 days after the addition, respectively. The samples were store at 4 °C after removing plant residues and analyzed as soon as possible.
Nitrate reductase activity was analyzed based on Abdelmagid and Tabatabai (1987). KNO3 was applied as the reaction medium and 2,4 p-nitro phenol was added to prohibit the reduction of produced nitrite. After a reaction period of 24 h at 25 °C, nitrite generated was determined using a spectrophotometer at 520 nm. Dehydrogenase activity was detected by adding 0.5% aqueous solution of triphenyltetrazolium chloride (TTC) to CW substrate and then incubating at 37 °C for 24 h (Tabatabai 1994). Triphenylformazan (TPF) with reddish color can be produced due to the microbial reduction of TTC and determined colorimetrically at 485 nm after extracted by methanol. CM-cellulase activity was analyzed by using CM-cellulose as a reaction medium at 37 °C for 72 h, and the produced reducing sugars were assayed colorimetrically at 540 nm after reacting with anthrone (Schinner and von Mersi 1990). β-Glucosidase activity was assayed using p-nitro phenol-β-d-glucoside solution as a reaction medium and the mixture was incubated at 37 °C for 1 h. Then, the released p-nitro phenol was determined colorimetrically at 400 nm (Xu and Zheng 1986). Urease activity was analyzed by using 10% aqueous urea as substrate, and the released ammonium after an incubation period of 48 h at 37 °C was assayed colorimetrically at 460 nm (Klose and Tabatabai 2000). As for the determination of protease, casein (2%, w/v) was added to the CW media and placed in an incubator at 37 °C for 24 h. Then, the produced tyrosine (TYR) was determined colorimetrically at 680 nm after it reacted with the Folin-Ciocalteu reagent (Xu and Zheng 1986; Tabatabai 1994). All enzyme activity assays were conducted in triplicate, corrected for blank, and calculated based on oven-dry weight.
2.5 Statistical Analysis
Nitrogen removal efficiency and area mass removal rate were calculated as follows based on the influent and effluent concentrations of the CWs as the evaporation rate during the experimental period (November to January) was very low and negligible.
where Cin and Ceff are the influent and effluent nitrogen concentrations (mg L−1), respectively, and HLR was set at 0.1 m3 m−2 day−1.
The differences of nitrogen removal rates and substrate enzyme activities between the BSFCWs were detected by one-way ANOVA followed by LSD test using SPSS 18.0 software package for Windows, and significant difference was regarded if p < 0.05.
3 Results and Discussion
3.1 Effect of Macrophyte Biomass Addition on the Nitrogen Removal Performance of the BSFCWs
The nitrate and TN removal efficiency of the BSFCWs are shown in Fig. 2 and Table 1.
It was seen that just a little amount of nitrate and TN were removed by CW 0, with mean efficiencies of 5.5 and 4.8%. This could be result of no organic carbon contained in the influent and added into the CW. High nitrate load and DO content in the CW (Fig. 4) can also lead to the low removal efficiency. The limited nitrogen removal could be attributed to plant uptake and denitrification fueled by internal carbon source from plant exudates and debris (Zhai et al. 2013). Nitrate reduction in CW can be restricted greatly by deficiency of available carbon source and prevailing aerobic conditions (Chang et al. 2013; Yang et al. 2016; Wang and Chu 2016). The NO3−-N and TN mass removal rate of 0.30 and 0.27 g N m−2 day−1 (Table 1) was in the lower end of values documented by others (Kadlec and Wallace 2009; Leverenz et al. 2010; Zhang et al. 2016a, b) despite of the varied operational conditions.
The addition of all macrophyte biomass significantly improved nitrogen removal rates of the BSFCWs (p < 0.05), although high efficiency declined largely after several days of operation. Available organic carbon supplied by the macrophyte biomass decreased quickly and was insufficient for denitrification. Similar trends were observed after each fresh biomass addition and the agitation (Fig. 2). A relatively low amount of macrophyte biomass was applied in this study to alleviate effluent secondary pollution derived from high releases of organic carbon and nitrogen in the start-up period after addition (Zhang et al. 2014; Chang et al. 2016). It was suggested that the macrophyte biomass could provide an effective carbon source for the denitrification process although the nitrogen removal was unstable. Nitrate reduction occurred along the entire CW microcosm (Fig. 3) mainly owing to the diffusion of carbon source. Furthermore, DO content and ORP values in all macrophyte biomass-added BSFCWs were notably lower than those in the control system (Fig. 4, just data of CWs 0 and 1 was shown), which is conducive to denitrification. DO in the inflow (5.5 ± 0.4 mg L−1) could be quickly consumed by microbial aerobic degradation of organic substance, creating a reducing environment within the CWs. This consumption was acceptable in view of low/no cost and easy availability of the macrophyte biomass. DO and ORP levels were lower at sampling points of A and C, probably due to the up-flow mode in these cells with less oxygen supplement by plant root release and air diffusion.
Variations of nitrogen concentrations along the length of BSFCW 1 on December 17. Details on the sampling points were shown in Fig. 1
DO and ORP values along the length of BHFCW 0 and 1 (n = 23). Details on the sampling points were shown in Fig. 1. The solid line and square within the box mark the median and the mean, and the upper and lower boundary of the box represent the 25th and 75th percentiles, respectively. Error bars above and below the box indicate the 95th and 5th percentiles and asterisks above and below the box indicate the maximum and minimum, respectively
Relatively low effluent organic matter residue and color degree were observed for the HSFCWs (Table 1) even in the initial phase after addition as a consequence of a low amount of macrophyte biomass applied in this study. Nevertheless, organic compounds in the effluent collected 1 day after the second addition could effectively inhibit the growth of cultured A. flos-aquae and M. aeruginosa, although the effect on M. aeruginosa was low (Fig. S1). The inhibition effect of outflow from BHFCW 3 was the highest in addition to its satisfactory nitrogen removal and low accumulations of nitrite and ammonium (Table 1), suggesting that reed biomass was a promising carbon source candidate for the treatment of nitrate-contaminated wastewater.
Accumulations of nitrite and ammonium occurred in the biomass-added BSFCWs due to nitrogen conversions (Table 1). The highest nitrite and ammonium contents were observed at sampling point A and limited decline along the length of the CW microcosm occurred (Fig. 3). The introduction of nitrogenous substances in macrophyte biomass could contribute to the accumulations, although the amount was much lower compared with that removed by the CWs (positive TN removal efficiency during the whole experiment, Fig. 2). In addition, dissimilatory nitrate reduction to ammonia (DNRA) process would contribute to the production of NH4+ (Van Rijn et al. 2006; Shen et al. 2013). Greenan et al. (2006) reported that less than 4% of nitrate removal was attributed to DNRA in denitrification biofilters filled with woodchips. Nitrite, one of intermediates during nitrate reduction, can accumulate under incomplete denitrification and was presented in the highest amount in the E.crassipes-added microcosm (Table 1). Many factors, including the amount and type of available organic carbon, HRT, and DO level can influence the nitrite accumulation (Gómez et al. 2000; Li et al. 2013; Shen et al. 2013). The effluent of the BSFCWs needed further treatment. Uneven release rate of natural solid biomass made it difficult to achieve a stably high nitrogen removal and low effluent secondary organic pollution. Hydrolyzation and fermentation of plant biomass to produce a low-cost liquid carbon source before feeding may be a solution although accurate control is required (Zhang et al. 2016a, b; Fu et al. 2017).
Due to the nitrogen import and transformations, TN removal of the BSFCWs was lower than that of NO3−-N (Fig. 2, Table 1). Overall, 2.15–2.80 g TN m−2 day−1 was removed by the carbon-added BSFCWs while the nitrate removal rates were 2.64–3.24 g NO3−-N m−2 day−1, which were comparable to other denitrification beds added with cellulose-rich carbon sources (Cameron and Schipper 2010; Warneke et al. 2011; Zhang et al. 2016a, b). Best performance was obtained in BSFCW 1 due to more effective carbon supplied by P. stratiotes. But its physical solid structure was easily destroyed to a pasty state after soaking, which may bring about some trouble for its replacement and management of the CW system. The E. crassipes performed worst, probably due to its low carbon content and more refractory component. Nevertheless, all of the macrophyte biomass, which distributed broadly in/around eutrophic surface waters with great trouble for disposal, could be used to improve nitrogen removal of CW systems when treating nitrate-contaminated wastewater. The excess organic matter leached from the macrophyte biomass may have some inhibition effect on algal growth although further study is still required.
3.2 Effect of Macrophyte Biomass Addition on Enzyme Activities of the BSFCWs
Activities of nitrate reductase, dehydrogenase, CM-cellulase, β-glucosidase, urease, and protease of the BSFCWs are depicted in Fig. 5 (just data of BSFCWs 0 and 1 was presented).
All of the enzyme activities in substrates of the BHFCWs were significantly enhanced by the addition of macrophyte biomass (p < 0.05), and almost increased after addition of fresh biomass. The introduction of solid carbon materials into the BSFCWs might be responsible for the increase by providing a variety of organic substances to stimulate microbial growth and higher activities in the microcosms. Choi et al. (2009) documented that organic matter from plant residues and root exudates played a significant role in enhancing sediment enzyme activities. After an operation period of several days, the amount of substances released from the biomass decreased, resulting in a decline of the enzyme activities.
Similar enzyme activities were obtained for the four biomass-added BSFCWs with no consistent variation trend among them (data not shown), suggesting that the species of macrophyte biomass had no significant influence on enzyme activities. Moreover, it was reported that enzyme activities were higher in the upper layer of CW substrate due to more availability of organic carbon, nutrients, and oxygen (Baddam et al. 2016), but it was not the case in this study (no significant difference between S1 and S2 in most cases) probably due to the modes of organic carbon addition and water flow of the BSFCWs.
Nitrate reductase, a vital enzyme catalyzing the first step of denitrification reducing nitrate to nitrite, was largely enhanced due to abundant available carbon from the macrophyte biomass and reducing environment facilitated the development and efficacy of denitrifiers. It was reported that denitrifying enzyme activity was positively correlated with dissolved organic carbon concentration (Song et al. 2011), and plant litter carbon could promote the growth of bacteria containing nitrate reduction gene in CWs (Chen et al. 2014; Fu et al. 2017). Accordingly, nitrate removal of the BHFCWs was largely improved by the macrophyte biomass addition. The highest values observed for S2 might be resulted from stronger reducing environment and more available carbon in the middle layer of the treatment zone.
Dehydrogenase activity, an indicator of organic matter mineralization capability and metabolism activities of viable microbes, was enhanced by the addition of organic carbon. CM-cellulase, β-glucosidase, urease, and protease were also enhanced, probably because organic compounds released from the macrophyte biomass, such as celluloses, carbohydrates, and proteins, induced the growth and excretion of extracellular enzymes of attached degrading microbes. It was reported that enzyme activities of CW sediment were closely dependent on the availability of organic carbon (Weaver et al. 2012; Chang et al. 2015; Baddam et al. 2016). The enhancement might be of significance for the enzymatic hydrolyzation of the insoluble macrophyte biomass to provide available carbon for diverse denitrifiers (Wang and Chu 2016). Solid organic carbon could enhance microbial diversity in CWs and increase the abundance of functional microorganisms involved in breakdown of complex organic materials (such as cellulose degraders) (Hiibel et al. 2011; Shen et al. 2013). This was crucial for the hydrolysis and subsequent utilization for denitrification of the plant biomass (Chu and Wang 2016).
4 Conclusion
Addition of P. stratiotes, E.crassipes, and P. australis biomass could all effectively enhance nitrogen removal performance of BSFCW microcosms treating nitrate-contaminated wastewater, with mean removal rates of 2.64–3.24 and 2.15–2.80 g N·m−2 day−1 achieved for nitrate and TN in comparison to 0.30 and 0.27 g N·m−2 day−1 for the control. Significantly lower DO and ORP values obtained in biomass-added microcosms were favorable for nitrate reduction. Effluent organic carbon concentration was low due to a low amount of biomass applied in this study, but a quick decrease of nitrate removal efficiency and nitrite accumulation was observed. Inhibition effect of the effluent on the growth of cultured M. aeruginosa and A. flos-aquae was detected, especially for A. flos-aquae, with the highest effect achieved by P. australis. Activities of nitrate reductase, dehydrogenase, CM-cellulase, β-glucosidase, urease, and protease were all significantly enhanced by the biomass addition, especially in the initial phase after addition, which corresponded to the increased nitrogen removal. This was probably of significance for the hydrolysis and utilization for denitrification of the macrophyte biomass. Overall, macrophyte biomass could be used as carbon source candidate for CW treating nitrate-rich runoff.
References
Abdelmagid, H.M., & Tabatabai, M.A. (1987). Nitrate reductase activity of soils. Soil Biology Biochemistry, 19, 421–427.
Baddam, R., Reddy, G. B., Raczkowski, C., & Cyrus, J. S. (2016). Activity of soil enzymes in constructed wetlands treated with swine wastewater. Ecological Engineering, 91, 24–30.
Cameron, S. G., & Schipper, L. A. (2010). Nitrate removal and hydraulic performance of organic carbon for use in denitrification beds. Ecological Engineering, 36(11), 1588–1595.
Chang, J. J., Wu, S. Q., Dai, Y. R., Liang, W., & Wu, Z. B. (2013). Nitrogen removal from nitrate-laden wastewater by integrated vertical-flow constructed wetland systems. Ecological Engineering, 58, 192–201.
Chang, J. J., Wu, S. Q., Liang, K., Wu, Z. B., & Liang, W. (2015). Responses of microbial abundance and enzyme activity in integrated vertical-flow constructed wetlands for domestic and secondary wastewater. Desalination and Water Treatment, 56(8), 2082–2091.
Chang, J. J., Lu, Y. F., Chen, J. Q., Wang, X. Y., Luo, T., & Liu, H. (2016). Simultaneous removals of nitrate and sulfate and the adverse effects of gravel-based biofilters with flower straws added as exogenous carbon source. Ecological Engineering, 95, 189–197.
Chen, Y., Wen, Y., Zhou, Q., & Vymazal, J. (2014). Effects of plant biomass on denitrifying genes in subsurface-flow constructed wetlands. Bioresource Technology, 157, 341–345.
Choi, H. C., Kang, H., & Park, S. S. (2009). Comparison of enzyme activities in vegetated and nonvegetated sediments. Journal of Environmental Engineering, 135(5), 299–305.
Chu, L. B., & Wang, J. L. (2016). Denitrification of groundwater using PHBV blends in packed bed reactors and the microbial diversity. Chemosphere, 155(3), 463–470.
Díaz, F. J., Anthony, T. O., & Dahlgren, R. A. (2012). Agricultural pollutant removal by constructed wetlands: Implications for water management and design. Agricultural Water Management, 104, 171–183.
Fu, G., Huangshen, L., Guo, Z., Zhou, Q., & Wu, Z. (2017). Effect of plant-based carbon sources on denitrifying microorganisms in a vertical flow constructed wetland. Bioresource Technology, 224, 214–221.
Gómez, M.A., González-López, J., Hontoria-Garcı́a, E. (2000). Influence of carbon source on nitrate removal of contaminated groundwater in a denitrifying submerged filter. Journal of Hazardous Materials, 80(1), 69–80.
Greenan, C. M., Moorman, T. B., Kaspar, T. C., Parkin, T. B., & Jaynes, D. B. (2006). Comparing carbon substrates for denitrification of subsurface drainage water. Journal of Environmental Quality, 35(3), 824–829.
Gruyer, N., Dorais, M., Alsanius, B. W., & Zagury, G. J. (2013). Use of a passive bioreactor to reduce water-borne plant pathogens, nitrate, and sulfate in greenhouse effluent. Journal of Environmental Science and Health, Part A, 48(13), 1740–1747.
Hang, Q., Wang, H., Chu, Z., Ye, B., Li, C., & Hou, Z. (2016). Application of plant carbon source for denitrification by constructed wetland and bioreactor: review of recent development. Environmental Science and Pollution Research, 23(9), 8260–8274.
Healy, M. G., Ibrahim, T. G., Lanigan, G. J., Serrenho, A. J., & Fenton, O. (2012). Nitrate removal rate, efficiency and pollution swapping potential of different organic carbon media in laboratory denitrification bioreactors. Ecological Engineering, 40, 198–209.
Hiibel, S. R., Pereyra, L. P., Breazeal, M. V. R., Reisman, D. J., Reardon, K. F., & Pruden, A. (2011). Effect of organic substrate on the microbial community structure in pilot-scale sulfate-reducing biochemical reactors treating mine drainage. Environmental Engineering Science, 28(8), 563–572.
Kadlec, R. H., & Wallace, S. D. (2009). Treatment wetlands (2nd ed.). Boca Raton: CRC Press.
Klose, S., & Tabatabai, M. A. (2000). Urease activity of microbial biomass in soils as affected by cropping systems. Biology and Fertility of Soils, 31(3), 191–199.
Leverenz, H. L., Haunschild, K., Hopes, G., Tchobanoglous, G., & Darby, J. L. (2010). Anoxic treatment wetlands for denitrification. Ecological Engineering, 36(11), 1544–1551.
Li, P., Zuo, J., Xing, W., Tang, L., Ye, X., Li, Z., Yuan, L., Wang, K., & Zhang, H. (2013). Starch/polyvinyl alcohol blended materials used as solid carbon source for tertiary denitrification of secondary effluent. Journal of Environmental Sciences, 25(10), 1972–1979.
Li, H., Chi, Z., Yan, B., Cheng, L., & Li, J. (2017). An innovative wood-chip-framework substrate used as slow-release carbon source to treat high-strength nitrogen wastewater. Journal of Environmental Sciences, 51, 275–283.
Lin, Y. F., Jing, S. R., Wang, T. W., & Lee, D. Y. (2002). Effects of s and external carbon sources on nitrate removal from groundwater in constructed wetlands. Environmental Pollution, 119(3), 413–420.
Park, J. B. K., Craggs, R. J., & Sukias, J. P. S. (2009). Removal of nitrate and phosphorus from hydroponic wastewater using a hybrid denitrification filter (HDF). Bioresource Technology, 100(13), 3175–3179.
Schipper, L. A., Robertson, W. D., Gold, A. J., Jaynes, D. B., & Cameron, S. C. (2010). Denitrifying bioreactors-an approach for reducing nitrate loads to receiving waters. Ecological Engineering, 36(11), 1532–1543.
Schinner, F., & Von Mersi, W. (1990) Xylanase-, CM-cellulose- and invertase activity in soil, an improved method. Soil biology Biochemistry, 22, 511–515.
Shen, Z., Zhou, Y., & Wang, J. (2013). Comparison of denitrification performance and microbial diversity using starch/polylactic acid blends and ethanol as electron donor for nitrate removal. Bioresource Technology, 131, 33–39.
Song, K., Lee, S. H., & Kang, H. (2011). Denitrification rates and community structure of denitrifying bacteria in newly constructed wetland. European Journal of Soil Biology, 47(1), 24–29.
State Environmental Protection Administration of China. (2002). Standard methods for water and wastewater monitoring and analysis (4th ed.). Beijing: China Environmental Science Press (In Chinese).
Tabatabai, M.A. (1994). Soil enzymes, in: Weaver, R.W., Angel, J.S., Bottomley, P.S.(Eds.), Methods of Soil Analysis, Part 2—Microbiological and Biochemical Properties. SSSA Book Series No. 5, Soil Science Society of America, Madison, WI, pp.775–833.
Tee, H. C., Lim, P. E., Seng, C. E., & Mohd-Asri, M. N. (2012). Newly developed baffled subsurface-flow constructed wetland for the enhancement of nitrogen removal. Bioresource Technology, 104, 235–242.
Van Rijn, J., Tal, Y., & Schreier, H. J. (2006). Denitrification in recirculating systems: theory and applications. Aquacultural Engineering, 34(3), 364–376.
Wang, J., & Chu, L. (2016). Biological nitrate removal from water and wastewater by solid-phase denitrification process. Biotechnology Advances, 34(6), 1103–1112.
Warneke, S., Schipper, L. A., Matiasek, M. G., Scow, K. M., Cameron, S., Bruesewitz, D. A., & McDonald, I. R. (2011). Nitrate removal, communities of denitrifiers and adverse effects in different carbon substrates for use in denitrification beds. Water Research, 45(17), 5463–5475.
Weaver, M. A., Zablotowicz, R. M., Krutz, L. J., Bryson, C. T., & Locke, M. A. (2012). Microbial and vegetative changes associated with development of a constructed wetland. Ecological Indicators, 13, 37–45.
Wen, Y., Chen, Y., Zheng, N., Yang, D., & Zhou, Q. (2010). Effects of plant biomass on nitrate removal and transformation of carbon sources in subsurface-flow constructed wetlands. Bioresource Technology, 101(19), 7286–7292.
Xu, G. H., & Zheng, H. Y. (1986). Handbook of analysis of soil microorganism. Beijing: Agriculture Press (In Chinese).
Yang, Y., Zhan, X., Wu, S., Kang, M., Guo, J., & Chen, F. (2016). Effect of hydraulic loading rate on pollutant removal efficiency in subsurface infiltration system under intermittent operation and micro-power aeration. Bioresource Technology, 205, 174–182.
Yi, X. H., Jing, D. D., Wan, J., Ma, Y., & Wang, Y. (2016). Temporal and spatial variations of contaminant removal, enzyme activities, and microbial community structure in a pilot horizontal subsurface flow constructed wetland purifying industrial runoff. Environmental Science and Pollution Research, 23(9), 8565–8576.
Zhai, X., Piwpuan, N., Arias, C. A., Headley, T., & Brix, H. (2013). Can root exudates from emergent wetland plants fuel denitrification in subsurface flow constructed wetland systems? Ecological Engineering, 61, 555–563.
Zhang, M., Zhao, L., Mei, C., Yi, L., & Hua, G. (2014). Effects of plant material as carbon sources on TN removal efficiency and N2O flux in vertical flow-constructed wetlands. Water Air and Soil Pollution, 225(11), 1–11.
Zhang, S. H., Xu, P. Y., & Chang, J. J. (2016a). Physiological responses of Aphanizomenon flos-aquae under the stress of Sagittaria sagittifolia extract. Bulletin of Environmental Contamination and Toxicology, 97(6), 870–875.
Zhang, C., Yin, Q., Wen, Y., Guo, W., Liu, C., & Zhou, Q. (2016b). Enhanced nitrate removal in self-supplying carbon source constructed wetlands treating secondary effluent: the roles of plants and plant fermentation broth. Ecological Engineering, 91, 310–316.
Acknowledgements
We thank the anonymous reviewers for their helpful comments, which greatly improved the quality of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (51408531), the Action Plans for Yunnan University Service to Yunnan Province (2016MS18), and Project of Science and Technology Program of Yunnan Province, China (2017FB090).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Fig. S1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Chang, J., Deng, S., Jia, W. et al. Nitrogen Removal Performance and Enzyme Activities of Baffled Subsurface-Flow Constructed Wetlands with Macrophyte Biomass Addition. Water Air Soil Pollut 229, 182 (2018). https://doi.org/10.1007/s11270-018-3837-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-3837-7