Abstract
Open or laparoscopic dismembered pyeloplasty (DP) is the gold standard procedure to treat UPJO since the procedure was first described by Anderson and Hynes (AHDP) in 1949 [1]. UPJO may be caused by intrinsic disorganization or by extrinsic compression from crossing vessels (CV); extrinsic causes often present symptomatically in older children. The association between UPJ obstruction and extrinsic aetiology by lower pole CV was first described by Von Rokitansky in 1842 [2]. UPJO due to CV, frequently observed in adults, is a rare condition in neonates and has a slight incidence in older children. An alternative approach to pure extrinsic UPJO was first described by Hellström [3] always in 1949; it involved displacing the lower pole vessels cranially and then anchoring them to the anterior pelvic wall using vascular adventitial sutures. Chapman [4] further modified this technique by securing a more superior position of the lower pole vessels within a wrap of the anterior redundant pelvic wall without the need for vascular adventitial sutures. This technique has since been described in children as an alternative to open DP, with the largest series reported in 1999 by Pesce [5]. Aberrant vessels usually cause intermittent UPJO. These cases present a normal perinatal history, followed by the subsequent onset of clinical signs and symptoms, often influenced by the child’s hydration status, characterized by intermittent hydronephrosis on imaging and normal kidney function. The CV typically cross over the UPJ to perfuse the lower pole of the affected kidney. Currently, there are no definitive imaging techniques or intraoperative procedures available to confirm the aetiology of UPJO. As noted by Schneider [6], frequently one encounters anatomic variability in the relationship between the renal pelvis and the lower pole vessels. Some authors have proposed DP to exclude intrinsic associated anomalies; others, in order to minimize technical difficulties and improve outcomes, have described simpler procedures that do not involve pyeloureteral anastomosis. We describe in this chapter the paediatric laparoscopic vascular hitch (LVH), a mini-invasive approach to UPJO by CV, suggesting a simple and uncomplicated intraoperative test, DT, to confirm the relief of the obstruction. This technique gives excellent results in our hands.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Open or laparoscopic dismembered pyeloplasty (DP) is the gold standard procedure to treat UPJO since the procedure was first described by Anderson and Hynes (AHDP) in 1949 [1]. UPJO may be caused by intrinsic disorganization or by extrinsic compression from crossing vessels (CV); extrinsic causes often present symptomatically in older children. The association between UPJ obstruction and extrinsic aetiology by lower pole CV was first described by Von Rokitansky in 1842 [2]. UPJO due to CV, frequently observed in adults, is a rare condition in neonates and has a slight incidence in older children. An alternative approach to pure extrinsic UPJO was first described by Hellström [3] always in 1949; it involved displacing the lower pole vessels cranially and then anchoring them to the anterior pelvic wall using vascular adventitial sutures. Chapman [4] further modified this technique by securing a more superior position of the lower pole vessels within a wrap of the anterior redundant pelvic wall without the need for vascular adventitial sutures. This technique has since been described in children as an alternative to open DP, with the largest series reported in 1999 by Pesce [5]. Aberrant vessels usually cause intermittent UPJO. These cases present a normal perinatal history, followed by the subsequent onset of clinical signs and symptoms, often influenced by the child’s hydration status, characterized by intermittent hydronephrosis on imaging and normal kidney function. The CV typically cross over the UPJ to perfuse the lower pole of the affected kidney. Currently, there are no definitive imaging techniques or intraoperative procedures available to confirm the aetiology of UPJO. As noted by Schneider [6], frequently one encounters anatomic variability in the relationship between the renal pelvis and the lower pole vessels. Some authors have proposed DP to exclude intrinsic associated anomalies; others, in order to minimize technical difficulties and improve outcomes, have described simpler procedures that do not involve pyeloureteral anastomosis. We describe in this chapter the paediatric laparoscopic vascular hitch (LVH), a mini-invasive approach to UPJO by CV, suggesting a simple and uncomplicated intraoperative test, DT, to confirm the relief of the obstruction. This technique gives excellent results in our hands.
2 Preoperative Diagnosis and Preparation
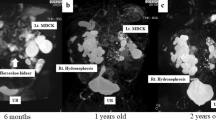
A preoperative diagnosis of extrinsic UPJO was based on complete medical history and a specific imaging examination. All patients with UPJO undergo, respectively, ultrasonography/Doppler scan and MAG3renogram, reserving functional magnetic resonance urography (fMRU) in case of suspected extrinsic obstruction (Fig. 52.1a–c). Suspicion of CV was based on a normal perinatal history with absence/non-significative renal pelvis dilation at prenatal ultrasound (as in our series), a late presentation with intermittent symptoms (vomiting, flank pain or renal colic), marked hydronephrosis at the time of pain with primarily extrarenal dilatation and an obstructed pattern on a diuretic MAG3renogram. Surgical indications included two or more of the following conditions: presence of clinical symptoms, obstruction on diuretic renogram (99mTc-MAG3), decrease on relative renal function, clear or suspected image of polar vessels on fMRU and worsening of intermittent hydronephrosis on follow-up. The patients are hospitalized 24 h before surgery and started with liquid diet and bowel cleansing with laxative and enemas to obtain bowel deflation and facilitate laparoscopic approach. All patients and their parents have to sign a specifically formulated informed consent before the procedure. Patients received a general anaesthesia and antibiotic prophylaxis with i.v. amoxicillin-clavulanic acid or cephalosporin.
3 Positioning
Considering the renal anatomy (aberrant polar vessel anteriorly to the renal pelvis), it is preferable a transperitoneal approach because this provides better anterior access to the renal pelvis and easier anterior CV hitching. In operatory theatre, patient is placed in a semilateral position (45°) at the edge of the surgical table. A bladder catheter and nasogastric tube are positioned before starting the procedure. The monitor is placed behind the patient. Surgeon’s position is in front of the abdomen of the patient with the assistant on his left/right trying to obtain for the surgical team the best possible ergonomy for the shoulders. The scrub nurse is on the side of the surgeon (on the right) (Fig. 52.2a, b).
4 Instrumentation
After an umbilical open approach, a 5 or 10 mm optical port is inserted (according to weight and age of the patient), and then an optical laparoscope is introduced to explore the abdominal cavity; usually a 30° scope is preferable to better visualize the different angulation of the operative field. Two other 3 mm working ports are then placed, one in the epigastrium and one in the ipsilateral iliac fossa at the midclavicular line, to allow an ideal triangulation during dissection of the CV and completion of the pelvic wrap. Sometimes could be useful to use a third 3 mm lateral operative port to move the colon or to suspend the aberrant vessels. Pneumoperitoneum is induced by insufflating CO2 at the minimal pressure to obtain an acceptable operative space (pressure varies from 5 to 10 mmHg).
5 Technique
The technique consisted in exposure of the lower aberrant CV via the transperitoneal approach without ipsilateral colon mobilization. This is usually obtained on the left side through a window in the mesocolon, while on the right side, by working just on the upper side of the colonic flexure that is freed (Fig. 52.3). Once the dilatation is identified and CV are visualized, we proceed with their dissection and mobilization off the UPJ or the proximal ureter. Diuretic test is then performed administering a bolus of normal saline (20 mL/kg IV) before complete vessel mobilization followed by furosemide (1 mg/kg IV) after complete mobilization (Fig. 52.4a, b). Full mobility of the UPJ is confirmed by moving freely the upper and lower portions of the anterior pelvis wall just behind the CV as a shoeshine (shoeshine manoeuvre). The UPJ is then carefully inspected for any intrinsic visible stenosis (significant narrowing). To be sure of a pure extrinsic obstruction, the CV must be temporarily transposed and the surgeon must observe the peristalsis associated with the easy urine passage across the junction and, finally, deflation of the pelvis. Once the test is successfully completed, the cranially displaced lower pole CV are then positioned away from the UPJ by performing a loose wrap of the anterior pelvic wall around these vessels using 3-4/0 polydioxanone or alternative polyglactin sutures (pyelo-pyelic sleeve). Two/three interrupted sutures may be necessary to achieve an adequate tunnel within the anterior pelvic wall (Fig. 52.5a, b). One possible tip is to pass the first suture transparietally, stabilizing and fixing the vascular bundle into the pelvic tunnel to assist the remaining suture. At the end of the procedure is very important to check the floppiness of the wrap and the absence of ischemia of the lower pole of the kidney. No double J stent or abdominal drain is required.
6 Postoperative Care
In the postoperative period, the patient can keep a normal decubitus.
A full oral feeding intake can start few hours after surgery. The analgesic requirement (paracetamol every 6–8 h) is generally limited to the first 24 postoperative hours. All patients are discharged on the second or maximum on the third postoperative day.
7 Results
Laparoscopic vascular relocation was feasible in all cases without open conversion. The median operative time was 95 min (range 45–125 min). The mean hospital stay was 3 days (range 2–4 days).
All patients underwent intraoperative DT in the first stages of laparoscopy, which showed reduction of hydronephrosis after the complete mobilization of the vessels in 45 children. We did not report intraoperative neither postoperative complications in our series of an 11-year period.
All patients had clinical evaluation and a renal US at 1–6 months, and diuretic renogram at 6 months following surgery. Follow-up (range 12–132 months) showed complete resolution of symptoms (pain, haematuria) and decrease in hydronephrosis grade. Although none of the children displayed significant improvement in relative renal function, all of them showed improved drainage on 99mTc-MAG3 renogram and became unobstructed. One had recurrent symptoms of flank pain associated with recurrent pelvic dilatation 18 months after surgery. She underwent successful laparoscopic-AHDP 2 years after the original LVH procedure.
8 Tips and Tricks
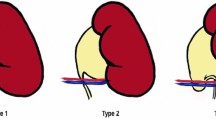
During laparoscopy, each case must be carefully evaluated regarding the presence and position of CV, appearance of the UPJ, ureter course and DT response of the dilated pelvis after vessel displacement. The main criteria to apply VH were the following: (I) hydronephrosis with the presence of obstructing lower pole CV, (II) normal UPJ on inspection and (III) DT response with emptying of the dilated pelvis after vessel displacement in order to confirm release of the obstruction and to exclude intrinsic UPJ anomalies. We divided our patients into two groups on the basis of anatomical relationships between CV, renal pelvis, UPJ and the ureter according to Schneider’s classification [6].
These are the AHDP group with the vessels placed in front of the UPJ which present a really intrinsic stenosis (Schneider’s second type) and the LVH group (45 patients), in which the vessels cross inferiorly the UPJ, resulting in variable ureteral kinking (defined as a ureteral curl or bend around the polar vessels similar to a swan neck ureter), observing intraoperatively peristalsis and demonstrating the absence of intrinsic UPJO (Schneider’s third type). In particular, the very low incidence of relapse suggests that intraoperative DT must be done correctly in every suspected extrinsic UPJO (after CV transposition) to exclude associated intrinsic obstruction.
9 Discussion
Usually UPJO is caused by the presence of an aperistaltic dysplastic segment of the UPJ. Besides this intrinsic aetiology, extrinsic factors, as aberrant lower pole CV, may be the causative factor. Although there are no studies to date, crossing the UPJ by an aberrant vessel may be the most common extrinsic cause of UPJO above all in older children. CV are thought to cause from 40% to over 50% of extrinsic UPJO in adults; they are more often ventrally located than dorsally to the UPJ. These CV are usually normal morphologic vessels of the lower pole segment, which can be divided into additional renal arteries arising from the aorta and accessory renal segment, which can be divided into additional renal arteries arising from the aorta and accessory renal arteries arising from branches of the aorta. The controversy regarding the functional significance of vessels crossing at the UPJ is not a new one, although the debate has been resurrected in recent years because of improved detection due to the advent of advanced imaging techniques such as CT scan and fMRU.
The CV incidence in the aetiology of UPJO in children has been reported to range from 11 to 15% but was as high as 58% in a series of older children with symptomatic UPJO and a history of normal antenatal renal ultrasonography.
Open AHDP is the gold standard procedure to treat UPJO in children, but laparoscopic approach has shown similar outcomes. Laparoscopic pyeloureteral anastomosis in small children remains a challenging task, although robotic pyeloplasty in the last year has been felt to be technically easier. Although some authors have proposed AHDP to exclude intrinsic associated anomalies, an alternative approach to pure extrinsic UPJO is laparoscopic vascular transposition. In literature, there are few published series of laparoscopic relocation of lower pole CV in children with extrinsic UPJO. The most recent series reported by Schneider [6] and Miranda [7] with a successful outcome in up to 95% [8] and by Chiarenza-Bleve, successful in 97% of patients, provided a careful selection of candidates [9]. Meng and Stoller (in 2003) were the first authors reporting vascular relocation using the Hellström technique via laparoscopic approach. They reported this procedure in nine adults, with resolution in all cases. These authors observed that the herniation and subsequent ureteral kinking were responsible for the obstruction and stated that changing the geometry may be enough to alleviate the obstacle [10]. Another important condition is the existence of several anatomic variations as studied by Sampaio [11]. These double vascular bundles form a vascular window and could facilitate a UPJ prolapse with increasing obstruction. Vascular compression in these cases is not in the UPJ but in the proximal ureter. Therefore, the junction is certainly healthy, and correcting the herniation is all that is needed [6, 8]. This observation is supported by histological analysis of the UPJ and CV. Normal muscle density was found and suggests an inherently different UPJ configuration between intrinsic and extrinsic obstruction. Only patients with pure extrinsic UPJO can be treated with this procedure, so any associated intrinsic UPJ abnormality must be ruled out. Some authors, as Janetschek, have recommended that the UPJ should always be explored by a longitudinal incision in order to rule out such associated intrinsic anomalies, which they report in up to 33% of their patients [12]. Some reports analysed the histology of resected UPJ tissue and have showed evidence of intrinsic fibrosis and inflammation in cases where CV was thought to be the aetiology of the obstruction. Lower pole vessels may predispose the UPJ to the narrowing that favours infection or inflammatory episodes or that causes tension and ischemia, thus resulting in fibrosis and stenosis of the urothelium. The presence of this UPJ fibrosis could be one cause of hypothetical failure of the VH procedure [6,7,8,9,10,11,12,13], even though there is no evidence to suggest that the fibrosis is progressive. In addition, electron microscopy studies of extrinsically obstructed UPJ tissue demonstrate no significant structural changes in muscle or collagen content or in nerve distribution, immunohistochemically, when compared to normal controls. Conversely, intrinsically obstructed tissue showed thinning of muscle fascicles with dense collagenous deposits when compared with controls. Careful selection of patients is essential to maintain a high success rate with LVH procedure; it is based on three criteria: preoperative patient selection, accurate diagnostic studies and performance of intraoperative DT to confirm extrinsic obstruction. Preoperatory various imaging modalities have been used, but none have an accuracy of 100% in the diagnosis of pure extrinsic UPJO by CV. Therefore, we believe that an accurate clinical history remains the basis for correct selection. No patients had history of prenatal hydronephrosis. They all presented with intermittent colicky flank pain, sometimes associated with vomiting or haematuria. All showed marked hydronephrosis with a dilated pelvis but relatively mild calyceal dilatation when they were symptomatic that resolved shortly after they became asymptomatic. Godbole [13] reported success with a similar procedure in 12/13 patients with a median age of 10 years; Esposito C, Chiarenza S.F. and Bleve C. et al. were successful in all 51 patients [14]. On our experience, we believe that a success rate >90% may be achieved with LVH procedure, but that close cooperation between surgeon and anaesthesiologists is required to perform the intraoperative diuretic test correctly. The saline bolus needs to be timed so that the renal pelvis is well dilated prior to vessel dissection and mobilization, and with IV furosemide administration, the operator will observe rapid emptying of the bloated renal pelvis, followed by normal ureteral peristalsis and urine passage. If UPJ has intrinsic abnormalities, pelvic dilatation remains even after furosemide administration. The test is crucial because it allows to discriminate a variability of cases that can occur, related to the location of the abnormal vessels and their relations with the ureter and UPJ, the size of the vessels, the presence of hydronephrosis with sufficient tissue to consent the VH (index of the presence of an obstruction), the size of the junction and the presence of ureteral peristalsis. Some authors have suggested the use of pelvic distension with saline by direct puncture of the pelvis or an intraoperative pelvic pressure measurement with laparoscopic visualization prior to ureteral dissection inserting percutaneously into the renal pelvis a needle evaluating the ureteral opening pressure with a column device before and after the procedure was completed [6, 7]. One of the great advantages of the LVH procedure is to preserve the UPJ integrity, eliminating the risk of leakage or urinoma and preserving the physiologic pyeloureteral motility and ureteral peristalsis; in addition operative time is shorter. In several cases, it was possible to observe the pyeloureteral peristalsis after the vessel mobilization. LVH is also particularly indicated and recommended in patients with symptomatic hydronephrosis due to CV in particular anatomic condition as horseshoe kidney. In these cases the UPJ anatomy is disadvantageous to a resection/re-anastomosis between the ureter and renal pelvis [15]. As for the technical point of view, in our mind laparoscopy is the procedure of choice to perform this procedure, but it is important that surgeons have a strong experience. We recommend careful patient selection based on preoperative clinical and radiologic findings that are diagnostic of extrinsic UPJO, combined with intraoperative DT, to confirm the appropriate selection of corrective procedure.
References
Anderson JC, Hynes W. Retrocaval ureter; a case diagnosed pre-operatively and treated successfully by a plastic operation. Br J Urol. 1949;21:209–14.
Von Rokitansky CF. Handbuch der Pathologischen Anatomie. Vienna: Braumüller und Seidel; 1842.
Hellström J, Giertz G, Lindblom K. Pathogenesis and treatment of hydronephrosis. In: Presented at VIII Congreso de la Sociedad International de Urologia, Paris, France; 1949.
Chapman TL. Urology in outline. Edinburgh, London: Churchill Livingstone; 1959. p. 82.
Pesce C, Campobasso P, Costa L, Battaglino F, Musi L. Ureterovascular hydronephrosis in children: is pyeloplasty always necessary? Eur Urol. 1999;36:71–4.
Schneider A, Gomes Ferreira C, Delay C, Lacreuse I, Moog R, Becmeur F. Lower pole vessels in children with pelviureteric junction obstruction: laparoscopic vascular hitch or dismembered pyeloplasty? J Pediatr Urol. 2013;9:419–23.
Miranda ML, Pereira LH, Cavalaro MA, Carvalho Pegolo P, De Oliveira-Filho AG, Murray Bustorff-Silva J. Laparoscopic transposition of lower pole crossing vessels (vascular hitch) in children with pelviureteric junction obstruction: how to be sure of the success of the procedure? J Laparoendosc Adv Surg Tech A. 2015;25:847–51.
Gundeti MS, Reynolds WS, Duffy PG, et al. Further experience with the vascular hitch (laparoscopic transposition of lower pole crossing vessels): an alternate treatment for pediatric ureterovascular ureteropelvic junction obstruction. J Urol. 2008;180:1832–6; discussion 1836.
Chiarenza SF, Bleve C, Fasoli L, et al. Ureteropelvic junction obstruction in children by polar vessels. Is laparoscopic vascular hitching procedure a good solution? Single center experience on 35 consecutive patients. J Pediatr Surg. 2016;51:310–4.
Meng MV, Stoller ML. Hellström technique revisited: laparoscopic management of ureteropelvic junction obstruction. Urology. 2003;62:404–8; discussion 408–9.
Sampaio FJ. The dilemma of the crossing vessel at the ureteropelvic junction: precise anatomic study. J Endourol. 1996;10:411–5.
Janetschek G, Peschel R, Franscher F. Laparoscopic pyeloplasty. Urol Clin North Am. 2000;27:695e704.
Godbole P, Mushtaq I, Wilcox DT, et al. Laparoscopic transposition of lower pole vessels—the ‘vascular hitch’: an alternative to dismembered pyeloplasty for pelvi-ureteric junction obstruction in children. J Pediatr Urol. 2006;2:285–9.
Esposito C, Bleve C, Escolino M, Caione P, Gerocarni Nappo S, Farina A, Caprio MG, Cerulo M, La Manna A, Chiarenza SF. Laparoscopic transposition of lower pole crossing vessels (vascular hitch) in children with pelviureteric junction obstruction. Transl Pediatr. 2016;5(4):256–61.
Bleve C, Bucci V, Conighi ML, Battaglino F, Costa L, Fasoli L, Zolpi E, Chiarenza SF. Horseshoe kidney and uretero-pelvic-junction obstruction in a pediatric patient. Laparoscopic vascular hitch: a valid alternative to dismembered pyeloplasty? Pediatr Med Chir. 2017;39(4):178. https://doi.org/10.4081/pmc.2017.178.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chiarenza, S.F., Bleve, C. (2019). Laparoscopic Management of Extrinsic Ureteropelvic Junction Obstruction (UPJO) by Crossing Vessels. In: Esposito, C., Becmeur, F., Steyaert, H., Szavay, P. (eds) ESPES Manual of Pediatric Minimally Invasive Surgery . Springer, Cham. https://doi.org/10.1007/978-3-030-00964-9_52
Download citation
DOI: https://doi.org/10.1007/978-3-030-00964-9_52
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-00963-2
Online ISBN: 978-3-030-00964-9
eBook Packages: MedicineMedicine (R0)