Abstract
Pelvi-ureteric junction obstruction corresponds to an impairment of urinary transport that can lead to renal dysfunction if not treated. Several mechanisms can cause the obstruction of the ureter including intrinsic factors or extrinsic factors such as the presence of crossing vessels. The treatment of the disease relies on surgical approaches, pyeloplasty being the standard reference. The technique consists in removing the pathologic ureteric segment and renal pelvis and transposing associated crossing vessels if present. The vascular anatomy of the pelvi-ureteric junction is complex and varies among individuals, and this can impact on the disease development and its surgical treatment. In this review, we summarize current knowledge on vascular anatomic variations in the pelvi-ureteric junction. Based on anatomic characteristics, we discuss implications for surgical approaches during pyeloplasty and vessel transposition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pelvi-ureteric junction (PUJ) obstruction corresponds to an impairment of urinary transport from the renal pelvis to the ureter. The disease can lead to progressive hydronephrosis or renal dysfunction and can favor calculus formation and pyelonephritis [1]. The obstruction can be caused by several mechanisms including intrinsic factors such as a ureteric stenosis, an aperistaltic ureteral segment, infoldings of the ureteral mucosa or extrinsic factors including fibrous bands or crossing vessels [1, 2]. When symptomatic or associated with complications, the treatment of PUJ obstruction is indicated and relies on surgical approaches. Several techniques have been developed, and pyeloplasty as described by Anderson and Hynes remains the reference standard [1, 3]. The technique consists in removing the pathologic ureteric segment and renal pelvis and transposing associated crossing vessels or removing calculus if present. This can be performed via open surgery or via minimally invasive procedures including laparoscopic or robotic pyeloplasty [1, 3]. Even if pyeloplasty remains the gold standard, an exclusive crossing vessel transposition can be proposed in some cases. Vascular anatomy of the pelvi-ureteric junction varies among individuals, and the causal link between crossing vessels and PUJ obstruction can be difficult to evaluate. The aim of this review is to summarize current knowledge on vascular anatomic variation in the PUJ. In the limelight of anatomic characteristics, we discuss consequences for surgical approach during pyeloplasty and vessel transposition.
Anatomic characteristics of the PUJ
Anatomy of the PUJ region
Kidneys are retroperitoneal organs located in the lumbar region (Fig. 1). Renal hilus is formed by several structures including the renal veins and arteries, the renal pelvis as well as neurologic and lymphatic structures. The renal artery originates from the left and the right side of abdominal aorta, below the superior mesenteric artery [4]. Right and left renal arteries can originate from the abdominal aorta at the same level, around the first or the second lumbar vertebra, or in some cases, the left renal artery originates at an upper level. Renal arteries move toward the rear and the right renal artery is longer and behind the vena cava. Classically, renal arteries divide into anterior and posterior branches (Fig. 2). The anterior branch further divides into segmental superior, segmental inferior, segmental antero-superior, segmental antero-inferior and antero-superior ureteric arteries. The posterior branch divides into segmental postero-superior, segmental postero-inferior and postero-superior ureteric arteries. Arterial renal vascularization has the peculiarity to be terminal which means that in case of damage of a segmental artery, there is no supply by another trunk which can lead to a segmental renal infarct and renal dysfunction. Other collateral branches that originate from the renal artery include the adipo-capsular artery, the inferior suprarenal artery and the pelvi-ureteric artery which vascularize the renal capsule, the suprarenal gland and the ureter.
Anterior retroperitoneal representation of kidneys. (1) Inferior vena cava, (2) right suprarenal gland, (3) right suprarenal vein, (4) right renal vein (and artery behind), (5) right kidney, (6) superior mesenteric artery, (7) right genital vein, (8) right ureter, (9) celiac artery, (10) abdominal aorta, (11) left suprarenal gland, (12) left kidney, (13) left inferior suprarenal vein, (14) left renal artery, (15) left renal vein, (16) left genital vein, (17) inferior mesenteric artery, (18) left ureter
Classic division of renal arteries. a Anterior frontal section of the kidney. (1) Segmental superior artery, (2) segmental antero-superior artery, (3) posterior branch, (4) renal artery, (5) anterior branch, (6) segmental antero-inferior artery, (7) ureteric antero-superior artery, (8) segmental inferior artery. b Posterior frontal section of the kidney. (1) Segmental postero-superior artery, (2) anterior branch, (3) renal artery, (4) posterior branch, (5, 7) segmental postero-inferior arteries, (6) postero-superior ureteric artery. c Superior axial section of the kidney. (1) Posterior branch, (2) renal artery, (3) anterior branch, (4) antero-superior ureteric artery
Renal veins originate from venous trunks that drain segmental veins [5]. They are oriented upward and inward to join the inferior vena cava (Fig. 1). The left renal vein is longer and crosses in front of the abdominal aorta, just below the origin of the superior mesenteric artery. The left renal vein drains the genital veins as well as the inferior suprarenal vein. On the right side, genital veins and the inferior suprarenal vein directly flow into the inferior vena cava [6]. Renal lymphatic system is formed by a subcapsular cortical network and a deep network that drain into latero-cava and latero-aortic lymph nodes [6]. The renal innervation depends on the renal plexus. Parasympathetic afferences originate from vagal nerves, and sympathetic afferences come from splanchnic nerves.
Arterial anatomic variation in the PUJ
Vascular anatomy of the PUJ can differ among individuals, and these variations may potentially be involved in PUJ obstruction and can impact on its surgical treatment.
Several anatomists observed that renal artery is not always unique (Fig. 3) and reported a duplicity of the renal artery in at least one third of the cases examined [7]. When present, it is estimated that the duplicity of the renal artery is also observed on the contralateral side in half of the cases [7]. Additional renal arteries originate from the abdominal aorta and can vascularize the hilum, the superior pole or the inferior pole (Fig. 3b, c). When present, a unique additional artery is more frequently observed than two additional renal arteries [5]. Some authors have estimated the incidence of first and second additional arteries at 23.2 and 4.5%, respectively [8]. These results are in accordance with other anatomic descriptions that reported a duplicity of renal artery in 24–26% of cases and a triplicity of a renal artery in approximately 3% of cases [5, 6]. Interestingly, additional arteries were more frequently observed on the left side (32% of cases versus 23.3%) and significant differences were observed depending on sex and ethnic origin. The morphometry of additional vessels was analyzed, and the mean lengths of first and second additional renal arteries were, respectively, 4.5 and 3.8 cm (right side), 4.9 and 3.7 cm (left side). The mean diameters were, respectively, 0.4 and 0.3 (right side), and 0.3 and 0.3 cm (left side). While renal artery can be unique or multiple, its division into segmental branches can also varies among individuals. To analyze functional kidney vascularization, a proportional analysis of each renal arterial segment was performed in 49 cases by injecting each arterial segmental branch with colored resin [9]. The presence of five arterial segments was more frequently observed than four arterial segments (respectively, 61.2 and 38.8% of cases). The median proportional areas of the superior, antero-superior, antero-inferior segments were, respectively, 13.02, 21.36 and 17.18%. The anterior segment was present in 38.8% of cases and had a median proportional area of 28.44%. The inferior segment was present in all cases and had a median proportional area of 22.65%. The posterior segment was also present in 100% of cases and was the segment with the greatest median proportional area (33.76%). These results reveal that anatomy of renal artery and its division into branches differ between individuals and induce variations in renal vascularization. Other authors addressed the anatomic variations in the upper segmental renal artery [10]. Based on the observation of 50 human kidneys, they found that the upper segmental renal artery was present in 98% of cases and described four types of variation in arterial pattern of upper segmental artery and two variations in the anatomic relations with the collecting system.
Variations in renal arterial vascularization (adapted from Henry et al. [6] and Boudghene et al. [5]). a Classic renal arterial vascularization: presence of one renal artery. b Presence of one additional renal artery. (1) Oriented to the hilum, (2) oriented to the superior pole, (3) oriented to the lower pole. C Presence of two additional renal arteries. (1) Additional renal arteries oriented to the hilum, (2) additional renal arteries oriented, respectively, to the superior and the inferior pole. d Presence of polar arteries originating from the renal artery. (1) Polar superior artery, (2) polar inferior artery
Polar arteries represent vessels which can arise from the renal artery or directly from the abdominal aorta (Fig. 3b-2, b-3, d-1, d-2). They are not systematically present, and the criteria used to characterize them are not clearly defined. Some define it based on their origin, whereas others characterize it based on their vascular ending to the renal pole, which can induce bias when comparing the studies. When defining polar arteries as vessels which originate from the abdominal aorta that goes to the renal pole, Henry et al. [6] estimated the prevalence of a polar superior and inferior artery in, respectively, 8 and 6% of cases. Other authors defined polar arteries as vessels that arise from the renal artery and terminate in the renal pole and reported the presence of a polar superior artery in 13% of cases and a polar inferior artery in 2% of cases [5]. To better characterize the origin of polar arteries from the division of the renal artery, Ternon et al. [7] classified the different configurations when renal artery is unique (Fig. 4). They showed that the polar inferior artery can originate directly from the renal artery (type 1) or can arise at the division of the renal artery to the anterior and posterior branch (type 2), or can originate from the anterior branch (type 3). In type 4, polar artery arises from the posterior branch. At last, in type 5, no inferior polar artery is observed. Hence, the presence or not of a polar artery contributes to vascular anatomic variation among individuals and can potentially impact on PUJ obstruction.
Variation in origin of polar artery when it arises from a unique renal artery (adapted from Ternon et al. [7]). a Type 1 configuration. b Type 2 configuration. c Type 3 configuration. d Type 4 configuration. e Type 5 configuration
To better understand the vascular anatomy of the PUJ, some investigators analyzed 546 kidneys harvested from cadaveric donors [11]. Sampaio et al. [11] revealed that in 65% of cases, a prominent artery, vein or both were in close relation to the ventral surface of the PUJ. In 45% of these cases, this was in relation to an inferior segmental artery and in only 6.8% of the cases an inferior polar artery crossed anteriorly the PUJ. These findings corroborate the low frequency of polar arteries observed in other studies [5, 6]. A direct relation between a large vessel and the dorsal surface of the PUJ was much less frequent and observed in 6.2% of cases [11]. Considering the inferior surface, a vessel crossing lower than 1.5 cm above the posterior surface of the PUJ was observed in 20.5% of cases. The observation of a close relation between vessels to PUJ led investigators to study the prevalence of crossing vessels. Based on analyses of CT angiography and endoluminal ultrasonography, clinical studies reported a prevalence of crossing vessels from 22.7 to 71% [12,13,14,15,16]. To go further in the characterization of crossing vessels, Leavitt et al. [15] analyzed computed tomography angiography images from asymptomatic patients with a radiographically normal PUJ. They reported that crossing vessels were more frequently left-sided (in 60.1% of cases versus 39.9%) and an artery was most frequently involved (81% of cases). The location of the crossing vessel relative to the PUJ varied and included anterior (25.8%), antero-lateral (36.8%), medial (14.6%), antero-medial (2.5%), lateral (12.9%) and posterior (7.4%). Similarly, Zelster et al. [14] also found that crossing vessels were more frequently anterior than posterior. At last, the mean diameter and the mean distance of the crossing vessels from the PUJ were, respectively, 3.3 mm and 1.8 mm.
Consequences for clinical practice
In the limelight of studies on vascular anatomic variation in the PUJ, several points should be taken into consideration. First, no consensus has been established to clearly define crossing vessels, additional renal arteries and polar arteries. An additional renal artery corresponds to an artery other than the main renal artery which arises from the abdominal aorta and terminates in the kidney. However, the terms “accessory,” “aberrant,” “anomalous,” “supernumerary,” “multiple,” “accessory aortic hilar” arteries have also been used to describe additional arteries. Besides, polar arteries have been described as vessels that go to the superior and inferior poles, whatever their origin from the renal artery or the abdominal aorta. In the latest case, polar arteries could in fact correspond to additional arteries as defined by other authors (Fig. 3b3, b4, d). This could have led to heterogeneity among different studies and contributes to confusion in the literature regarding nomenclature. Second, the consequences of the presence of crossing vessels and its direct causal link with PUJ obstruction can be difficult to evaluate. As suggested by Sampaio et al., it is possible that many of the vessels in close relation to the PUJ could be in fact normal segmental arteries that do not cause PUJ obstruction [11]. To assess the impact of crossing vessels as etiology of PUJ obstruction, Stern et al. [17] performed an intraoperative Whitaker test to infuse saline in the renal pelvis and measured bladder and renal pelvic pressures before and after complete mobilization of the PUJ. They did not find changes of renal pelvic pressure after mobilization of the renal pelvis in patients without crossing vessels, whereas the mean pelvic pressure significantly declined after vessel repositioning in patients with crossing vessels [17]. Their results suggest that lower pole crossing vessels directly contributes to PUJ obstruction by causing extrinsic compression. Nevertheless, the number of patients included is low and these results cannot be extrapolated to the general population. In clinical practice, PUJ obstruction is diagnosed based on injected CT scan images which can be complemented with dynamic tests such as diuretic 99 mTc-mercaptoacetyltriglycine (MAG3) dynamic scintigraphy or Tc-99m DTPA dynamic renal scintigraphy [1]. These tests represent useful tools to evaluate the functional renal consequences and the severity of the disease, but cannot formally determine the etiology of the compression. To go further in the assessment of crossing vessels involvement in the PUJ obstruction, it would be interesting to precisely evaluate their distance and their diameter from the PUJ. This could help to determine predictive factors to assess the impact of crossing vessels on PUJ obstruction.
Implication for surgical approaches
The precise knowledge of renal vasculature is of valuable contribution for surgical approaches.
As described by anatomists, the anterior surface of the PUJ is highly vascularized and requires a precise dissection to avoid any arterial damage. Several techniques have been developed to treat PUJ obstruction, among which pyeloplasty remains the reference standard [1, 3]. The technique was first described by Foley in 1937 and was modified by Anderson and Hynes [1, 3, 18, 19]. Anderson–Hynes technique was originally performed via open surgery and evolved since the development of minimally invasive approaches including laparoscopic or robot-assisted procedures. Both retroperitoneal and transperitoneal approaches can be performed to remove the pathological ureter and the pathologic renal pelvis. When present, crossing vessels are dissected and transposed behind the PUJ [19]. At the end of the procedure, a pelvi-ureteric anastomosis is created and temporarily protected using a double-J catheter. While the choice between retroperitoneal and transperitoneal approaches mainly depends on training and experience of the surgeons, the transperitoneal approach has the advantage to provide familiar anatomic landmarks and larger working space to suture. However, the retroperitoneal approach offers a rapid and direct access to the PUJ by simple elevation of the lower pole of the kidney and allows better detection of crossing vessels [19,20,21]. Using laparoscopic approaches, the rate of ureteric transposition of the PUJ anterior to the lower pole has been reported in 42% of cases [19, 21]. Several authors suggest that this rate may be higher than in open surgery due to minimal mobilization of the kidney needed to access the PUJ when using laparoscopic approach. Open pyeloplasty may require a mobilization of the entire kidney and may modify the relations of the PUJ to lower pole vessels, reducing the possibility to identify crossing vessels as a potential cause of obstruction.
Both laparoscopic pyeloplasty and robot-assisted pyeloplasty have proven efficiency to treat PUJ obstruction [19, 20]. A meta-analysis revealed that the rates of postoperative complications and success were similar between the two approaches, but robot-assisted pyeloplasty was associated with a 10-min operative time reduction and a significant shorter hospital stay [22]. Vascular outcome may not significantly differ between the two approaches as revealed by similar mean blood loss and low frequency of vascular complications (Table 1). Besides, the identification of a crossing vessel was similar among the groups, with rates ranging from 42 to 57.1% in the laparoscopic approach and from 30 to 48.9% in the robot-assisted procedure [21, 23,24,25,26,27,28,29,30,31].
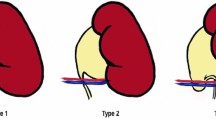
While pyeloplasty represents the surgical approach the most commonly used to treat PUJ obstruction, the exclusive transposition of crossing vessels initially developed by Hellström et al. has been proposed as a therapeutic alternative and has proved efficiency in selected cases [32,33,34,35,36]. Compared to pyeloplasty, the technique has the advantage to be less technically challenging, to require minimal suturing and no need for incising the renal pelvis leaving the collecting system intact [34]. However, the challenge of this approach is to evaluate its indications as to date, no imaging techniques or intraoperative procedures are available to formally confirm that the crossing vessels are the unique cause of obstruction of the PUJ. The indications of the technic have been based on preoperative images as well as perioperative empirical judgment. Zhang et al. evaluated the morphological and functional status of the PUJ and defined the following criteria to perform the laparoscopic Hellström technique: a normal appearance of the PUJ, transmission of peristaltic wave across the PUJ and complete drainage of urine after the relief of oppression [34]. Similarly, intraoperative decision to perform the Hellström technique by other authors was based on the presence of the crossing vessels, a grossly normal appearance of the ureter and PUJ as well as a small renal pelvis [32]. Nevertheless, these criteria can be subjective and potentially lack of sensitivity to detect intrinsic causes of PUJ obstruction. This could partly explain some cases that report failure of Hellström technique [37]. At last, Schneider et al. proposed an anatomic classification based on the location of polar vessels that may help to choose between a laparoscopic vascular hitch and a dismembered pyeloplasty. In their study, polar vessels were located in front of the dilated pelvis in type 1; in type 2 in front of the PUJ in type 2; and under the PUJ in type 3, resulting in ureteral kinking. Based on their experience, the authors suggest that only patients with type 3 anatomic variation and with a normal PUJ should be proposed for the laparoscopic vascular hitch and that in other cases dismembered pyeloplasty should remain the standard treatment option. Clinical studies performed so far underline the lack of clear objective criteria to choose the most appropriate surgical approach. Further detailed morphological studies may be useful to identify anatomic criteria of crossing vessels that could be useful parameters to evaluate indications of each surgical technique. The diameter of crossing vessels, their location and their distance to the PUJ could potentially represent attractive tools to evaluate the need to transpose crossing vessels and whether it should be associated with pyeloplasty.
Conclusion
Vascular anatomy of the PUJ differs among individuals and the nomenclature used in the literature to define crossing vessels and polar arteries is not clear. This led to heterogeneity among different studies highlighting the real need to standardize the definitions. The existence of crossing vessels in the PUJ has several implications for clinical practice. First, caution should be taken when dissecting the region to avoid any arterial damage which could lead to ischemic lesions. Second, the identification and the visualization of crossing vessels may be impacted depending on the surgical approach performed to treat PUJ obstruction. At last, the link between the presence of crossing vessels and the etiology of PUJ obstruction may be difficult to assess and the indications of exclusive vessel transposition over its association with dismembered pyeloplasty remain to be precisely defined. Further studies should be oriented to better characterize morphology and relations of crossing vessels to the PUJ based on imaging as well as perioperative observations. We believe that this could potentially lead to identify predictive factors that would be useful to help to choose the most appropriate surgical approach in context of PUJ obstruction.
Abbreviations
- PUJ:
-
Pelvi-ureteric junction
References
Descotes JL (2013) Management of adult uretero-pelvic junction obstruction. Prog Urol 23(14):1172–1176
Boylu U, Oommen M, Lee BR, Thomas R (2009) Ureteropelvic junction obstruction secondary to crossing vessels-to transpose or not? The robotic experience. J Urol 181(4):1751–1755
Manikandan R, Saad A, Bhatt RI, Neilson D (2005) Minimally invasive surgery for pelviureteral junction obstruction in adults: a critical review of the options. Urology 65(3):422–432
Poirier et Charpy. Traité d’Anatomie Humaine. Vol. tome 5. Paris: Masson
Imagerie de l’aorte abdominale normale. F. Boudghene MTEM
Anatomie des reins et de la voie excrétrice supérieure. N. Henry PSEEM
Anatomie de l’abdomen tome II, G. Doin et Cie Saint-Étienne. Couinaud, 1963
Satyapal KS, Haffejee AA, Singh B, Ramsaroop L, Robbs JV, Kalideen JM (2001) Additional renal arteries: incidence and morphometry. Surg Radiol Anat 23(1):33–38
Sampaio FJ, Schiavini JL, Favorito LA (1993) Proportional analysis of the kidney arterial segments. Urol Res 21(6):371–374
Mishra GP, Bhatnagar S, Singh B (2015) Anatomical variations of upper segmental renal artery and clinical significance. J Clin Diagn Res 9(8):AC01-3
Sampaio FJ (1996) The dilemma of the crossing vessel at the ureteropelvic junction: precise anatomic study. J Endourol 10(5):411–415
Nakada SY, Wolf JS Jr, Brink JA, Quillen SP, Nadler RB, Gaines MV et al (1998) Retrospective analysis of the effect of crossing vessels on successful retrograde endopyelotomy outcomes using spiral computerized tomography angiography. J Urol 159(1):62–65
Quillin SP, Brink JA, Heiken JP, Siegel CL, McClennan BL, Clayman RV (1996) Helical (spiral) CT angiography for identification of crossing vessels at the ureteropelvic junction. AJR Am J Roentgenol 166(5):1125–1130
Zeltser IS, Liu JB, Bagley DH (2004) The incidence of crossing vessels in patients with normal ureteropelvic junction examined with endoluminal ultrasound. J Urol 172(6 Pt 1):2304–2307
Leavitt DA, Nicholson AF, Ortiz-Alvarado O, Maust TJ, Rutledge GM, Walker SP et al (2013) Nature of crossing vessels in patients with radiographically normal ureteropelvic junctions: prevalence and anatomic characteristics. Urology 81(6):1168–1172
Sampaio FJ (1998) Vascular anatomy at the ureteropelvic junction. Urol Clin North Am 25(2):251–258
Stern JM, Park S, Anderson JK, Landman J, Pearle M, Cadeddu JA (2007) Functional assessment of crossing vessels as etiology of ureteropelvic junction obstruction. Urology 69(6):1022–1024
Anderson JC, Hynes W (1949) Retrocaval ureter; a case diagnosed pre-operatively and treated successfully by a plastic operation. Br J Urol 21(3):209–214
El-Shazly MA, Moon DA, Eden CG (2007) Laparoscopic pyeloplasty: status and review of literature. J Endourol 21(7):673–678
Eden CG (2007) Minimally invasive treatment of ureteropelvic junction obstruction: a critical analysis of results. Eur Urol 52(4):983–989
Moon DA, El-Shazly MA, Chang CM, Gianduzzo TR, Eden CG (2006) Laparoscopic pyeloplasty: evolution of a new gold standard. Urology 67(5):932–936
Braga LH, Pace K, DeMaria J, Lorenzo AJ (2009) Systematic review and meta-analysis of robotic-assisted versus conventional laparoscopic pyeloplasty for patients with ureteropelvic junction obstruction: effect on operative time, length of hospital stay, postoperative complications, and success rate. Eur Urol 56(5):848–857
Wagner S, Greco F, Inferrera A, Hoda MR, Kawan F, Hamza A et al (2010) Laparoscopic dismembered pyeloplasty: technique and results in 105 patients. World J Urol 28(5):615–618
Turk IA, Davis JW, Winkelmann B, Deger S, Richter F, Fabrizio MD et al (2002) Laparoscopic dismembered pyeloplasty—the method of choice in the presence of an enlarged renal pelvis and crossing vessels. Eur Urol 42(3):268–275
Soulie M, Salomon L, Patard JJ, Mouly P, Manunta A, Antiphon P et al (2001) Extraperitoneal laparoscopic pyeloplasty: a multicenter study of 55 procedures. J Urol 166(1):48–50
Inagaki T, Rha KH, Ong AM, Kavoussi LR, Jarrett TW (2005) Laparoscopic pyeloplasty: current status. BJU Int 95(Suppl 2):102–105
Chuanyu S, Guowei X, Ke X, Qiang D, Yuanfang Z (2009) Retroperitoneal laparoscopic dismembered Anderson–Hynes pyeloplasty in treatment of ureteropelvic junction obstruction (report of 150 cases). Urology 74(5):1036–1040
Patel V (2005) Robotic-assisted laparoscopic dismembered pyeloplasty. Urology 66(1):45–49
Mufarrij PW, Woods M, Shah OD, Palese MA, Berger AD, Thomas R et al (2008) Robotic dismembered pyeloplasty: a 6-year, multi-institutional experience. J Urol 180(4):1391–1396
Schwentner C, Pelzer A, Neururer R, Springer B, Horninger W, Bartsch G et al (2007) Robotic Anderson–Hynes pyeloplasty: 5-year experience of one centre. BJU Int 100(4):880–885
Sivaraman A, Leveillee RJ, Patel MB, Chauhan S, Bracho JE 2nd, Moore CR et al (2012) Robot-assisted laparoscopic dismembered pyeloplasty for ureteropelvic junction obstruction: a multi-institutional experience. Urology 79(2):351–355
Meng MV, Stoller ML (2003) Hellstrom technique revisited: laparoscopic management of ureteropelvic junction obstruction. Urology 62(3):404–408 (discussion 8–9)
Esposito C, Bleve C, Escolino M, Caione P, Gerocarni Nappo S, Farina A et al (2016) Laparoscopic transposition of lower pole crossing vessels (vascular hitch) in children with pelviureteric junction obstruction. Transl Pediatr 5(4):256–261
Zhang X, Xu K, Fu B, Zhang J, Lang B, Ai X et al (2007) The retroperitoneal laparoscopic Hellstrom technique for pelvi-ureteric junction obstruction from a crossing vessel. BJU Int 100(6):1335–1338
Schneider A, Ferreira CG, Delay C, Lacreuse I, Moog R, Becmeur F (2013) Lower pole vessels in children with pelviureteric junction obstruction: laparoscopic vascular hitch or dismembered pyeloplasty? J Pediatr Urol 9(4):419–423
Godbole P, Mushtaq I, Wilcox DT, Duffy PG (2006) Laparoscopic transposition of lower pole vessels–the ‘vascular hitch’: an alternative to dismembered pyeloplasty for pelvi-ureteric junction obstruction in children. J Pediatr Urol 2(4):285–289
Nerli RB, Jayanthi VR, Reddy M, Koura A (2009) Pelvi-ureteric junction obstruction with crossing renal vessels: a case report of failed laparoscopic vascular hitch. J Pediatr Urol 5(2):147–150
Palese MA, Munver R, Phillips CK, Dinlenc C, Stifelman M, DelPizzo JJ (2005) Robot-assisted laparoscopic dismembered pyeloplasty. JSLS 9(3):252–257
Gupta NP, Nayyar R, Hemal AK, Mukherjee S, Kumar R, Dogra PN (2010) Outcome analysis of robotic pyeloplasty: a large single-centre experience. BJU Int 105(7):980–983
Bernie JE, Venkatesh R, Brown J, Gardner TA, Sundaram CP (2005) Comparison of laparoscopic pyeloplasty with and without robotic assistance. JSLS 9(3):258–261
Link RE, Bhayani SB, Kavoussi LR (2006) A prospective comparison of robotic and laparoscopic pyeloplasty. Ann Surg 243(4):486–491
Weise ES, Winfield HN (2006) Robotic computer-assisted pyeloplasty versus conventional laparoscopic pyeloplasty. J Endourol 20(10):813–819
Author information
Authors and Affiliations
Contributions
All authors confirmed that they have contributed to the intellectual content of this article and have approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Panthier, F., Lareyre, F., Audouin, M. et al. Pelvi-ureteric junction obstruction related to crossing vessels: vascular anatomic variations and implication for surgical approaches. Int Urol Nephrol 50, 385–394 (2018). https://doi.org/10.1007/s11255-017-1771-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-017-1771-z