Abstract
The algal growth and physiological characters of Aphanizomenon flos-aquae were studied under the stress of Sagittaria sagittifolia extract. The results showed that the growth of A. flos-aquae was significantly inhibited by S. sagittifolia extract. The exopolysaccharide (EPS), total soluble protein, intracellular phosphorus (o-PO4-P) contents and malondialdehyde (MDA) contents in A. flos-aquae cells increased significantly. These results suggested that A. flos-aquae can adapt to stress by increasing its normal metabolic activity. The algal cellular antioxidant enzymes, superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD), were triggered to different degrees when exposed to S. sagittifolia extract. The MDA contents and activities of SOD, CAT and POD in algal cells suggested that oxidative damage induced by S. sagittifolia extract via the oxidation of ROS (O2·−) might be an important factor responsible for the inhibition of the growth of A. flos-aquae. In addition, SOD may be an important site for the inhibition of S. sagittifolia extract on A. flos-aquae cells. These results indicate that S. sagittifolia may be a good candidate for controlling A. flos-aquae blooms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Cyanobacterial blooms are ubiquitous phenomenon in eutrophic aquatic ecosystems, having deleterious effects on the biodiversity and equilibrium of aquatic ecosystems. Therefore, the control and elimination of the growth of cyanobacterial blooms is a crucial step in the recovery and protection of aquatic ecosystems, especially lakes. Natural algicides from natural biomaterials have received attention as alternatives to chemical agents, as algicides are likely to be specific and biodegradable and may, therefore, offer an environmentally friendly method for the control of algal blooms (Yi et al. 2012). The polyphenols produced by aquatic plants influenced the activity of different extracellular or membrane-bound enzymes produced by M. aeruginosa (Dziga et al. 2009). Linoleic acid (LA) could also influence the antioxidant enzymes of M. aeruginosa (Ni et al. 2015).
Aphanizomenon flos-aquae is a bloom-forming cyanobacteria species and can release toxins into the surrounding water (Liu et al. 2006; Zhang et al. 2015b). In Dianchi Lake, occurrences of Microcystis blooms alternate with Aphanizomenon blooms, and A. flos-aquae is the dominant species from March to May (Liu et al. 2006). Although there have been several studies involving the isolation of natural algicides for the control of Microcystis blooms (Nakai et al. 2000; Hong et al. 2009; Zhang et al. 2012, 2016), less attention has been paid to the search for algicides against A. flos-aquae. Sagittaria sagittifolia is an emergent macrophyte used in the ecological restoration of eutrophic bodies of water. In our previous study, the extract of S. sagittifolia showed inhibitory activity on the growth of A. flos-aquae (Zhang et al. 2015b). The aim of the present study is to further explore the mechanisms of S. sagittifolia extract activity on A. flos-aquae.
Materials and Methods
Sagittaria sagittifolia was collected from a small lake in a suburb of Kunming City, Yunnan Province, China, dried at normal ambient temperature. Powdered S. sagittifolia (20 g) was immersed in hexane (600 mL) for 3 days at room temperature and was filtered with filter paper to remove the insoluble residue. The filtrate was concentrated under vacuum to remove hexane and to yield the extract. The extract was then stored in a freezer (−20°C). The cyanobacteria species A. flos-aquae (FACHB 1170) was provided by the Freshwater Algae Culture Collection of the Institute of Hydrobiology, the Chinese Academy of Sciences, and was pre-cultivated in BG11 medium at 25 ± 2°C with a 14 h:10 h light/dark cycle, with illumination at 43.2 μmol photons m−2 s−1 provided by cool-white fluorescent lamps. The algae were shaken three times each day. In the study, organisms in the exponential growth phase were used. The bacterial biomass in the cultures was negligible.
The growth inhibition of A. flos-aquae was performed using the ISO 8692 method (1989) with some modifications. Conical flasks (250 mL) were prepared and autoclaved, with each containing 100 mL culture media. After its dissolution into dimethyl sulfoxide (DMSO), the initial concentration gradients of macrophyte extract were as follows: 0, 5, 10, 20, 50 and 100 mg L−1. Media with only DMSO were used as the controls. The DMSO level in the test flasks did not exceed 0.1 % (v/v). Prior to these experiments, we found that 0.1 % (v/v) DMSO had no obvious effect on the growth and other tested physiological characteristics of A. flos-aquae. The initial algal density (IADs) was 1 × 106 cells m L−1. Each experiment included triplicate treatments, and the experiments were repeated twice.
The chlorophyll a concentrations of A. flos-aquae were measured using the colorimetric method (SEPA 2002), and the algal cell number was examined with a light microscope (Olympus CX23) and hemocytometers (STD, 20 mm × 20 mm, 0.1mL), with 10 µm defined as one unit (Larson and Passy 2012). These measurements were performed every day.
The algal cell-free enzyme extract was obtained according to the procedure described by Zhang et al. (2015a) and it was used to measure the enzyme activities and malondialdehyde (MDA). The activity of superoxide dismutase (SOD) was determined using the nitroblue tetrazolium (NBT) photoreduction (Beauchamp and Frodovich 1971) test. The antioxidant enzyme activities of catalase (CAT) and peroxidase (POD) were determined according to the method of Rao et al. (1996) and Dias and Costa (1983) respectively. The MDA content was assayed by the method described by Shiu and Lee (2005).
The total soluble protein content was measured according to the Coomassie brilliant blue G-250 dye-binding method (Bradford 1976) using bovine serum albumin as a standard. The total exopolysaccharide (EPS) concentration was obtained by measurement of the bound and the soluble exopolysaccharide according to the methods described by Hellebust and Craigie (1978) and Staats et al. (1999). The orthophosphate concentration in the cells of A. flos-aquae was obtained and measured according to the method described by Shi et al. (2003).
The mass units of the enzymatic activities and non-enzymatic substance contents in this study were defined using the cell-counting method. All experiments were performed in triplicate, and the data were expressed as the means ± standard deviation (SD). Analysis for significance was performed with SPSS software (13.0) (SPSS Inc., Chicago, IL, USA) using the independent-samples t-test, and the differences were considered to be significant at p < 0.05.
Results and Discussion
The growth inhibition of A. flos-aquae caused by the hexane extract from S. sagittifolia is illustrated in Fig. 1. After 3 days, the cell densities of A. flos-aquae exposed to 5, 10, 20, 50, and 100 mg L−1 macrophyte extract were 87 %, 67 %, 26 %, 16 % and 8 %, relative to the controls, respectively. The changes in the algal Chl-a concentration showed the same tendency as that of the algal densities. Zhang et al. (2012) found that methanol extract of Hydrilla verticillata (Linn.f.) Royle had the highest inhibition on the growth of Anabaena flos-aquae and Chlorella pyrenoidosa. Thalia dealbata root aqueous extract showed growth inhibition against Anabaena flos-aquae, M. aeruginosa and natural phytoplankton assemblages (Zhang et al. 2011). In this study, both the algal cells and Chl-a concentration decreased obviously in the S. sagittifolia extract treatment (Fig. 1, p < 0.05).
Carbohydrate and cellular proteins are two basic indicators of the physiological state of cells (Henderson et al. 2008). Phytoplankton can adapt to stress by increasing their normal metabolic activity (Prado et al. 2009). One of the functions of EPS is to form a protective layer to resist toxins or other environmental stresses. The EPS concentration of Phormidium autumnale increased significantly to defend against three ciliate grazer species (Pajdak-Stos et al. 2001). In our study, EPS showed a concentration-dependent increase in response to S. sagittifolia hexane extract exposure (Fig. 2). The bound, soluble and total EPS contents of A. flos-aquae increased with increasing S. sagittifolia extract concentration after 24 h exposure. The increased EPS concentrations suggested that new polysaccharides were synthesized to resist environmental stress. Soluble proteins in Scenedesmus obliquus cells are accumulated under heavy metal stress (Mohamed et al. 2004). In the present experiment, the total soluble protein content of A. flos-aquae cells increased significantly with the increasing exposure concentration of S. sagittifolia hexane extract (Fig. 3a). Accumulation of protein may be one of the ways through which A. flos-aquae abolishes the toxic effects of the S. sagittifolia hexane extract.
Effects of the Sagittaria sagittifolia hexane extract on the contents of bound (a), soluble (b) and total exopolysaccharides (EPS) (c) of Aphanizomenon flos-aquae. (Data are given as the means ± SD of three replicates, *p < 0.05 indicates significant differences compared with the corresponding controls without S. sagittifolia extract)
Soluble protein (a) and phosphorus (o-PO4-P) (b) contents in Aphanizomenon flos-aquae cells exposed to varying concentrations of Sagittaria sagittifolia hexane extract. (Data are given as the means ± SD of three replicates, *p < 0.05 indicates significant differences compared with the corresponding controls without S. sagittifolia extract)
The change in phosphorus (o-PO4-P) (B) content (Fig. 3b) showed a similar pattern to that of the soluble protein content in that the o-PO4-P content increased after exposure to all treatment concentrations between 24 and 72 h. Orthophosphate (PO4 3−) is a substance in high-energy phosphate-bond synthesis. Kasemets et al. (2006) showed that the addition of low concentrations of octanoic and decanoic acids increased QATP (specific ATP production rate) and m e (maintenance energy requirement for growth) in Saccharomyces cerevisiae. Nonanoic acid stress stimulated the phosphorus uptake of M. aeruginosa (Shao et al. 2009); thus, the S. cerevisiae and M. aeruginosa required more energy to cope with the stress of allelochemicals. The contents of o-PO4-P in A. flos-aquae cells showed a concentration-dependent increase in response to S. sagittifolia extract exposure. We speculate that A. flos-aquae, similar to M. aeruginosa and S. cerevisiae, requires more energy to cope with S. sagittifolia extract stress, and more high-energy phosphate bonds were required under different doses of S. sagittifolia extract, suggesting that S. sagittifolia extract stress stimulated phosphorus uptake by A. flos-aquae.
Enhanced MDA content is a vital sign of cellular oxidative damage (Shao et al. 2009). In the present study, MDA showed a concentration-dependent increase in response to S. sagittifolia hexane extract exposure (Fig. 4d). The MDA contents of the control group remained relatively steady, but those of the treatment groups exhibited significant changes. Increases in the MDA concentrations of A. flos-aquae were observed after 24 h incubation with increased exposure concentration. In addition, the MDA concentration in A. flos-aquae cells exposed to low concentrations of S. sagittifolia extract increased sharply at first and then decreased to slightly higher than that of the control. These results indicated that the S. sagittifolia extract caused algal cell membrane damage, and the oxidative damage might be reduced by the cellular defense system under exposure to low concentrations.
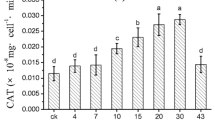
Activities of superoxide dismutase (a), catalase (b), peroxidase (c), and malondialdehyde content (d) in Aphanizomenon flos-aquae exposed to varying concentrations of Sagittaria sagittifolia hexane extract. The y-axis represents the activities of the enzymes (or the content of MDA) expressed as the mean ± SD of three replicate cultures. The asterisks indicate statistically significant differences compared with the corresponding control, with p < 0.05
Scavenging of ROS occurs mainly through antioxidant pathways that consist of enzymatic and non-enzymatic scavenging systems. Antioxidant enzymes in algal cells were activated when exposed to allelochemicals (Latifi et al. 2009; Zhang et al. 2015a). Our results indicated that algal cellular antioxidant enzymes were triggered to different degrees when exposed to S. sagittifolia extract (Fig. 4a–c). The change in SOD activity was different depending on the S. sagittifolia hexane extract concentration (Fig. 4a). With exposure to lower concentrations (5, 10 and 20 mg L−1) of S. sagittifolia extract, the SOD activity increased throughout the 72 h period. In contrast, the SOD activities increased at first and then decreased when exposed to the 50 and 100 mg L−1 concentrations of S. sagittifolia extract. This result is in accordance with the effects of the algicidal bacterium Pseudomonas mendocina on the antioxidant system of A. flos-aquae (Shi et al. 2009). However, the effects of S. sagittifolia hexane extract on CAT and POD activities were different (Fig. 4b, c). The CAT and POD activities both increased initially after exposure to S. sagittifolia hexane extract, and increased as the S. sagittifolia extract concentration increased and exposure time extended.
The CAT and POD activities in the treated group remained at high levels throughout the 72 h cultivation period. The SOD activity in the treated A. flos-aquae showed significant increases during the first 24 h, thereafter, the SOD activity showed a declining tendency (Shi et al. 2009). The resistance through SOD disappeared later due to the durative or acute increase of O2·− (Hong et al. 2009). Therefore, the decrease in SOD activities in A. flos-aquae cells under higher exposure concentrations is a result of the acute increase in ROS in cells. The major function of CAT and POD in cells is the direct removal of H2O2 to protect from oxidative damage (Latifi et al. 2009). The CAT and POD activities in treated cells were significantly higher than those in the control under all the treatment concentrations, indicating that CAT and POD were also active in eliminating ROS directly or indirectly. The major ROS include the superoxide radical (O2·−), hydrogen peroxide (H2O2), and their derivatives. Primarily, O2·− is converted into H2O2 by superoxide dismutase (SOD). Subsequently, H2O2 is scavenged by hydroperoxidases. Therefore, SOD is the first line of resistance against ROS. The S. sagittifolia hexane extract increased SOD activity in A. flos-aquae cells after 24 h incubation with increased exposure concentration, and SOD activity transitioned from an increase to a decrease with the prolongation of exposure time when exposed to 50 and 100 mg L−1 concentrations of S. sagittifolia extract. These results are consistent with previous reports in which the O2·− in cells increased and SOD activities decreased when treated with allelochemicals (Park et al. 2007; Hong et al. 2009). Therefore, our results suggest that oxidative damage induced by S. sagittifolia hexane extract via the oxidation of ROS (O2·−) might be an important factor responsible for the inhibition of the growth of A. flos-aquae. The results also suggest that SOD is an important site for the activities of S. sagittifolia hexane extract in A. flos-aquae cells.
Aphanizomenon flos-aquae is rarely used as the target algae in the search for the algicides. In our previous study, the extract of S. sagittifolia showed inhibitory activity on the growth of A. flos-aquae (Zhang et al. 2015b), but the mechanisms for the inhibition were not explored. As far as we know, this is the first report elucidating the effects of S. sagittifolia extract on the physiological characteristics of A. flos-aquae. In conclusion, the growth of A. flos-aquae was inhibited by S. sagittifolia hexane extract. On the basis of the results for EPS, protein, o-PO4-P, our present study suggests that A. flos-aquae could adapt to stress by increasing the normal metabolic activity. The MDA contents and SOD, CAT and POD activities in algal cells suggested that oxidative damage induced by S. sagittifolia extract via the oxidation of O2·− is an important factor responsible for the inhibition of the growth of A. flos-aquae and that SOD is an important site of action of S. sagittifolia extract in A. flos-aquae cells. These results indicate that S. sagittifolia would be a potential species for A. flos-aquae blooms control.
References
Beauchamp C, Frodovich I (1971) Superoxide dismutase: improved assays and an assays applicable acrylamide gels. Anal Biochem 44:276–287
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Dias M-A, Costa M-M (1983) Effect of low salt concentrations on nitrate reductase and peroxidase of sugar beet leaves. J Exp Bot 34:537–543
Dziga D, Goral T, Bialczyk J (2009) Extracellular enzymes of the Microcystis aeruginosa PCC 7813 strain are inhibited in the presence of hydroquinone and pyrogallol, allelochemicals produced by aquatic plants. J Phycol 45:1299–1303
Hellebust J, Craigie J (eds) (1978) Handbook of phycological methods. Physiological and biochemical methods. Cambridge University, Cambridge
Henderson R-K, Baker A, Parsons S-A, Jefferson B (2008) Characterisation of algogenicorganic matter extracted from cyanobacteria, green algae and diatoms. Water Res 42:3435–3445
Hong Y, Hu H-Y, Xie X, Sakoda A, Sagehashi M, Li F-M (2009) Gramine-induced growth inhibition, oxidative damage and antioxidant responses in freshwater cyanobacterium Microcystis aeruginosa. Aquat Toxicol 91:262–269
ISO 8692 (1989) Water quality - Fresh water algal growth inhibition test with Scenedesmus subspicatus and Selenastrum capricornutum. International Organization for Standardization, Geneva
Kasemets K, Kahru A, Laht T-M, Paalme T (2006) Study of the toxic effect of short and medium-chain monocarboxylic acids on the growth of Saccharomyces cerevisiae using the CO2-auxo-accelerostat fermentation system. Int J Food Microbiol 111:206–215
Larson C-A, Passy S-I (2012) Taxonomic and functional composition of the algal benthos exhibits similar successional trends in response to nutrient supply and current velocity. FEMS Microbiol Ecol 80:352–362
Latifi A, Ruiz M, Zhang C-C (2009) Oxidative stress in cyanobacteria. FEMS Microbiol Rev 33:258–278
Liu Y-M, Chen W, Li D-H, Shen Y-W, Li G-B, Liu Y-D (2006) First report of aphantoxins in China – waterblooms of toxigenic Aphanizomenon flos-aquae in Lake Dianchi. Ecotoxic Environ Safety 65:84–92
Mohamed E-H-O, EI-Naggar A-H, EI-Sheekh M-M, EI-Mazally E-E (2004) Differential effects of Co2+ and Ni2+ on protein metabolism in Scenedesmus obliquus and Nitzschia perminuta. Environ Toxicol Pharmacol 16:169–178
Nakai S, Inoue Y, Hosomi M, Murakami A (2000) Myriophyllum spocatum-released allelopathic polyphenols inhibiting growth of blue-green algae Microcystis aeruginosa. Water Res 34:3026–3032
Ni L, Jie X, Wang P, Li S, Wang G, Li Y, Acharya K (2015) Effect of linoleic acid sustained-release microspheres on Microcystis aeruginosa antioxidant enzymes activity and microcystins production and release. Chemosphere 121:110–116
Pajdak-Stos A, Fialkowska E, Fyda J (2001) Phormidium autumnale (Cyanobacteria) defense against three ciliate grazer species. Aquat Microb Ecol 23:237–244
Park W-H, Han Y-H, Kim S-H, Kim S-Z (2007) Pyrogallol, Ros generator inhibits As4.1 juxtaglomerular cells via cell cycle arrest of G2 phase and apoptosis. Toxicology 235:130–139
Prado R, Garcia Rioboo C, Herrero C, Abalde J, Cid A (2009) Comparision of the sensitivity of different toxicity test endpoints in a microalga exposed to the herbicide paraquat. Environ Int 35:240–247
Rao M-V, Paliyath G, Ormrod D-P (1996) Ultraviolet-B and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol 110:125–136
Shao J-H, Wu X-Q, Li R-H (2009) Physiological responses of Microcystis aeruginosa PCC7806 to nonanoic acid stress. Environ Toxicol 24:610–617
Shi X, Yang L, Niu X, Xiao L, Kong Z, Qin B, Gao G (2003) Intracellular phosphorus metabolism of Microcystis aeruginosa under various redox potential in darkness. Microbiol Res 158:345–352
Shi S, Tang D, Liu Y (2009) Effects of an algicidal bacterium Pseudomonas mendocina on the growth and antioxidant system of Aphanizomenon flos-aquae. Curr Microbiol 59:107–112
Shiu C-T, Lee T-M (2005) Ultraviolet-B-induced oxidative stress and responses of the ascorbate–glutathione cycle in a marine macroalga Ulva fasciata. J Exp Bot 56:2851–2865
Staats N, De Winder B, Stal L-J, Mur L-R (1999) Isolation and characterization of extracellular polysaccharides from the epipelic diatoms Cylindrotheca closterium and Navicula salinarum. Eur J Phycol 34:161–169
State Environmental Protection Administration (SEPA) of China (2002) Monitor and analysis method of water and wastewater. Chinese Environmental Science Publication House, Beijing
Yi Y-L, Lei Y, Yin Y-B, Zhang H-Y, Wang G-X (2012) The antialgal activity of 40 medicinal plants against Microcystis aeruginosa. J Appl Phycol 24:847–856
Zhang T-T, Wang L-L, He Z-X, Zhang D (2011) Growth inhibition and biochemical changes of cyanobacteria induced by emergent macrophyte Thalia dealbata roots. Biochem Syst Ecol 39:88–95
Zhang T-T, He M, Wu A-P, Nie L-W (2012) Inhibitory effects and mechanisms of Hydrilla verticillata (Linn.f.) royle extracts on freshwater algae. Bull Environ Contam Toxicol 88:477–481
Zhang S-H, Chang J-J, Cao J-Y, Yang C-L (2015a) Comparative studies on growth and physiological responses of unicellular and Colonial Microcystis aeruginosa to Acorus calamus. Bull Environ Contam Toxicol 94:225–231
Zhang S-H, Guo L, Cao J-Y, Chang J-J (2015b) Allelopathic activities of three emergent macrophytes on several monospecific cyanobacterial species and natural phytoplankton assemblages. Pol J Environ Stud 24:397–402
Zhang S-H, Zhang S-Y, Li G (2016) Acorus calamus root extracts to control harmful cyanobacteria blooms. Ecol Eng 94:95–101
Acknowledgments
This work was supported by grants from the National Science Fund of China (51468066); the Science and Technology Program of Yunnan Province, China (2014FB105); and the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (2013BAB06B03).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, SH., Xu, PY. & Chang, JJ. Physiological Responses of Aphanizomenon flos-aquae Under the Stress of Sagittaria sagittifolia Extract. Bull Environ Contam Toxicol 97, 870–875 (2016). https://doi.org/10.1007/s00128-016-1948-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-016-1948-7