Abstract
The involvement of the endocannabinoid system in the brain functions is likely the conclusion of its capability to interact with specific neurotransmitters in several brain regions. The present study was designed to examine the role of the glutamatergic system on cannabinoid-induced hyperphagia in chicken. In this survey 10 experiments designed to investigate interaction of cannabinoidergic and glutamatergic systems on feeding behavior in neonatal chickens. In experiment 1, chicken were intracerebroventricular (ICV) injected with saline, 2-AG (2-Arachidonoylglycerol, 5.28 nmol, CB1 receptors agonist), MK-801(NMDA receptor antagonist, 15 nmol) and co-administration of 2-AG + MK-801. In experiment 2, injection of saline, 2-AG (5.28 nmol), CNQX) AMPA/kainate receptor antagonist, 390 nmol) and their combination (2-AG + CNQX) was done. In Experiment 3, injections were saline, 2-AG (5.28 nmol), AIDA)mGluR1 antagonist, 2 nmol) and 2-AG + AIDA. Experiments 4 and 5 were similar to experiment 3, except birds injected with LY341495 (mGLUR2 glutamate antagonist, 150 nmol) and UBP1112 (mGLUR3 glutamate antagonist, 2 nmol) instead of AIDA. Experiments 6–10 followed the procedure similar to experiments 1–5, except chickens received ICV injection of CB65 (CB2 receptor agonist, 3 nmol), instead of 2-AG. Then the cumulative food intake measured until 120 min post injection. According to the results, ICV injection of 2-AG and CB65 significantly increased food intake (P < 0.001). Co-injection of 2-AG and MK-801 significantly amplified hyperphagic effect of CB1 receptors agonist(P < 0.001). Moreover, co-administration of CB65 plus CNQX significantly increased CB65- induced hyperphagia in FD3 neonatal layer-type chickens (P < 0.001). These results suggest there is an interaction between endocannabinoids and glutamatergic systems via NMDA and AMPA receptors in feeding behavior of neonatal layer-type chickens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several factors with complicated neurological mechanisms are responsible for appetite in animals (Levine 2006). Central food intake regulation is modulated by neurochemical mediators known as neurotransmitters in several parts of the brain, e.g. striatum, hypothalamus, amygdala, nucleus tractus solitaries (NTS) and arcuate nucleus (ARC) (Parker et al. 2014). Cannabinoids (CBs) were originally isolated as psychoactive components of marijuana (Δ9-tetrahydrocannabinol; THC). Interestingly, endocannabinoids (ECBs) are produced in the animal and human body where tissues express at least two cannabinoidergic (CBergic) receptors: CB1 and CB2 belong to the G protein coupled receptors (GPCRs) (Kangas et al. 2013). ECBs has a deniable role in numerous physiological processes such as the control of movement, nociception, learning, memory and feeding (Parker et al. 2014; Sharkey et al. 2014).
CB1 receptors are mainly expressed in presynaptic nerve terminals of inhibitory and excitatory nerves in the central nervous system (CNS) in mammalian and birds (Novoseletsky et al., 2011; Sharkey et al. 2014) and controls neurotransmitter release (Pertwee, 2005). CB2 receptors are plentiful in the peripheral nervous system (PNS), immune cells and tissues (Pertwee, 2005) but also expressed in the brain (Onaivi et al. 2012). It is know there are differences on role of neurotransmitters between avian and mammals (Zendehedel and Hassanpour, 2014). For example, microinjection of DAMGO (a μ-opioid receptors agonist) decreased food intake in chicks (Bungo et al. 2005; Alimohammadi et al. 2015) but increased in rat (Kaneko et al. 2012). Furthermore, interestingly comparative physiological studies suggested there are differences on appetite regulation pathways between the meat-type (broiler) and layer-type (hens) chickens (Denbow, 1994; Shiraishi et al. 2011). In this regard CB1 and CB2 receptors have hyperphagic effects in mammals (Pertwee, 2005) and neonatal layer type chickens(Alizadeh et al. 2015; Hassanpour et al. 2015) while food intake increases after ICV injection of CB2 receptor agonists in broiler chicken but CB1 receptors have no effect(Emadi et al. 2011).
Feeding behavior is not regulated using sole neurotransmitter and a complex of neurotransmitters using a wide disturbed network acts to regulate appetite. Glutamate is the most excitatory amino acid in the mammalian and avian brain (Danbolt, 2001). Two major families of glutamate receptors identified: the ionotropic (iGluR) and metabotropic (mGluR) glutamate receptors. The previously is subdivided into N-methyl-D-aspartic acid (NMDA), alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate receptors (Reid and Bliss, 2000), and the latter subdivided into group I, group II and group III receptors (Reid and Bliss, 2000). Although glutamate receptors are widely distributed in the avian brain and scarce information exist on the role of glutamate and GluRs on feeding behavior in the domestic fowl (Zeni, 2000, Baghbanzadeh and Babapour, 2007). Glutamate attenuates food intake in chicken and this effect is probably mediated by both ionotropic and metabotropic receptors (Zeni et al. 2000; Baghbanzadeh and Babapour 2007; Zendehdel et al. 2009). In addition, injection of NMDA and AMPA-kainate receptor antagonists into the ventral striatal and ventral pallidal areas of the pigeon induced feeding behavior (Da Silva et al. 2003).
The ECBs has ability to interact with other neurotransmitters in several brain regions. In the synaptic area, ECBs have a modulatory role than a function as a classic transmitter (Sánchez-Blázquez et al. 2013a; Vicente-Sánchez et al. 2013). For example, presynaptic CB1 receptors directly influence synthesis, release or reuptake of neurotransmitters such as glutamate, nitric oxide, opioid and GABA in the brain (Cota et al. 2003; Hassanpour et al., 2015). Pharmacological, electrophysiological and immunohistochemical studies revealed ECBs acts as retrograde signal molecules in glutamatergic neurons (Irving et al. 2002). Based on the literature, no report was found on the interaction of cannabinoidergic (CBergic) and glutamatergic systems on feeding behavior in neonatal layer-type chickens. So, the present study designed to investigate the possible canabinoid-glutamate interaction on feeding behavior in neonatal layer-type chicks.
Materials and methods
Animals
In this study to determine relation of central glutamatergic and CBergic systems in control of food intake, 440 day-old layer-type (Hy-Line) chickens used (Morghak Co. Iran). Animal handling and experimental procedures performed according to the Guide for the Care and Use of Laboratory animals by the National Institutes of Health (USA) and the current laws of the Iranian government for animal care. Birds at first were kept for 2 days as flocks. Then, randomly distributed into individual cages at a temperature of 30 ± 1 °C with 50 ± 2 % humidity until 5 days of age (Olanrewaju et al. 2006). A mesh diet contains 21 % crude protein and 2850 kcal/kg of metabolizeable energy (Chineh Co. Iran) provided for animals. During the study birds had ad libitum access to food and fresh water. A 3-h prior the intracerebroventricular (ICV) injections, animals were food deprived (FD3) but given free access to water. ICV injections done on day 5 of age.
Experimental drugs
2-AG (2-Arachidonoylglycerol, a selective CB1 receptors agonist), CB65 (a selective CB2 receptors agonist), MK- 801 (NMDA receptor antagonist), CNQX (AMPA/kainate receptor antagonist), AIDA (mGluR1 antagonist), LY341495 (mGluR2 antagonist), UBP1112 (mGluR3 antagonist) and Evans blue purchased from Sigma Co. (Sigma, USA) and Tocris Co. (Tocris, Uk). Drugs, except CB1 receptor agonist (2-AG), dissolved in absolute dimethyl sulphoxide and then diluted with 0.85 % saline containing Evans blue at a ratio of 1:250. 2- AG diluted in saline containing Evans blue.
ICV injection protocol
Before the initiation of the study, chickens weighed and allocated into treatment groups based on their body weight. Therefore, the average weight among the groups was made as uniform as possible. In this study, 10 experiments (each includes 4 treatment groups within 11 replicates in each group; n = 44 birds per experiment) designed to assume the role of central glutamatergic and CBergic systems on food intake in neonatal layer-type chicken. ICV injections were done using a Microsyringe (Hamilton, Switzerland) without anesthesia in accordance to Davis et al. (1979) and Furuse et al. (1997). Briefly in this technique, head of the bird was held with an acrylic device which the bill holder was 45° and calvarium parallel to the surface of the table (Van Tienhoven and Juhasz 1962). A hole was drilled in a plate. This plate was overlaid on the skull immediately over the right lateral ventricle. Then a microsyringe was inserted into the ventricle through the hole and the test solution was injected. Only 4 mm below the skin of the skull was penetrated by the top of the needle. The procedure does not cause physiological stress in neonatal chicks (Saito et al. 2005; Furuse et al. 1999). Each chick received an ICV injection (with vehicle or drug solution) in a volume of 10 μL (Furuse et al. 1999). After injection, the chick was immediately returned to its cage and fresh food and water were supplied. All experimental procedures were done from 8:00 A.M. until 3:30 P.M.
Feeding experiments
Experiment 1 designed to examine the effect of ICV injection of NMDA receptor antagonist on the food intake induced by CB1 receptor agonist in chickens. So, FD3 chickens were injected with 15 nmol MK-801)NMDA receptor antagonist), 5.28 nmol 2-AG (CB1 receptor agonist) and co-administration of MK- 801 + 2-AG.
In experiment 2, FD3 chickens received 390 nmol of CNQX )AMPA/kainate receptor antagonist), 2-AG (5.28 nmol) and combination of CNQX +2-AG. Experiment 3 was designed to examine the effect of ICV injection of 2 nmol AIDA) mGluR1 receptor antagonist), 5.28 nmol 2- AG and AIDA +2-AG on food intake in chickens. Birds in experiment 4, received ICV injection mGluR2 receptor antagonist (LY341495, 150 nmol), 2-AG (5.28 nmol) and LY341495 + 2-AG. In experiment 5, birds injected through ICV with mGluR3 receptor antagonist (UBP1112, 2 nmol), 2-AG (5.28 nmol) and combination of UBP1112 + 2-AG.
In experiment 6, chickens were injected with 15 nmol of MK- 801)NMDA receptor antagonist), 3 nmol of CB65 (CB2 receptor agonist) and combination of MK-801 + CB65. Experiment 7 was carried on with CNQX (390 nmol), CB65 (3 nmol) and combination of CNQX + CB65. In experiment 8, birds injected with AIDA (2 nmol), CB65 (3 nmol) and combination of AIDA + CB65. In experiment 9, chicken received ICV injection of LY341495 (150 nmol), CB65 (3 nmol) and LY341495 + CB65. In experiment 10, chickens were injected with 2 nmol of UBP1112, CB65 (3 nmol) and combination of UBP1112 + CB65. Then chickens were transferred to their individual cages with water and pre-weighed food. Food consumption was measured at 30, 60 and 120 min post injection. Food consumption is expressed as a percent of body weight that body weight impact on the amount of food intake to a minimum. At the end of the experiments, to recognize accuracy of injection, the chicks were sacrificed by decapitation. Only data from individual chicks were used for analysis that were confirmed by the existence of Evans blue color in the lateral ventricle. In each experiment, control groups were injected like treatment groups with 10 μl saline containing Evans blue (n = 11). Each group included at least 11 chicks. Each bird was injected once only. All doses of drugs were calculated based on previous and pilot studies (Zeni et al. 2000; Chen et al. 2006; Baghbanzadeh and Babapour 2007; Irwin et al. 2008; Onaivi et al. 2008; Emadi et al. 2011; Novoseletsky et al. 2011; Seyedali Mortezaei et al. 2013; Alimohammadi et al. 2015; Alizadeh et al. 2015; Hassanpour et al. 2015).
Statistical analysis
Cumulative food intake as percent of body weight was analyzed by repeated measure two-way analysis of variance (ANOVA) using SPSS 16.0 for Windows (SPSS, Inc., Chicago, IL, USA). Data is presented as mean ± SEM. For treatment showing a main effect by ANOVA, means compared by Tukey-Kramer test. P < 0.05 was considered as significant differences between treatments.
Results
Effects and interactions of central CBergic and glutamatergic systems on cumulative food intake in FD3 neonatal layer- type chicks are shown in Figs. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10.
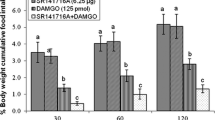
Effect of ICV injection of 2-AG (5.28 nmol), MK- 801(15 nmol) and their combination on cumulative food intake (% BW) in neonatal chickens. 2-AG: CB1 receptors agonist, MK- 801: NMDA receptor antagonist. Data are expressed as mean ± SEM. F and P value for within and between subject factors are as follows: Time, F(2,86) = 78.53, P < 0.001; 2-AG, F(l,43) = 154.21, P < 0.001; MK- 801, F(l,43) = 0.08, P > 0.05; MK- 801 × 2-AG interaction, F(l,43) = 154.29, P < 0.001. Different letters (a, b and c) indicate significant differences between treatments (P < 0.001)
Effect of ICV injection of 2-AG (5.28 nmol), CNQX(390 nmol) and their combination on cumulative food intake (% BW) in neonatal chickens. 2-AG: CB1 receptors agonist, CNQX: AMPA/kainate receptor antagonist. Data are expressed as mean ± SEM. F and P value for within and between subject factors are as follows: Time, F(2,37) = 51.07, P < 0.001; 2-AG, F(l,24) = 74. 98, P < 0.001; CNQX, F(l,24) = 1.07, P > 0.05; CNQX × 2-AG interaction, F(l,24) = 0.16, P > 0.05. Different letters (a and b) indicate significant differences between treatments (P < 0.001)
Effect of ICV injection of 2-AG (5.28 nmol), AIDA (2 nmol) and their combination on cumulative food intake (% BW) in neonatal chickens. 2-AG: CB1 receptors agonist, AIDA: mGluR1 antagonist. Data are expressed as mean ± SEM. F and P value for within and between subject factors are as follows: Time, F(2,24) = 81.25, P < 0.001; 2-AG, F(l,37) = 73.51, P < 0.001; AIDA, F(l,37) = 1.05, P > 0.05; AIDA × 2-AG interaction, F(l,37) = 0.44, P > 0.05. Different letters (a and b) indicate significant differences between treatments (P < 0.001)
Effect of ICV injection of 2-AG (5.28 nmol), LY341495 (150 nmol) and their combination on cumulative food intake (% BW) in neonatal chickens. 2-AG: CB1 receptors agonist, LY341495: mGluR2 antagonist. Data are expressed as mean ± SEM. F and P value for within and between subject factors are as follows: Time, F(2,47) = 64.37, P < 0.001; 2-AG, F(l,46) = 149.31, P < 0.001; LY341495, F(l,46) = 1.29, P > 0.05; LY341495 × 2-AG interaction, F(l,46) = 0.01, P > 0.05. Different letters (a and b) indicate significant differences between treatments (P < 0.001)
Effect of ICV injection of 2-AG (5.28 nmol), UBP1112(2 nmol) and their combination on cumulative food intake (% BW) in neonatal chickens. 2-AG: CB1 receptors agonist, UBP1112: mGluR3 antagonist. Data are expressed as mean ± SEM. F and P value for within and between subject factors are as follows: Time, F(2,56) = 95.14, P < 0.001; 2-AG, F(l,32) = 81.09, P < 0.001; UBP1112, F(l,32) = 1.48, P > 0.05; UBP1112 × 2-AG interaction, F(l,32) = 0.07, P > 0.05. Different letters (a and b) indicate significant differences between treatments (P < 0. 001)
Effect of ICV injection of CB65 (3 nmol), MK-801(15 nmol) and their combination on cumulative food intake (% BW) in neonatal chickens. CB65: CB2 receptors agonist, MK-801: NMDA antagonist. Data are expressed as mean ± SEM. F and P value for within and between subject factors are as follows: Time, F(2,94) = 59.83, P < 0.001; CB65, F(l,43) = 81. 35, P < 0.001; MK-801, F(l,43) = 1.35, P > 0.05; MK-801× CB65 interaction, F(l,43) = 0.05, P > 0.05. Different letters (a and b) indicate significant differences between treatments (P < 0.001)
Effect of ICV injection of CB65 (3 nmol), CNQX (390 nmol) and their combination on cumulative food intake (% BW) in neonatal chickens. CB65: CB2 receptors agonist, CNQX: AMPA antagonist. Data are expressed as mean ± SEM. F and P value for within and between subject factors are as follows: Time, F(2,56) = 41.35, P < 0.001; CB65, F(l,14) = 79. 13, P < 0.001; CNQX, F(l,14) = 0.17, P > 0.05; CNQX × CB65 interaction, F(l,14) = 127.01, P < 0.001. Different letters (a, b and c) indicate significant differences between treatments (P < 0.001)
Effect of ICV injection of CB65 (3 nmol), AIDA (2 nmol) and their combination on cumulative food intake (% BW) in neonatal chickens. CB65: CB2 receptors agonist, AIDA: mGluR1antagonist. Data are expressed as mean ± SEM. F and P value for within and between subject factors are as follows: Time, F(2,61) = 70.12, P < 0.001; CB65, F(l,59) = 40. 91, P < 0.001; AIDA, F(l,59) = 0.34, P > 0.05; AIDA × CB65 interaction, F(l,59) = 0.04, P > 0.05. Different letters (a and b) indicate significant differences between treatments (P < 0.001)
Effect of ICV injection of CB65 (3 nmol), LY341495 (150 nmol) and their combination on cumulative food intake (% BW) in neonatal chickens. CB65: CB2 receptors agonist, LY341495: mGluR2 antagonist. Data are expressed as mean ± SEM. F and P value for within and between subject factors are as follows: Time, F(2,84) = 63.40, P < 0.001; CB65, F(l,39) = 73. 48, P < 0.001; LY341495, F(l,39) = 1.02, P > 0.05; LY341495 × CB65 interaction, F(l,39) = 0.09, P > 0.05. Different letters (a and b) indicate significant differences between treatments (P < 0.001)
Effect of ICV injection of CB65 (3 nmol), UBP1112 (2 nmol) and their combination on cumulative food intake (% BW) in neonatal chickens. CB65: CB2 receptors agonist, UBP1112: mGluR3antagonist. Data are expressed as mean ± SEM. F and P value for within and between subject factors are as follows: Time, F(2,74) = 85.27, P < 0.001; CB65, F(l,56) = 51.45, P < 0.001; UBP1112, F(l,56) = 0.18, P > 0.05; UBP1112 × CB65 interaction, F(l,56) = 0.06, P > 0.05. Different letters (a and b) indicate significant differences between treatments (P < 0.001)
In experiment 1, ICV injection of 2-AG (a selective CB1 receptors agonist, 5.28 nmol) significantly increased food intake compared to control group in chickens [F(l,43) = 154.21, P < 0.001]. ICV injection of 15 nmol MK- 801 (NMDA receptor antagonist) had no significant effect on cumulative food intake (% BW) in comparison with control group [F(l,43) = 0.08, P > 0.05]. Co-administration of 2-AG plus MK-801 significantly amplified hyperphagic effect of CB1 receptors agonist [Time, F(2,86) = 78.53, P < 0.001; MK- 801 × 2-AG interaction, F(l,43) = 154.29, P < 0.001] (Fig. 1).
In experiment 2, there was no significant effect on food intake after ICV injection of 390 nmol CNQX (AMPA/kainate receptor antagonist)[F(l,24) = 1.07, P > 0.05] and co-administration of CNQX plus 2-AG had no effect on 2-AG induced hyperphagia [Time, F(2,37) = 51.07, P < 0.001; 2-AG, F(l,24) = 74. 98, P < 0.001; CNQX × 2-AG interaction, F(l,24) = 0.16, P > 0.05] (Fig. 2).
According to the results of experiment 3, AIDA (mGluR1 antagonist, 2 nmol) had no significant effect on cumulative food intake (% BW) in comparison with control group [F(l,37) = 1.05, P > 0.05]. Also, co-administration of AIDA plus 2-AG was not able to alter the hyperphagic effect of CB1 receptors agonist[Time, F(2,24) = 81.25, P < 0.001; 2-AG, F(l,37) = 73.51, P < 0.001; AIDA × 2-AG interaction, F(l,37) = 0.44, P > 0.05](Fig. 3).
In experiment 4, ICV injection of 2-AG (5.28 nmol) significantly increased the amount of food intake [2-AG, F(l,46) = 149.31, P < 0.001]; but the sub-effective dose of LY341495 (mGluR2 antagonist, 150 nmol) had no effect on food intake [F(l,46) = 1.29, P > 0.05]. Furthermore, co-injection of LY341495 (150 nmol) and 2-AG (5.28 nmol) had no effect on the orexigenic effect of 2-AG [Time, F(2,47) = 64.37, P < 0.001; LY341495 × 2-AG interaction, F(l,46) = 0.01, P > 0.05].
In experiment 5, ICV administration of 2-AG (5.28 nmol) significantly increased cumulative food intake [F(l,32) = 81.09, P < 0.001]; while the sub-effective dose of UBP1112 (mGluR3 antagonist, 2 nmol) had no effect on food intake [F(l,32) = 1.48, P > 0.05]. Furthermore, the orexigenic effect of 2-AG (5.28 nmol) on food intake was not affected by UBP1112 (2 nmol) [Time, F(2,56) = 95.14, P < 0.001; UBP1112 × 2-AG interaction, F(l,32) = 0.07, P > 0.05] (Fig. 5).
The results of experiment 6 showed CB65 (a selective CB2 receptors agonist, 3 nmol), significantly increased cumulative food intake [F(l,43) = 81. 35, P < 0.001]; but the sub-effective dose of MK- 801 (15 nmol) had no effect on feeding [F(l,43) = 1.35, P > 0.05]. In addition, the orexigenic effect of CB65 (3 nmol) did not alter in the co-injection of MK- 801 (15 nmol) and CB65 (3 nmol) [Time, F(2,94) = 59.83, P < 0.001; MK-801× CB65 interaction, F(l,43) = 0.05, P > 0.05] (Fig. 6).
In experiment 7, administration of 390 nmol CNQX (AMPA/kainate receptor antagonist) had no significant effect on cumulative food intake (% BW) in comparison with control group [F(l,14) = 0.17, P > 0.05]; while co-administration of CB65 (3 nmol) plus CNQX significantly increased hyperphagic effect of CB65 in FD3 neonatal layer-type chickens[Time, F(2,56) = 41.35, P < 0.001; CB65, F(l,14) = 79. 13, P < 0.001; CNQX × CB65 interaction, F(l,14) = 127.01, P < 0.001] (Fig. 7).
In experiment 8, injection of 3 nmol CB65 significantly increased the amount of cumulative food intake [F(l,59) = 40. 91, P < 0.001]. Also, administration of AIDA (mGluR1 antagonist, 2 nmol) had no significant effect on food intake in neonatal chick [F(l,59) = 0.34, P > 0.05]. However, co-injection of CB65 plus AIDA had no effect on CB65 induced hyperphagia in layer-type chickens [Time, F(2,61) = 70.12, P < 0.001; AIDA × CB65 interaction, F(l,59) = 0.04, P > 0.05] (Fig. 8).
In experiment 9, administration of LY341495 (mGluR2 antagonist, 150 nmol) had no effect on food intake in neonatal chick [F(l,39) = 1.02, P > 0.05]. Furthermore, co-injection of LY341495 (150 nmol) and CB65 (3 nmol) had no effect on CB65 (3 nmol) induced hyperphagia [Time, F(2,84) = 63.40, P < 0.001; CB65, F(l,39) = 73. 48, P < 0.001; LY341495 × CB65 interaction, F(l,39) = 0.09, P > 0.05] (Fig. 9).
In experiment 10, ICV injection of the effective dose of CB65 (3 nmol) significantly increased cumulative food consumption [F(l,56) = 51.45, P < 0.001]; but UBP1112 (mGluR3 antagonist) at dose of 2 nmol had no effect on food intake [F(l,56) = 0.18, P > 0.05]. Additionally, the hyperphagic effect of CB65 (3 nmol) was not affected by co-injection of UBP1112 and CB65 [Time, F(2,74) = 85.27, P < 0.001; UBP1112 × CB65 interaction, F(l,56) = 0.06, P > 0.05] (Fig. 10).
Discussion
The present survey support physiologically relevant interactions between CBergic and glutamatergic systems on food intake in FD3 neonatal layer-type chickens. Despite psychoactive constituent of Cannabis sativa (marijuana) is known scince past decades, the role of CBergic system is not fully elicited. ECBs produced from arachidonic acid on the cell membranes. Difference exist between ECBs and other neurotransmitters. Almost all neurotransmitters are water-soluble and stored in high concentrations in vesicles. After depolarization, neurotransmitter releases from the presynaptic terminal into the synaptic cleft, binds to its specific receptors on the postsynaptic neuron (Nicoll and Alger 2004). CB1 receptor identified in the both inhibitory and excitatory terminals in central and peripheral neurons and glial cells (D’Addario et al. 2014). As observed in this study food intake increased via both CB1 and CB2 receptors in FD3 layer-type chicks which was similar to mammals (Di Marzo et al. 2001; Chen et al. 2006; López, 2010; Wiley et al. 2012) but dissimilar to broilers which only CB2 receptors affect feeding (Emadi et al. 2011; Novoseletsky et al. 2011). This inconsistency might due to the differences in localization, affinity or expression of CB1 receptors between layer- and broilers (Emadi et al. 2011; Novoseletsky et al. 2011). CB2 receptors act on the immune system and indirectly altered feeding behavior (Emadi et al. 2011). CB2 receptors are primarily expressed in the cells and organs of the immune system but identified in the CNS (Onaivi et al. 2008). Previously, Fowler et al. (2001) reported that a CB2-like protein exists in the CNS of neonatal chicks and disappears in adult chickens (Fowler et al. 2001). The suggested mechanism for hyperphagic effect of CBergic system in mammals is that CB receptors impress their orexigenic effect by blocking POMC neurons and stimulation of NPY neurons in the ARC nucleus (Verty et al. 2004; Lim et al. 2010; D’Addario et al. 2014).
In this study, we used of sub effective dose of antagonists which blocks receptor but without effect on food intake to assay possible interaction between glutamatergic and CBergic systems in food intake of chicken. To our knowledge this paper is the first report on the specific role of glutamatergic receptors on feeding behavior induced by CBergic system in neonatal layer-type chicken. Glutamate, is the main excitatory neurotransmitter in the CNS of mammals and other vertebrates (Lin et al., 2015) and glutamatergic transmission is important for controlling neuronal activity and involved in processes such as plasticity, learning and memory, neural development and appetite (Zeni et al., 2000; Tasca et al., 2004). Glutamate plays an important role in food intake control and manipulation of its vesicular concentration affects feeding behavior in broilers (Baghbanzadeh and Babapour 2007). Several researches reported glutamate attenuates food intake in broiler cockerels and this effect is probably mediated by both ionotropic and metabotropic receptors (Zeni et al. 2000; Baghbanzadeh and Babapour 2007; Zendehdel et al. 2009). Evidence shown NMDA receptors may mediate some aspects of eating and satiety (Duva et al., 2005). Neurons use glutamate as a co-transmitter which acts via AMPA/kainate-mediated excitatory post synaptic potentials (EPSPs) (Ciranna 2006; Liu and Salter 2010). Glutamate probably attenuates food intake via ionotropic and metabotropic receptors in broiler cockerels (Baghbanzadeh and Babapour 2007). Ionotropic glutamate receptor antagonist increased food intake and decreased in latency of birds to start feeding while metabotropic glutamate receptor antagonist, severely reduced food intake and increased the latency to start feeding (Baghbanzadeh and Babapour 2007).
Our results showed MK-801 (NMDA receptor antagonist) significantly amplified hyperphagic effect of 2-AG (CB1 receptors agonist). In this regard, our previous study showed that the glutamate via NMDA receptors dose dependently decreased food consumption in FD3 broiler cockerels (Taati et al. 2011). Also, Da Silva et al.(2003) reported microinjections of NMDA and AMPA-kainite receptor antagonists into ventral striatal and ventral pallidal areas of the pigeon induced feeding behavior (Da Silva et al. 2003). Thus, glutamate maybe impress its hypophagic effect through NMDA and AMPA-kainite receptors in birds. On the basis of our results, hyperphagic effect of CBergic system is maybe mediated via NMDA and AMPA/Kainate receptors. The CBergic system not only modulates neurotransmitters release, but it may also influence the expression and/or release of other feeding related neurotransmitters (Emadi et al. 2011). Previously, it is reported the reduction in corticostriatal synaptic transmission during CB1 receptor activation mediated by a presynaptic decrease in glutamate release (Gerdeman and Lovinger, 2001; Huang et al., 2001). CBs are able to activate a comparatively greater number of G-proteins per occupied receptor in brain. Activation of CB1 receptors inhibits adenylate cyclase, N- and P/Q-type Ca2 + channels, activate mitogen-activated protein kinase and enhance inwardly rectifying K+ channels (Sánchez-Blázquez et al. 2013a). CB1 receptors are present at high density on the presynaptic terminals of glutamatergic synapses and stimulation of CB1 receptors associated with a reduction in glutamate release (Ferna’ndez-Ruiz, 2010).
CBs produce their effects by reducing the pre synaptic release of glutamate or interfering with post-synaptic NMDAR-regulated signaling pathways in several physiological function(Sánchez-Blázque et al. 2013b). CB1 receptor has direct interactions with NMDARs (Hampson et al. 2011) in post synaptic neurons (Liu et al. 2009). However direct mechanism for how CB1 receptor interacts with glutamatergic neurons, suggested activation of CB1 receptor inhibits glutamatergic synaptic transmission through a presynaptic site of action (Sánchez-Blázquez et al. 2013a). Thus, endocannabinoids in the synaptic function appeared more agreeable with a modulatory role rather than with a function as a classic transmitter. CBs acts on presynaptic Cav2.1 (P/Q-type) channels via Ca2+ channel function or secondary by modulation of protein kinase. This might consequently altered voltage-dependent Ca2+ channel phosphorylation (Vicente-Sánchez et al. 2013). Also, activation of CB1 receptor produce long-lasting neurochemical and functional changes in glutamatergic system (Hampson et al. 2011). CB1 and NMDARs colocalize on neuronal bodies and dendritic processes in the nervous system suggesting for possible interconnection. For instance, activation of CB1 receptor protects NMDAR-mediated neurotoxicity and stimulates the removal of excess cytosolic Ca2+ (Liu et al. 2009). Moreover, CB1 receptor blocks endogenous increase in Ca2+ via direct inhibition of NMDAR Ca2+ influx (Liu et al. 2009). Thus, besides interacting with distant signaling pathways, cannabinoids can also directly affect the NMDAR calcium channel.
Our results indicated that CB2-induced hyperphagia is probably mediated via AMPA-kainite receptors. In this regard, Suarez et al. (2008) suggested the decreased expressions of AMPA glutamate receptors induced by developmental THC exposure could lead to functional alterations via inhibit glutamatergic neurotransmission and clearly demonstrate an interaction between CBs and the glutamatergic system. Despite AMPA/kainate receptors not allow to penetrating enough Ca2+ to cells, the large flux of Na+ leads to depolarization of the cell. This phenomenon activates voltage-sensitive Ca2+ channels and facilitate NMDA receptor activation and indirectly lead to accumulation of intracellular Ca2+ levels (Hampson et al. 2011). In vitro studies revealed THC protect neurons from NMDA receptor toxicity which suggests CB neuroprotection might independent of CB receptor activation (Hampson et al. 2011). CB1 couples to NMDA receptor via histidine triad nucleotide-binding protein 1 proteins (HINT1), then CBs stimulate their cointernalization. So, CBs decrease NMDA receptor activity and provides neuroprotection (Sánchez-Blázquez et al. 2013a). The CB regulation of NMDA receptor function is lost in the absence of HINT1 or protein kinase A (Sánchez-Blázquez et al. 2013a).
CBs are abundant at presynaptic sites, they are also present at postsynapses. In this context, CB and NMDA receptor colocalize on neuronal bodies and dendritic processes in certain areas of the nervous system. CB and NMDA receptor co-localize at postsynapses in the brain. CBs reduce the primary Ca2+ influx through activated NMDA receptor. Secondly, CBs stimulates removal of excess cytosolic Ca2+ and decrease cell permability to Ca2+ and in this manner reduce the exocytosis of glutamet from the gulatamatergic neurons. In this scenario, CBs agonists disassemble NMDA receptor through the co-internalization CB1 receptor with NR1 subunit (Sánchez-Blázquez et al. 2013a). Perhaps the role of CBs on gulamatrgic receptors on feeding behavior modulates via this theory, however the accuracy of this idea is still unclear. In fact, interactions exist among CBergic and glutamatergic systems, however, in this study we were not able to find a study to compare the obtained results with it.
To our knowledge we think there is interaction between CBergic and glutamatergic systems on central food intake regulation via CB1 and CB2 recepos with NMDA and AMPA receptors in chicks. The findings of current study can use as basic information and further research required to clarify any direct interaction of cellular and molecular signaling pathways in the interconnection between CBergic and glutamatergic systems on feeding behavior in avian.
References
Alimohammadi S, Zendehdel M, Babapour V (2015) Modulation of opioid-induced feeding behavior by endogenous nitric oxide in neonatal layer-type chicks. Vet Res Commun 39(2):105–113
Alizadeh A, Zendehdel M, Babapour V, Charkhkar S, Hassanpour S (2015) Role of cannabinoidergic system on food intake in neonatal layer-type chicken. Vet Res Commun 39:151–157
Baghbanzadeh A, Babapour V (2007) Glutamate ionotropic and metabotropic receptors affect feed intake in broiler cockerels. J Vet Res 62(4):125–129
Bungo T, Kawamura K, Izumi T, Dodo K, Ueda H (2005) Effects of various lμ-, δ- and κ-opioid ligands on food intake in the meat type chick. Physiol Behav 85:519–523
Chen RZ, Frassetto A, Fong TM (2006) Effects of the CB1 cannabinoid receptor inverse agonist AM251 on food intake and body weight in mice lacking μ-opioid receptors. Brain Res 1108:176–178
Ciranna L (2006) Serotonin as a modulator of glutamate- and GABAmediated neurotransmission: implications in physiological functions and in pathology. Curr Neuropharmacol 4:101–114
Cota D, Marsicano G, Lutz B, Vicennati V, Stalla GK, Pasquali R, Pagotto U (2003) Endogenous cannabinoid system as a modulator of food intake. Int J Obes 27:289–301
D’Addario C, Micioni Di Bonaventura MV, Pucci M, Romano A, Gaetani S, Ciccocioppo R, Cifani C, Maccarrone M (2014) Endocannabinoid signaling and food addiction. Neurosci Biobehav Rev 47:203–224
Da Silva AA, Marino-Neto J, MA P (2003) Feeding induced by microinjections of NMDA and AMPA–kainite receptor antagonists into ventral striatal and ventral pallidal areas of the pigeon. Brain Res 966:76–83
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1–105
Davis JL, Masuoka DT, Gerbrandt LK, Cherkin A (1979) Autoradiographic distribution of L-proline in chicks after intracerebral injection. Physiol Behav 22:693–695
Denbow DM (1994) Peripheral regulation of food intake in poultry. J Nutr 124:1349S–1354S
Di Marzo VGS, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G (2001) Leptin regulated endocannabinoids are involved in maintaining food intake. Nature 410:822–825
Duva MA, Siu A, Stanley BG (2005) The NMDA receptor antagonist MK-801 alters lipoprivic eating elicited by 2-mercaptoacetate. Physiol Behav 83:787–791
Emadi L, Jonaidi H, Hosseini Amir Abad E (2011) The role of Central CB2 cannabinoid receptors on food intake in neonatal chicks. J Comp Physiol A 197:1143–1147
Ferna’ndez-Ruiz J, Herna’ndez M, JA R (2010) Cannabinoid–dopamine interaction in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther 16:72–91
Fowler CJ, Nilsson O, Andersson M, Disney G, Jacobsson SO, Tiger G (2001) Pharmacological properties of cannabinoid receptors in the avian brain: similarity of rat and chicken cannabinoid1 receptor recognition sites and expression of cannabinoid2 receptor-like immunoreactivity in the embryonic chick brain. Pharmacol Toxicol 88:213–222
Furuse M, Ando R, Bungo T, Ao R, ShimoJO M, Masuda Y (1999) Intracerebroventricular injection of orexins does not stimulate food intake in neonatal chicks. Br Poult Sci 40:698–700
Furuse M, Matsumoto M, Saito N, Sugahara K, Hasegawa S (1997) The central corticotropin-releasing factor and glucagon-like peptide-1 in food intake of the neonatal chick. Eur J Pharmacol 339:211–214
Gerdeman G, Lovinger DM (2001) CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J Neurophysiol 85:468–471
Hampson RE, Miller F, Palchik G, Deadwyler SA (2011) Cannabinoid receptor activation modifies NMDA receptor mediated release of intracellular calcium: implications for endocannabinoid control of hippocampal neural plasticity. Neuropharmacology 60:944–952
Hassanpour S, Zendehdel M, Babapour V, Charkhkar S (2015) Endocannabinoid and nitric oxide interaction mediates food intake in neonatal chicken. Br Poult Sci 56(4):443–451
Huang CC, Lo SW, Hsu KS (2001) Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. J Physiol Lond 532:731–748
Irving AJ, Rae MG, Coutts AA (2002) Cannabinoids on the brain. Sci World J 2:632–648
Irwin N, Hunter K, Frizzell N, Flatt PR (2008) Antidiabetic effects of sub-chronic administration of the cannabinoid receptor (CB1) antagonist, AM251, in obese diabetic (ob/ob) mice. Eur J Pharmacol 581:226–233
Kaneko K, Yoshikawa M, Ohinata K (2012) Novel orexigenic pathway prostaglandin D2-NPY system-involvement in orally active orexigenic δ opioid peptide. Neuropeptides 46:353–357
Kangas BD, Delatte MS, Vemuri VK, Thakur GA, Nikas SP, Subramanian KV, Shukla VG, Makriyannis A, Bergman J (2013) Cannabinoid discrimination and antagonism by cb1 neutral and inverse agonist antagonists. J Pharmacol Exp Ther 344:561–567
Levine AS (2006) The animal model in food intake regulation: examples from the opioid literature. Physiol Behav 89:92–96
Lim CT, Kola B, Korbonits M (2010) AMPK as a mediator of hormonal signalling. J Mol Endocrinol 44:87–97
Lin TY, Lu CW, Wu CC, Huang SK, Wang SJ (2015) Palmitoylethanolamide inhibits glutamate release in rat cerebrocortical nerve terminals. Int J Mol Sci 16:5555–5571
Liu Q, Bhat M, Bowen WD, Cheng J (2009) Signaling pathways from cannabinoid receptor-1 activation to inhibition of N-methyl-D-aspartic acid mediated calcium influx and neurotoxicity in dorsal root ganglion neurons. J Pharmacol Exp Ther 331:1062–1070
Liu XJ, Salter MW (2010) Glutamate receptor phosphorylation and trafficking in pain plasticity in spinal cord dorsal horn. Eur J Neurosci 32:278–289
López HH (2010) Cannabinoid–hormone interactions in the regulation of motivational processes. Horm Behav 58:100–110
Nicoll RA, Alger BE (2004) The brain’s own marijuana. Sci Am 291:68–75
Novoseletsky N, Nussinovitch A, Friedman-Einat M (2011) Attenuation of food intake in chicks by an inverse agonist of cannabinoid receptor1 administered by either injection or ingestion in hydrocolloid carriers. Gen Comp Endocrinol 170:522–527
Olanrewaju HA, Thaxton JP, Dozier WA, Purswell J, Roush WB, Branton SL (2006) A review of lighting programs for broiler production. Int J Poult Sci 5(4):301–308
Onaivi ES, Carpio O, Ishiguro H, Schanz N, Uhl GR, Benno R (2008) Behavioral effects of CB2 cannabinoid receptor activation and its influence on food and alcohol consumption. Ann N Y Acad Sci 1139:426–433
Onaivi ES, Ishiguro H, Gu S, Liu QR (2012) CNS effects of CB2 cannabinoid receptors: beyond neuro-immuno-cannabinoid activity. J Psychopharmacol 26:92–103
Parker KE, Johns HW, Floros TG, Will MJ (2014) Central amygdala opioid transmission is necessary for increased high-fat intake following 24-h food deprivation, but not following intraaccumbens opioid administration. Behav Brain Res 260:131–138
Pertwee RG (2005) Pharmacological actions of cannabinoids. Handb Exp Pharmacol 168:1–51
Reid CA, Bliss TVP (2000) Learning about kainate receptors. Tips 21:159–160
Saito ES, Kaiya H, Tachibana T, Tomonaga S, Denbow DM, Kangawa K, Furuse M (2005) Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chicks. Regul Pept 125:201–208
Sánchez-Blázquez P, Rodríguez-Muñoz M, Vicente-Sánchez A, Garzón J (2013a) Cannabinoid receptors couple to NMDA receptors to reduce the production of NO and the mobilization of zinc induced by glutamate. Antioxid Redox Signal 19:1766–1782
Sánchez-Blázque P, Rodríguez-Muño M, Garzón J (2013b) The cannabinoid receptor 1 associates with NMDA receptors to produce glutamatergic hypofunction: implications in psychosis and schizophrenia. Front Pharmacol 4:169. doi:10.3389/fphar.2013.00169
Seyedali Mortezaei S, Zendehdel M, Babapour V, Hasani K (2013) The role of glutamatergic and GABAergic systems on serotonin- induced feeding behavior in chicken. Vet Res Commun 37:303–310
Sharkey KA, Darmani NA, Parker LA (2014) Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system. Eur J Pharmacol 722:134–146
Shiraishi J, Yanagita K, Fukumori R, Sugino T, Fujita M, Kawakami S, McMurtry JP, Bungo T (2011) Comparisons of insulin related parameters in commercial-type chicks: evidence for insulin resistance in broiler chicks. Physiol Behav 103:233–239
Suarez J, Bermudez-Silva FJ, Mackie K, Ledent C, Zimmer A, Cravatt BF, de Fonseca FR (2008) Immunohistochemical description of the endogenous cannabinoid system in the rat cerebellum and functionally related nuclei. J Comp Neurol 509:400–421
Taati M, Nayebzadeh H, Zendehdel M (2011) The effects of DLAP5 and glutamate on ghrelin-induced feeding behavior in 3- h food-deprived broiler cockerels. J Physiol Biochem 67:217–223
Tasca CI, Santos TG, Tavares RG, Battastini AM, Rocha JB, Souza DO (2004) Guanine derivatives modulate L-glutamate uptake into rat brain synaptic vesicles. Neurochem Int 44(6):423–431
Van Tienhoven A, Juhasz LP (1962) The chicken telencephalon, diencephalon and mesencephalon in sterotaxic coordinates. J Comp Neurol 118:185–197
Verty ANA, McFarlane JR, McGregor IS, Mallet PE (2004) Evidence for an interaction between CB1 cannabinoid and melanocortin MCR-4 receptors in regulating food intake. Endocrinol 145(7):3224–3231
Vicente-Sánchez A, Sánchez-Blázquez P, Rodríguez-Muñoz M, Garzón J (2013) HINT1 protein cooperates with cannabinoid 1 receptor to negatively regulate glutamate NMDA receptor activity. Mol Brain 6:42. doi:10.1186/1756-6606-6-42
Wiley JL, Marusich JA, Zhang Y, Fulp A, Maitra R, Thomas BF, Mahadevan A (2012) Structural analogs of pyrazole and sulfonamide cannabinoids: effects on acute food intake in mice. Eur J Pharmacol 695:62–70
Zendehdel M, Baghbanzadeh A, Babapour V, Cheraghi J (2009) The effects of bicuculline and muscimol on glutamate-induced feeding behaviour in broiler cockerels. J Comp Physiol A 195:715–720
Zendehdel M, Hassanpour S (2014) Ghrelin-induced hypophagia is mediated by the β2 adrenergic receptor in chicken. J Physiol Sci 64:383–391
Zeni LA, Seidler HB, De Carvalho NA, Freitas CG, Marino-Neto J, Paschoalini MA (2000) Glutamatergic control of food intake in pigeons: effects of central injections of glutamate, NMDA, and AMPA receptor agonists and antagonists. Pharmacol Biochem Behav 65(1):67–74
Acknowledgments
This research was supported by a grant from the Research Council of the Faculty of Veterinary Medicine, University of Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Informed Consent
This manuscript does not contain any studies with human subjects performed by any of the authors.
Human and Animal Rights
All experiments executed according to the Guide for the Care and Use of Laboratory Animals and approved by the institutional animal ethics committee.
Rights and permissions
About this article
Cite this article
Keyshams, N., Zendehdel, M., Babapour, V. et al. Cannabinoid–glutamate interactions in the regulation of food intake in neonatal layer- type chicks: role of glutamate NMDA and AMPA receptors. Vet Res Commun 40, 63–71 (2016). https://doi.org/10.1007/s11259-016-9655-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-016-9655-8