Abstract
Many constructed wetland systems are facing the problem of low dissolved oxygen (DO) and reduced nitrogen removal efficiency. In this study, an experimental constructed wetland system is designed and used to investigate the effect of biochar (rice husk biochar (RHB), coconut shell biochar (CSB), and wood biochar (WB) and earthworm on DO concentration, nitrogen transformation, and ammonia nitrogen removal. Specifically, effects of different biochar and earthworm on NH4+-N in wastewater, N content of Phragmites australis, NH4+-N and NO3--N content in substrates, microbial nitrification and denitrification potentials, and the DO concentration were investigated. Results show that the addition of biochar and earthworm increased the removal efficiency of NH4+-N from wastewater. The addition of RHB and WB significantly increased the concentration of DO by 21.4% and 25.7% (P < 0.05) respectively in the constructed wetland. The addition of earthworm significantly increased the DO concentration in the constructed wetland system by an average of 30.35% (P < 0.05).The N content of P. australis increased when biochar and earthworm were introduced into the constructed wetland system, with higher relative N content observed in the above-ground biomass. NO3−-N content increased, but NH4+-N decreased in the substrate. Addition of both biochar and earthworm increased nitrification and denitrification potentials. However, no significant increase in denitrification potential was observed when only biochar was added. The removal efficiency of NH4+-N from wastewater is significantly positively correlated with the DO, nitrification, and denitrification potential, and nitrogen content of above-ground part of P. australis (P < 0.05). Results suggest that the DO concentration in constructed wetland systems could be improved by the addition of biochar and earthworm. These findings imply that both biochar and earthworm could be added into constructed wetlands to solve the low DO concentration and improve the removal efficiency of nitrogen.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ammonia nitrogen, including free ammonia (NH3) and ammonium ions (NH4+), is mainly derived from nitrogenous organic matter in domestic sewage, such as fertilizer, excrement of aquatic animals, and decomposition of animal carcasses. Ammonia can be present in water as ionized ammonia nitrogen (NH4+-N) and unionized ammonia nitrogen (NH3 -N), with the ionized form being most common. The NH3 can react with water to form a weak base and the reaction is reversible (Goldshleger et al. 2018). The ratio of NH3-N to NH4+-N is normally in equilibrium depending on water pH and temperature. Aquatic organisms may be at risk of poisoning from ammonia (Egnew et al. 2019; Lu et al. 2019). Zhou and Claude (2015) showed that biological membranes are more permeable to NH3 than to NH4+, and Cheng et al. (2019) demonstrated that NH3-N was more toxic to aquatic organisms, especially for benthic crustaceans and mollusks due to its diffusion ability across the gill membranes. However, Liew et al. (2013) reported that NH4+ has some degree of toxicity because the high NH4+ concentration in the water interferes with the outward movement of ammonia through the gills. Wang et al. (2020) showed that annual emissions of ammonia in China are approximately 2.3 million tons. Therefore, removal of NH4+-N from wastewater is potentially very important to protect human health.
The main methods for removing NH4+-N from wastewater include adsorption, chemical precipitation, and biological action. The adsorption method has been widely used in the treatment of wastewater containing NH4+-N because it has many advantages, such as simple operation, no secondary pollution, and the potential to regenerate the adsorbent (Xu et al. 2019). However, the high cost of adsorbent regeneration and usually low adsorption capacity are disadvantages. NH4+-N can also be removed from wastewater by chemical precipitation. For example, the MgNH4PO4·6H2O (MAP) precipitate occurs when adding Mg2+ and PO43− into the wastewater with NH4+-N, which is a compound fertilizer and has good economic benefits (Ueno and Fujii 2001). However, chemical precipitation requires large amounts of chemical agents, resulting in increased treatment cost. NH4+-N can also be removed from wastewater by nitrification-denitrification by microorganisms. This method has many advantages, such as low investment cost and no significant secondary pollution. However, the purifying effectiveness is significantly influenced by temperature and certain substances (pH and toxic metals, etc.) in wastewater.
Constructed wetland systems (CWs) consisting of substrate, aquatic plants, and microorganisms, have an efficient pollutant purification capacity by synergy of physical, chemical, and biological interactions (Ilyas and Masih 2017). Biochar refers to a fine-grained, porous carbon material formed by high-temperature pyrolysis of carbon-rich biomass under anaerobic conditions. Biochar can possess strong adsorption and anti-biodegradation properties (Manyà et al. 2018). At present, biochar has been used in constructed wetlands to increase purification capacity. Kuno et al. (2018) found that addition of wood biochar into CWs increased the removal of total nitrogen (TN) by 20.0%. Zhou et al. (2017) demonstrated that addition of bamboo biochar into intermittently aerated vertical flow CWs improved removal of NH4+-N and TN by 99% and 53%, respectively. de Rozari et al. (2018) found an 81% reduction in ammonia following the addition of biochar to a CWs. This improvement was attributed to the following main factors: (1) Improved nitrification by biochar. Nitrification is the first step in the nitrogen transformation process in NH4+-N for nitrogen removal of wastewater, and the high DO concentration can promote nitrifying bacteria for nitrification (Lu et al. 2016). Compared to the traditional media, biochar has an extensive surface area for the adsorption of pollutants, microorganism growth, and development to improve the pollutant degradation (Feng et al. 2020). (2) Increasing adsorption NH4+-N by biochar due to its composition of coarse and heterogeneous surfaces, shallow channels (Kasak et al. 2018), and its porosity (Li et al. 2018). (3) Enhanced plant uptake NH4+-N by biochar. Uptake of NH4+-N by Lythrum Salicaria improved growth and the removal of NH4+-N provided improved conditions (in terms of oxygen secretion and root surface) for nitrification (Kasak et al. 2018). Therefore, the much larger specific surface area, total pore volume, and micropore volume of biochar were favorable for NH4+-N adsorption for subsequent nitrification and uptake by plant.
Earthworm is a common terrestrial annelid in soil and plays a key role in the ecosystem as decomposers. Previous research shows that adding earthworm into CWs effectively improves the clogging problem of wetland systems and increases the purification efficiency (Davison et al. 2005). Li et al. (2011) showed that the quantity of solid particles accumulated in the substrate was reduced by 50%, when earthworm was added into a subsurface flow CWs. The addition of earthworm can also affect the growth of wetland plants, which means more N could be absorbed and continuously removed by harvesting of mature plants. Xu et al. (2013a, b) found that the absorption of N in the above-ground part of P. australis, Typha, and Canna in CW increased by 185%, 216%, and 108%, respectively. Therefore, the removal of N improved with the addition of earthworms into CWs.
In general, NH4+-N removal efficiency is mainly related to nitrification in aerobic systems, and the improvement of dissolved oxygen (DO) content, e.g., through mechanical aeration, is conducive to the enhancement of removal of NH4+-N by nitrification. DO content is therefore a significant factor for effective NH4+-N removal in CWs. In CWs, substrate layers also play a vital role in the elimination of pollutants from wastewater. Substrates such as biochar, earthworms, gravel, activated carbon, and zeolite have been used in CWs (Abedi and Mojiri 2019; Xu et al. 2013a, 2013b). Previous studies have, however, tended not to look at the combined impact of biochar and earthworms in CWs, and there is a little information on the effect of DO content on removal of NH4+-N in CWs where both biochar and earthworm have been added. Therefore, the objectives of this study were to (1) analyze changes in DO content in CWs with biochar and earthworms; (2) determine the response of removal efficiency of NH4+-N to CWs with biochar and earthworms; (3) assess NH4+-N, NO3−-N content, and nitrification and denitrification potentials in CWs with biochar and earthworms.
Materials and methods
Materials
Biochar, including rice husk biochar (RHB), coconut shell biochar (CSB), and wood biochar (WB), were obtained from a market. Sand, with diameter less than 3 mm, was obtained from a local building supply company. Phragmites australis was chosen as the test plant and the seedlings were selected from the pond of Nanjing University of Information Science and Technology. Earthworms (Eisenia fetida) were purchased from a local farming market and E. fetida was chosen as the test earthworm because it has proven to be effective in vermifiltration (Taylor et al. 2003). E. fetida with an average weight of 0.2–0.3 g were selected.
Design of horizontal subsurface flow constructed wetland
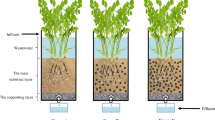
An experimental CWs consisting of two plastic basins was designed (Fig. 1). All CWs were placed outdoors under a transparent shelter at Agriculture and Meteorology Experimental Station, Nanjing University of Information Sciences and Technology, Jiangsu Province, China (32.160 N, 118.860 E). Four holes in the bottom of the inner basin (each 1 cm in diameter) allowed free movement of water between the basins. Within the inner basin, a plastic tube with holes (dissolved oxygen tube) was placed inside a rhizobag made from nylon mesh (< 30 μm) to allow exchange of water and air. The dissolved oxygen tube could effectively detect the DO content in substrate of CWs. Eight versions of the system were constructed using different combinations of substrate, biochar, and earthworm, as follows (Table 1). In each version of the system, 1 L and 7 L of the tested substrate were added to the inside and outside of the rhizobag, respectively. Two seedlings of P. australis were planted in the rhizobag after 1–2 weeks of laboratory cultivation. E. foetida with an average weight of 0.2–0.3 g were selected and added to the inner basin of the relevant systems (0.75 g/L) after 3–5 days of training in laboratory. The inner basin was then placed in the outer basin containing synthetic wastewater. The quality index of the synthetic wastewater is shown in Table 2 and is similar to the pollutant concentration of domestic wastewater in southeast China.
The synthetic wastewater was added during the operation to ensure the normal growth of the P. australis, and 8 L of wastewater was added into outer basin in May 2017. Water was sampled from the CWs after a 7-day hydraulic retention time (HRT) to analyze NH4+-N content as well as dissolved oxygen (DO) content on October 20, 2017. The DO content of CWs was measured by dissolved oxygen analyzer (JPB-607A, INESA, China).
The rhizobag was taken from CWs on November 5, 2017. The substrate and P. australis were taken from root bags, respectively. Some substrate samples were used to measure the exchanged NO3− and NH4+. Other substrate samples were prepared to determine nitrification and denitrification potentials. P. australis were washed thoroughly with tap water and cleaned with deionized water. Fresh P. australis was dried at 60 °w in an oven for 48 h to a constant weight. The roots (under-ground), stems and leaves (above-ground) samples were sub-sampled and ground to powder for the determination of the N concentrations.
Analytical methods
Measure removal efficiency of NH4+-N in wastewater
The NH4+-N concentration in wastewater was analyzed according to standard methods (APHA 1992), and its removal efficiency was then calculated by the following equation:
where R is removal efficiency (%), Iv is initial volume (L), Ic is initial concentration (mg/L), Ev is remaining volume (L), Ec is remaining concentration (M).
Measure N content of plants
Subsamples of 0.2 g were digested with H2SO4-H2O2 at 260 °C. The N concentration in the digest was then determined by the Kjeldahl method (Lu 1999).
Measure NH4+-N and NO3−-N content in substrates
The concentration of NH4+-N and NO3−-N in substrates was determined after mixing 10 g freshly sampled substrate with 50 mL 2 M KCl solution and allowing it to equilibrate overnight (Robertson et al. 1999). The supernatant was filtered and then analyzed for NH4+-N and NO3−-N contents. NH4+-N was determined at a wavelength of 697 nm using spectrophotometer according to the salicylic acid method (Ministry of Environmental Protection 2006). NO3−-N contents were determined at a wavelength of 220 and 275 nm in ultraviolet spectrophotometer (UV-752N, YOKE, China).
Determine nitrification and denitrification potentials in substrates
Nitrification potentials were carried out as described by Hart et al. (1994) with slight modifications. Ten grams of substrate were put in 500-mL Erlenmeyer flasks containing 100 mL of pH 7.2 of incubation solution (in M) (volume ratio was 3:7:30 for 0.2 KH2PO4, 0.2 K2HPO4, and 0.05 (NH4)2SO4). Erlenmeyer flasks were plugged by absorbent cotton and were rotary shaken at 25 °. (140 rpm). The concentration of NO3−-N was measured after 48 h incubation. Nitrification potentials were analyzed using the following equation:
where N is nitrification potential (mg/(kg h)), C2 and C1 were NO3−-N concentration after 48-h incubation solution and initial concentration of substrate (mg/L). V1 and V2 represent the volume of incubation solution and the volume of water in substrate (L). T is incubation time. M is dry weight of substrate (g).
Denitrification potentials were carried out as described by Mathew et al. (1982). Briefly, 10 g of substrate was put in 500-mL Erlenmeyer flasks containing 100 mL of pH 7.2 of incubation solution (in M) (volume ratio was 3:7:30:10 for 0.2 KH2PO4, 0.2 K2HPO4, 0.03 KNO3, and 0.02 glucose). The C/N ratio of incubation solution was 3:1. Erlenmeyer flasks were plugged by rubber and incubated at 20 °r. The denitrification potential was analyzed according to the change in nitrate concentration. Denitrification potentials use a similar approach as Eq. (2).
Statistical analysis
Data was processed with Microsoft Office Excel 2016, OriginLab 2018, and SPSS 22.0 software. The effect of different treatments was tested by analysis of one-way ANOVA, and comparisons were made using mean ± standard deviation (LSD’s multiple range). Significance values are for P ≤ 0.05 unless stated otherwise. The correlation between removal efficiency of NH4+-N, NH4+-N, NO3−-N, microbial nitrification and denitrification potential in substrate, nitrogen content of above-ground and under-ground parts of plants, and DO content were also analyzed in this study.
Results and discussion
Effect of biochar and earthworm on DO content in CWs
Content of DO in different CWs ranged from 3.5 to 5.9 mg/L (Fig. 2). Compared to the system with only sand as substrate, the addition of RHB and WB significantly increased the concentration of DO by 21.4% and 25.7% (P < 0.05), respectively. The content of DO significantly increased by 31.4%, 30.6%, 25.3%, and 34.1% (P < 0.05), respectively with the addition of earthworm into the four systems containing only sand, sand and RHB, sand and CSB, and sand and WB. Therefore, the addition of both biochar and earthworm may significantly improve the DO content of CWs. Some DO in CWs come from atmosphere by atmospheric diffusion. Kizito et al. (2017) demonstrated that there is higher total pore volume and micropore volume for biochar than ceramsite. Therefore, the increasing DO content in CWs could be related to higher pore volume of biochar than those of sand. In addition, porosity of CWs increased by earthworm excavation resulted in increasing DO content.
The concentration of dissolved oxygen (DO) in different constructed wetlands. S, S + RHB, S + CSB, and S + WB represent constructed wetlands with different substrates of sand, sand with rice husk biochar, sand with coconut shell biochar, sand with wood biochar, respectively. S + E, S + E + RHB, S + E + CSB, and S + E + WB represent constructed wetlands with earthworms added and substrates of sand, sand with rice husk biochar, sand with coconut shell biochar, sand with wood biochar, respectively. Different lowercase letters indicate the difference with significance value for P < 0.05. Data are means ± SD (n = 3)
Effect of biochar and earthworm on nitrogen content of plant
The effect of different biochars on N content in above-ground part of P. australis varied (Fig. 3a). The addition of RHB and WB significantly increased N content by 54.3% and 69.4% (P < 0.05), respectively. Compared with the CWs with only sand (S), the addition of different biochars (RHB, CSB, and WB) increased the N content in the under-ground part of P. australis by 9.0%, 22.1%, and 22.9% (Fig. 3b), respectively. Addition of earthworms into CWs with only sand as substrate increased the N content of above-ground part of P. australis by 28.6%. This result was similar to those of Xu et al. (2013a, b), who demonstrated that addition of earthworms into CWs increased the N content of plant. However, the N content in above-ground part of P. australis was increased on average by 7.56% when earthworms were added to CWs with biochar. Mahtab et al. (2012) showed that content of nitrogen of biochar is 1.14–1.91%, but N is not usually found in sand. Therefore, the response of increased concentration of N in above-ground part of P. australis to addition of earthworm was higher in CWs with only sand as substrate than with sand and biochar. Similarly, the increased N concentration in the under-ground part of P. australis was lower when earthworms were added into CWs with only sand as substrate or with sand and biochar. Results showed that the effect of biochar and earthworm on N content of P. australis differs.
Nitrogen content in above-ground (a) and under-ground (b) part of P. australis. S, S + RHB, S + CSB, and S + WB represent constructed wetlands with only sand as substrate, sand and rice husk biochar, sand and coconut shell biochar, and sand and wood biochar, respectively. S + E, S + E + RHB, S + E + CSB, and S + E + WB represent constructed wetlands with sand and earthworms, with sand, earthworm and rice husk biochar, with sand, earthworm and coconut shell biochar, and with sand, earthworm, and wood biochar, respectively. Different letters indicate the difference with significance value for P < 0.05. Data are means ± SD (n = 3)
Addition of biochar into CWs obviously increased the N content of P. australis. Chan et al. (2007) showed that biochar could improve soil properties, such as porosity. Blackwell et al. (2010) observed that biochar could increase the parasitism of mycorrhizal fungi, thus enhancing the absorption of nutrients. In addition, Kolton et al. (2011) reported that the addition of biochar could effectively alter the population structure of soil microbes, which contributes to plant growth. Therefore, the increasing N content of P. australis could be related to improving substrate properties, or microorganism growth after addition of biochar into CWs. The N content of P. australis was not obviously increased by the addition of earthworm into CWs with biochar.
It can be seen from Fig. 3, that the addition of biochar into the constructed wetland systems increased the N content of P. australis, especially in the above-ground part. Zhu et al. (2019) demonstrated that addition of biochar into soil will increase migration of NO3−-N from roots to shoots. Therefore, the higher level of N in the above-ground part compared to the under-ground part of P. australis can be attributed to greater migration of NO3−-N from roots to shoots due to adding biochar to the constructed wetland system. These results therefore suggest that the harvesting of plants could be an effective and sustainable way of removing N from wastewater in constructed wetlands due to the higher N content in above-ground than under-ground part of P. australis.
Effect of biochar and earthworm on nitrification potentials in substrates
Compared to the CWs with only sand as substrate, the nitrification potential significantly increased by 45.5% and 226.2% (P < 0.05), respectively for CWs with addition of RHB and WB (Fig. 4). Zhou et al. (2018) found that adding wheat straw biochar into metal-contaminated farmland significantly increased the nitrification potential. Ball et al. (2010) demonstrated that the content of biochar was significantly positively correlated with the number of ammoniated bacteria in soil. Deluca et al. (2006) reported that biochar could adsorb some nitrification inhibitors in soil, thereby alleviating the inhibition of nitrification and promoting the nitrification process of microorganisms. In this study, it can be concluded that biochar can enhance the nitrification potential of substrate in CWs.
Nitrification potentials in substrates. S, S + RHB, S + CSB, and S + WB represent constructed wetlands with different substrates of sand, sand with rice husk biochar, sand with coconut shell biochar, and sand with wood biochar, respectively. S + E, S + E + RHB, S + E + CSB, and S + E + WB represent constructed wetlands with earthworms added and substrates of sand, sand with rice husk biochar, sand with coconut shell biochar, and sand with wood biochar, respectively. Different lowercase letters indicate the difference with significance value for P < 0.05. Data are means ± SD (n = 3)
The nitrification potentials increased by 62.4%, 151.9%, 61.8%, and 26.4%, respectively for addition of earthworm into the CWs with only sand as substrate, sand and RHB, sand and CSB, and sand and WB. These results are similar to those of Xu et al. (2013a, b), who demonstrated that nitrification increased with the addition of earthworm into CWs. Increased aeration and consequently DO concentration from the addition of both biochar and earthworm into CWs resulted in higher rates of nitrification (Fig. 2).
Effect of biochar and earthworm on denitrification potentials in substrates
Denitrification potentials in substrate of different CWs range from 4.38 to 19.41 mg/(kg h) (Fig. 5). The denitrification potential significantly increased by 184.7%, 127.2%, and 270.6%, respectively with the addition of RHB, CSB, and WB into CWs (P < 0.05). The denitrification process mainly refers to the biochemical process in which bacteria in soil convert nitrate (NO3−) into nitrogen (N2) through a series of intermediate products (NO2, NO, N2O). The porous structure of biochar can provide a suitable habitat for the growth and reproduction of denitrifying bacteria (Shamim and Whalen 2016), thereby enhancing the denitrification process. Results suggest that biochar can significantly affect denitrification potential in substrates in CWs. The denitrification potentials increased by 23.2%, 28.6%, 28.1%, and 19.7%, respectively for addition of earthworms into CWs with only sand as substrate, sand and RHB, sand and CSB, and sand and WB. However, in this study, addition of earthworm did not significantly increase denitrification potential.
Denitrification potentials in substrate. S, S + RHB, S + CSB, and S + WB represent constructed wetlands with different substrates of sand, sand with rice husk biochar, sand with coconut shell biochar, and sand with wood biochar, respectively. S + E, S + E + RHB, S + E + CSB, and S + E + WB represent constructed wetlands with earthworms added and substrates of sand, sand with rice husk biochar, sand with coconut shell biochar, and sand with wood biochar, respectively. Different lowercase letters indicate the difference with significance value for P < 0.05. Data are means ± SD (n = 3)
Effect of biochar and earthworm on ammonia and nitrate content in substrate
Figure 6 a shows that the NH4+-N content in substrate of different CWs ranges from 0.36 to 0.48 mg/kg. Addition of RHB and WB into the CWs significantly decreased NH4+-N content in substrate (P < 0.05). The content of NH4+-N in substrate was decreased on average by 8.9% with the addition of earthworm into CWs. Wu et al. (2015) reported that NH4+-N in CWs is mainly influenced by DO content, and NH4+-N can be easily converted to other forms of nitrogen through nitrification and oxidation reactions where sufficient DO is present. Therefore, the addition of biochar and earthworm into the CWs decreased the NH4+-N content, which could be related to increasing DO content (Fig. 2).
The content of NH4+-N (a) and NO3−-N (b) in substrate. S, S + RHB, S + CSB, and S + WB represent constructed wetlands with different substrates of sand, sand with rice husk biochar, sand with coconut shell biochar, and sand with wood biochar, respectively. S + E, S + E + RHB, S + E + CSB, and S + E + WB represent constructed wetlands with earthworms added and substrates of sand, sand with rice husk biochar, sand with coconut shell biochar, and sand with wood biochar, respectively. Different lowercase letters indicate the difference with significance value for P < 0.05. Data are means ± SD (n = 3)
According to Fig. 6b, the addition of WB into the CWs significantly increased NO3−-N content (P < 0.05). Knowles et al. (2011) demonstrated that biochar can reduce the leaching of NO3−-N in soil. Mizuta et al. (2004) also found that biochar can reduce NO3−-N leaching by direct adsorption. Therefore, the increasing NO3−-N content after the addition of biochar into the CWs could be related to NO3−-N adsorption by biochar.
The addition of earthworm significantly increased NO3−-N content by 54.9% and 38.4%, respectively for CWs with RHB and WB (P < 0.05). Earthworm activity enhanced DO content in the substrate (Fig. 2), which helps to stimulate the process of nitrification, and thereby NH4+-N can be rapidly oxidized and converted to NO3−-N under aerobic conditions. As shown in Fig. 6, the addition both earthworm and biochar into CWs decreased NH4+-N content, but increased NO3−-N content. This is similar to the findings of Fu et al. (2019), who found that with increasing biochar application rate into soil, the content of NH4+-N decreased, but the content of NO3−-N increased.
Effect of biochar and earthworm on removal efficiency of ammonia nitrogen
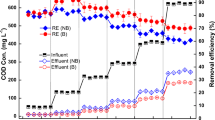
The addition of RHB, CSB, and WB into CWs significantly increased the removal efficiency of NH4+-N by 8.4%, 6.2%, and 9.6% (P < 0.05) (Fig. 7), respectively. These results show that the addition of biochar can significantly improve the removal efficiency of NH4+-N. The observed increase in removal of NH4+-N associated with the addition of biochar could be attributed to (1) biochar improving the physical and chemical properties of substrates, thus promoting the growth of plants, which is beneficial to improving N uptake by P. australis (Fig. 3), (2) biochar producing a carbon resource and habitat for microorganisms, thus enhancing nitrification and denitrification potentials (Figs. 4 and 5). As shown in Fig. 7, the addition of earthworm into CWs increased removal efficiency of NH4+-N by 5.3% on average. The improved removal efficiency of NH4+-N was related to increasing DO content caused by earthworm action in the CWs.
The removal efficiency of NH4+-N in wastewater. S, S + RHB, S + CSB, and S + WB represent constructed wetlands with different substrates of sand, sand with rice husk biochar, sand with coconut shell biochar, and sand with wood biochar, respectively. S + E, S + E + RHB, S + E + CSB, and S + E + WB represent constructed wetlands with earthworms added and substrates of sand, sand with rice husk biochar, sand with coconut shell biochar, and sand with wood biochar, respectively. Different lowercase letters indicate the difference with significance value for P < 0.05. Data are means ± SD (n = 3)
Response of removal efficiency of NH4+-N to DO, nitrification, and denitrification potential
The correlation coefficients between the removal efficiency of NH4+-N and DO in wastewater, NH4+-N, NO3−-N, nitrification and denitrification potential in substrate, and nitrogen content of uptake by P. australis are shown in Table 3. The removal efficiency of NH4+-N in wastewater is significantly negatively correlated with the content of NH4+-N in substrate (P < 0.01). However, it is significantly positively correlated with the DO content, nitrification and denitrification potential, and nitrogen content of above-ground part of P. australis (P < 0.05). Maltais-Landry et al. (2009) reported that artificial aeration supplied oxygen to the wetland substrate, stimulating nitrification and resulting in a higher removal of NH4+-N. Kasak et al. (2018) showed that the growth of Lythrum Salicaria was improved by biochar, and NH4+ removal benefited from plant uptake and the provision of improved conditions (in terms of oxygen secretion and root surface) for nitrification. Therefore, the addition of biochar and earthworm into constructed wetland systems increased the removal efficiency of NH4+-N, which can be attributed to higher DO, nitrification and denitrification potential, and uptake N by P. australis.
Conclusions
The addition of biochar and earthworms into CWs increased DO content, nitrification and denitrification potentials in substrates, and nitrogen accumulation in above-ground of P. australis. The addition of biochar and earthworm into CWs increased the removal efficiency of NH4+-N, and this was related to enhanced DO, nitrification and denitrification potentials, and N uptake by plant. The addition of biochar and earthworm could therefore be an effective option to improve removal of NH4+-N in CWs.
References
APHA (1992) Standard methods for the examination of water and wastewater, eighteenth edn. American Public Health Association, Washington, DC
Ball P, MacKenzie MD, Deluca TH, Holben WE (2010) Wildfire and biochar enhance nitrification and ammonium-oxidizing bacterial abundance in dry montane forest soils. Environ Qua l39(4):1243–1253
Blackwell P, Krull E, Bulter G, Herbert A, Solaiman Z (2010) Effect of banded biochar on dry land wheat production and fertiliser use in southwestern Australia: an agronomic and economic perspective. Aust J Soil Res 48(7):531–545
Chan KY, Van ZL, Meszaros I, Downie A, Joseph SD (2007) Agronomic values of green waste biochar as a soil amendment. Aust J Soil Res 45:629–634
Cheng CH, Ma HL, Su YL, Deng YQ, Feng J, Xie JW, Chen XL, Guo ZX (2019) Ammonia toxicity in the mud crab (Scylla paramamosain): the mechanistic insight from physiology to transcriptome analysis. Ecotoxicol Environ Saf 179:9–16
Davison L, Headley TR, Pratt K (2005) Aspects of design, structure and performance and operation of reed beds-eight years’ experience in northeastern New South Wales, Australia. Water Sci Technol 51(10):129–138
de Rozari P, Greenway M, El Hanandeh A (2018) Nitrogen removal from sewage and septage in constructed wetland mesocosms using sand media amended with biochar. Ecol. Eng. 111:1–10
Deluca TH, MacKenzie MD, Gundale MJ, Holben WE (2006) Wildfire-produced charcoal directly influences nitrogen cycling in ponderosa pine forests. Soil Sci Soc Am J 70(2):448–453
Egnew N, Renukdas N, Ramena Y, Yadav AK, Kelly AM, Lochmann RT, Sinha AK (2019) Physiological insights into largemouth bass (Micropterus salmoides) survival during long-term exposure to high environmental ammonia. Aquat Toxicol 207:72–82
Feng L, Wang R, Jia L, Wu H (2020) Can biochar application improve nitrogen removal in constructed wetlands for treating anaerobically-digested swine wastewater. Chem Eng J DOI 379:122273. https://doi.org/10.1016/j.cej.2019.122273
Fu Q, Yan JW, Li H, Li TX, Hou RJ, Liu D, Ji Y (2019) Effects of biochar amendment on nitrogen mineralization in black soil with different moisture contents under freeze-thaw cycles. Geoderma 353:459–467
Goldshleger N, Grinberg A, Harpaz S, Shulzinger A, Abramovich A (2018) Real-time advanced spectroscopic monitoring of ammonia concentration in water. Agricultural Engineering 83:103-108
Hart SC, Stark JM, Daridson EA, Firestone MK (1994) Nitrogen mineralization, immobilization, and nitrification. In: Weaver RW, Angle JS, Bottomley PS (eds) Method of Soil Analysis, Part 2. Microbiological and Biochemical Properties. SSSA Book Series, no. 5. SSSA, Madison, pp 987–1016
Ilyas H, Masih I (2017) The performance of the intensified constructed wetlands for organic matter and nitrogen removal: a review. J Environ Manag 198:372–383
Kasak K, Truu J, Ostonen I, Sarjas J, Oopkaup K, Paiste P, KoivVainik M, Mander Ü, Truu M (2018) Biochar enhances plant growth and nutrient removal in horizontal subsurface flow constructed wetlands. Sci Total Environ 639:67–74
Kizito S, Lv T, Wu S, Ajmal Z, Luo H, Dong R (2017) Treatment of anaerobic digested effluent in biochar-packed vertical flow constructed wetland columns: role of media and tidal operation. Sci Total Environ 592:197–205
Knowles OA, Robinson BH, Contangelo A, Clucas L (2011) Biochar for the mitigation of nitrate leaching from soil amended with biosolids. Sci Total Environ 409(17):3206–3210
Kolton M, Harel YM, Pasternak Z, Graber ER, Elad Y, Cytryn E (2011) Impact of biochar application to soil on the root-associated bacterial community structure of fully developed greenhouse pepper plants. Appl Environ Microb 77(14):4924–4930
Kuno K, Truu J, Ostonen I, Sarjas J (2018) Biochar enhances plant growth and nutrient removal in horizontal subsurface flow constructed wetlands. Sci Total Environ 639:67–74
Li HZ, Wang S, Ye JF, Xu ZX, Jin W (2011) A practical method for the restoration of clogged rural vertical subsurface flow constructed wetlands for domestic wastewater treatment using earthworm. Water Sci Technol 63(2):283–290
Li J, Fan J, Zhang J, Hu Z, Liang S (2018) Preparation and evaluation of wetland plant-based biochar for nitrogen removal enhancement in surface flow constructed wetlands. Environ Sci Pollut Res 25:13929–13937
Liew HJ, Sinha AK, Nawata CM, Blust R, Wood CM, Boeck G (2013) Differential responses in ammonia excretion, sodium luxes and gill permeability explain different sensitivities to acute high environmental ammonia in three fresh water teleosts. Aquat Toxicol 126:63–76
Lu RK (1999) Agrochemical analyzed method of soil. Agricultural Science and Technology Press of China, Beijing
Lu S, Zhang X, Wang J, Pei L (2016) Impacts of different media on constructed wetlands for rural household sewage treatment. J Clean Prod 127:325–330
Lu X, Luan S, Dai P, Luo K, Chen B, Cao B, Sun L, Yan Y, Kong J (2019) Insights into the molecular basis of immunosuppression and increasing pathogen infection severity of ammonia toxicity by transcriptome analysis in pacific white shrimp Litopenaeus vannamei. Fish Shellfish Immunol 88:528–539
Mahtab A, Sang SL, Dou XM, Dinesh M, Sung JK, Yang JE, Yong SO (2012) Effects of pyrolysis temperature on soybean stover and peanut shell derived biochar properties and TCE adsorption in water. Bioresour Technol 118:536–544
Maltais-Landry G, Maranger R, Brisson J, Chazarenc F (2009) Nitrogen transformations and retention in planted and artificially aerated constructed wetlands. Water Res 43:535–545
Manyà JJ, Azuara M, Manso JA (2018) Biochar production through slow pyrolysis of different biomass materials: seeking the best operating conditions. Biomass Bioenerg 117:115–123
Mathew K, Newman PWG, Ho GE (1982) Groundwater recharge with secondary sewage effluent. Australian Water Resource Council. Technical Paper, No.71, Canberra
Mizuta K, Matsumoto T, Hatate Y, Nishihara K, Nakanishi T (2004) Removal of nitrate–nitrogen from drinking water using bamboo powder biochar. Bioresour Technol l95(3):255–257
Robertson GP, Wedin D, Groffman PM, Blair JM, Holland EA, Nadelhoffer KJ, Harris D (1999) Soil carbon and nitrogen availability: nitrogen mineralization, nitrification, and soil respiration potentials. In: Robertson GP, Coleman DC, Bledsoe CS, Sollins P (eds) Standard soil methods for long-term ecological research. Oxford University Press, New York, pp 258–271
Shamim G, Whalen JK (2016) Biochemical cycling of nitrogen and phosphorus in biochar-amended soils. Soil Biol Biochem 103:1–15
Abedi T, Mojiri A (2019) Constructed wetland modified by biochar/zeolite addition for enhanced wastewater treatment. Environ Technol Inno 16:100472. https://doi.org/10.1016/j.eti.2019.100472
Taylor M, Clarke WP, Greenfield PF (2003) The treatment of domestic wastewater using small-scale vermicompost filter beds. Ecol Eng 21:197–203
Ueno Y, Fujii M (2001) Three years operating experience selling recovered struvite from full scale plants. Environ Technol 22(11):1373–1381
Wang XN, Li J, Chen J, Cui L, Li WW, Gao XY, Liu ZT (2020) Water quality criteria of total ammonia nitrogen (TAN) and un-ionized ammonia (NH3-N) and their ecological risk in the Liao River, China. Chemosphere 243:125328
Wu H, Fan J, Zhang J, Ngo HH, Guo W, Hu Z, Liang S (2015) Decentralized domestic wastewater treatment using intermittently aerated vertical flow constructed wetlands: impact of influent strengths. Bioresour Technol 176:163–168
Xu DF, Cao JM, Li YX, Howard A, Yu KW (2019) Effect of pyrolysis temperature on characteristics of biochars derived from different feedstocks: a case study on ammonium adsorption capacity. Waste Manage 87:652–660
Xu DF, Li YX, Howard A, Guan YD (2013b) Effect of earthworm Eiseniafetida and wetland plants on nitrification and denitrification potentials in vertical flow constructed wetland. Chemosphere 92:201–206
Xu DF, Li YX, Howard A (2013a) Influence of earthworm Eiseniafetida on removal efficiency of N and P in vertical flow constructed wetland. Environm Sci Pollut R 20:5922–5929
Zhou HM, Wang P, Chen D, Shi GL, Cheng K, Bian RJ, Liu XY, Zhang XH, Zheng JF (2018) Short-term biochar manipulation of microbial nitrogen transformation in wheat rhizosphere of a metal contaminated Inceptisol from North China plain. Sci Total Environ 640-641:1287–1296
Zhou L, Claude EB (2015) An assessment of total ammonia nitrogen concentration in Alabama USA ictalurid catfish ponds and the possible risk of ammonia toxicity. Aquaculture 437:263–269
Zhou X, Wang XZ, Zhang H, Wu HM (2017) Enhanced nitrogen removal of low C/N domestic wastewater using a biochar amended aerated vertical flow constructed wetland. Bioresour Technol 241:269–275
Zhu Y, Li H, Wu Y, Yin XA, Zhang GX (2019) Effects of surface-modified biochars and activated carbon on the transformation of soil inorganic nitrogen and growth of maize under chromium stress. Chemosphere 227:124–132
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Non-profit Research Institution of Chinese Academy of Forestry (CAFYBB2017MA027), Six Talent Peaks Project in Jiangsu Province (JNHB-057), QingLan Project (20161507), and Science and technology program of Ministry of Housing and Urban-Rural Development(2019-K-141).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Zhihong Xu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, L., Wang, J., Xu, D. et al. Responses of nitrogen transformation and dissolved oxygen in constructed wetland to biochar and earthworm amendment. Environ Sci Pollut Res 27, 29475–29484 (2020). https://doi.org/10.1007/s11356-020-09274-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09274-2