Abstract
Purpose
To investigate the diagnostic performance of urinary brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) as potential biomarkers for overactive bladder (OAB).
Methods
Ninety women diagnosed with OAB and 45 normal controls without OAB were enrolled. Urine samples were collected from all subjects. Urinary BDNF and NGF levels were measured using enzyme-linked immunosorbent assays. Results normalized by urinary creatinine (Cr) levels were compared between OAB groups and controls. Symptom severity was assessed using overactive bladder symptom score.
Results
Urinary BDNF and NGF levels were elevated in OAB groups but not in controls. Mean (SD) baseline BDNF and NGF levels normalized by Cr levels were significantly higher in OAB subjects than in controls (20.609 ± 23.932 vs. 1.779 ± 0.729, p < 0.01) and (0.258 ± 0.264 vs. 0.081 ± 0.028, p < 0.01), respectively. Urinary BDNF/Cr levels were 80-fold higher than NGF/Cr levels in OAB subjects. Receiver operating characteristic curves for assessing urinary BDNF/Cr levels in OAB groups showed sensitivity and specificity of 93.33 and 88.89 %, respectively. Urinary BDNF levels were associated with OAB symptom severity.
Conclusions
Urinary BDNF/Cr levels are elevated in women with OAB and are significantly associated with symptom severity. No elevation of BDNF is found in women without OAB. BDNF analysis has better sensitivity than NGF in detecting OAB in subjects without other lower urinary tract disorders. Results of the present study suggest a potential role for BDNF as an objective biomarker for OAB diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Overactive bladder (OAB) is one of the most common female urinary tract disorders, and it negatively affects women’s health-related quality of life, leading to anxiety, depression and treatment seeking [1]. The overall prevalence of OAB in women is reported to be 16.9 % in the USA [2].
The International Continence Society (ICS) defines symptomatic OAB as urinary urgency with urinary frequency and nocturia, with or without urge incontinence, in the absence of pathologic or metabolic factors [3]. Clinical diagnosis of OAB depends mainly on self-reported subjective symptoms such as urinary urgency and frequency, although other possible causes of these symptoms must be ruled out. Based on patients’ self-reports alone, it can be difficult to clinically identify patients who have increased bladder sensation or detrusor overactivity from patients who have OAB. While noninvasive urodynamic tests such as residual urine measurement and invasive tests such as cystometry or videourodynamics may provide objective evaluation of the functional status and efficiency of the lower urinary tract [4], the role of urodynamics in the setting of OAB is not well defined [5]. An objective and noninvasive test method to diagnose OAB and assess therapeutic outcomes is still evolving.
Experimental and clinical evidence has suggested a role for neurotrophic factors in the bladder and micturition pathways [6]. Fairly, recent studies have shown that NGF is higher in OAB subjects than in healthy subjects, suggesting that urinary NGF levels may be a potential diagnostic biomarker for OAB syndrome [7–9]. However, determination of NGF by enzyme-linked immunosorbent assay (ELISA) has demonstrated low sensitivity and specificity compared to assays for BDNF in a preliminary observational study [10]. Urinary BDNF levels are exceptionally high in OAB patients compared to normal controls [10] and are also associated with severity of symptoms [11]. The ELISA assay for BDNF analysis has exhibited higher sensitivity and specificity than that for NGF, and thus, BDNF has been suggested to be a more effective potential diagnostic biomarker for OAB [11]. Investigation of other potential diagnostic methods for OAB, including ultrasound measurement of detrusor wall thickness [12], and urine or serum biomarkers such as prostaglandins [13–15], urine cytokines [16] and C-reactive protein (CRP) [17], has not yet resulted in establishing a standard effective measurement. Although neurotrophic factor concentrations show promise as potential biomarkers for OAB, questions still remain about the variability of results among patients with similar complaints, lack of knowledge of urinary levels in normal populations, biochemical mechanisms such as binding to proteins or cells in urine and whether assays are sensitive enough to identify patients with specific pathologies [6]. The purpose of the present study was to investigate the diagnostic performance of urinary BDNF and NGF and determine their potential as objective diagnostic biomarkers for OAB.
Patients and methods
One hundred and thirty-five women from Wuhan Union Hospital were enrolled, including 90 women from the Department of Urology diagnosed with OAB and 45 women without OAB from the Departments of Otorhinolaryngology and Stomatology who served as normal controls. This study was approved by the ethics committee of our hospital. Informed consent was obtained from all subjects before collecting urine samples for measurement of NGF and BDNF and before any treatment was administered.
Study subjects
Included subjects were diagnosed with OAB based on the diagnostic criteria developed by the International Continence Society [3]. OAB subjects who were over age 55 years, pregnant or had bladder tumor, bladder stones, interstitial cystitis, tuberculosis of the bladder, bladder contracture, or diabetes insipidus were excluded. Female patients with OAB had characteristic symptoms, including urinary urgency and urinary frequency but no urge incontinence or cystoscopic findings such as follicular edema, papillary hyperplasia, or chronic inflammatory effects. All female patients with urgency-frequency without urge incontinence were diagnosed as OAB by a 3-day voiding diary and subjective symptoms. Cystoscopies were performed on all OAB patients, and biopsy specimens were taken to the clinical laboratory for pathological examination. Normal controls over age 55 years or who had lower urinary tract symptoms or any other urogenital disease were excluded from the study. Imaging examinations such as Doppler ultrasound, intravascular ultrasound (IVU), or CT were performed in all subjects to exclude occupying lesions or urethral stricture in the urinary tract.
Overactive bladder symptom score (OABSS)
Overactive bladder symptom score (OABSS) measures four symptoms of OAB: daytime frequency of urination, nighttime frequency, urgency and urgency incontinence [18]. Scores are based on secondary analysis of an epidemiologic database, with maximum scores for the four symptoms defined as 2, 3, 5 and 5, respectively. Each score is the sum (0–15) of symptoms of OAB, and higher scores at different levels (0–2, 3–5, 6–9, 9–11 and 12–14) indicate symptom severity, differentiating mild, moderate and severe OAB. OABSS was recorded for each OAB patient in this study when OAB was diagnosed.
Urine examination procedures
Urine samples were collected from the OAB group in the Department of Urology and from the controls in the Departments of Otorhinolaryngology or Stomatology. All urine samples were collected in the morning, and subjects had a strong desire to void. The urine samples were placed on ice immediately and centrifuged at 3,000 rpm at 4 °C for 10 min, and then, the supernatant was separated into aliquots in 1.5-mL tubes and preserved in a freezer at −80 °C. In addition, a 3-mL urine sample was taken to measure urinary creatinine (Cr) level as previously described [7]. Measurement of urinary NGF levels was performed by enzyme-linked immunosorbent assay (ELISA) (Wuhan Boster Biological Technology, Ltd., Wuhan, China) using nondiluted urine samples. Urinary NGF concentration was determined using an immunoassay system (BioTek Instruments, Inc., Winooski, VT, USA) using a specific and highly sensitive ELISA kit, with minimum sensitivity of 1 pg/mL. Assays were performed according to the manufacturer’s instructions. Urinary BDNF concentration was determined using an immunoassay system also, with a specific and highly sensitive ELISA kit, with minimum sensitivity of 2 pg/mL. According to the results of the preliminary experiments, urine specimens were diluted tenfold to fit the detection limit in the ELISA assay. All urine samples were detected in triplicate in the ELISA assays for NGF and BDNF. Total urinary NGF and BDNF levels (pg/mL) were further normalized by the concentrations of urinary creatinine (mg/dL) (NGF/Cr level and BDNF/Cr level), and the ratio of NGF/Cr and BDNF/Cr levels was used as normalized urinary NGF and BDNF levels. Then urinary NGF/Cr and BDNF/Cr levels (pg/mg) were compared between patients with OAB and normal controls as described previously [19].
Statistical analysis
Continuous data, age, are normally distributed and presented as mean and standard deviation (SD); nonnormal distributed continuous variables are presented as median and inter-quartile range (IQR). The two independent samples t test was performed to compare age between groups. Nonparametric Mann–Whitney test was performed to compare OABSS, Cr, BDNF/Cr and NGF/Cr between groups. Correlations between NGF/Cr and BDNF/Cr versus OABSS were performed by Spearman rank correlation coefficient. Receiver operating characteristic (ROC) curves were performed to evaluate the efficiency of OABSS, NGF/Cr and BDNF/Cr in diagnosing OAB as previously described [20, 21]. Higher area under the ROC curves (AUC) indicated higher efficiency in diagnosing OAB. The optimized cut-off points on the ROC curves were determined by Youden’s index (maximum of sensitivity + specificity-1). Statistical analyses were all two-sided, evaluated at the 0.05 level of significant difference and performed using SPSS 15.0 statistics software (SPSS Inc, Chicago, IL).
Results
Comparison of characteristics between subjects with and without OAB
A total of 135 women were enrolled in this study, including 90 subjects diagnosed with OAB and 45 without OAB or other urinary tract symptoms. No significant differences in age (p = 0.526) and Cr (p = 0.847) were observed in patients with and without OAB. Mean age of OAB patients was 36.5 ± 15.8 years (range 17–55 years), and mean age of controls was 38.2 ± 13.5 years (range 19–50 years).
The median OABSS of subjects was 9.0 for the OAB group and 1.0 for the control group (p < 0.001). BDNF/Cr and NGF/Cr levels were increased in the 90 OAB women compared with controls (medians of BDNF/Cr: 11.95 vs. 1.65, p < 0.001; medians of NGF/Cr: 0.15 vs. 0.08, p < 0.001) (Table 1).
Correlations of NGF/Cr and BDNF/Cr with symptom severity (OABSS)
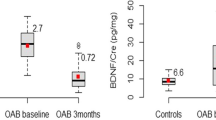
Significantly positive associations were observed between NGF/Cr and BDNF/Cr and symptom severity determined by OABSS. Median NGF/Cr levels were 0.077, 0.075, 0.108, 0.167 and 0.239 in OABSS symptom ranges of 0–2, 3–5, 6–9, 9–11 and 12–14, respectively. A Spearman correlation coefficient of 0.509 indicated a moderate positive correlation between NGF/Cr and OABSS (p < 0.0001). The medians of BDNF/Cr were 1.6, 2.5, 13.5, 14.1 and 13.1 in OABSS symptom ranges of 0–2, 3–5, 6–9, 9–11 and 12–14, respectively. A Spearman correlation coefficient of 0.649 indicated a moderate positive correlation between BDNF/Cr and OABSS (p < 0.0001) (Fig. 1).
Diagnosis for OAB by OABSS, NGF/Cr and BDNF/Cr
The areas under the ROC curve (AUC) of OABSS were significantly higher than those of NGF/Cr and BDNF/Cr (1.0 vs. 0.776 and 0.952, p < 0.0001 and p = 0.0186). The AUC of BDNF/Cr was significantly greater than that of NGF/Cr (0.952 vs. 0.776, p < 0.0001) (Fig. 2).
Table 2 summarizes the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) when using the optimized cut-off points of OABSS, NGF/Cr and BDNF/Cr in diagnosing OAB. OABSS ≥ 3 had the highest sensitivity (100.0) and NPV (100.0 %) compared to NGF/Cr and BDNF/C. BDNF/Cr ≥ 4.6 had the highest specificity (100.0 %) and PPV (100.0 %); sensitivity and NPV were 88.9 and 81.8 %, respectively (Table 2).
Discussion
In the present study, we measured urinary BDNF/Cr and NGF/Cr levels in female patients with OAB and female normal controls without OAB. Results revealed that urinary BDNF/Cr and NGF/Cr levels were both significantly increased in patients with OAB compared to controls, and the increase in BDNF/Cr levels was significantly greater than that of NGF/Cr levels. Furthermore, OABSS revealed significant associations between urinary BDNF/Cr levels and symptom severity in all OAB subjects; BDNF levels were markedly higher in patients with more severe symptoms. In addition, the sensitivity and specificity of urinary BDNF/Cr levels in detecting OAB among known OAB subjects were significantly higher than that of NGF/Cr. However, the specificity of urinary BDNF/Cr in diagnosing OAB must be confirmed in a population that also includes patients with other lower urinary tract disorders. Nevertheless, results of the present study suggest that BDNF may be more sensitive and therefore more useful than NGF as a potential objective biomarker for diagnosis of OAB.
A review study suggests that urinary neurotrophic factors play an important role in the complex pathophysiology of OAB, while urinary prostaglandins, cytokines and CRP are shown to be less specific to the OAB disease process [6]. The search for biomarkers able to identify the presence, severity, progression and response to medical treatment of OAB is ongoing, and prominent in this search is the demonstration, as in the present study, that NGF and BDNF appear to be the most promising biomarkers to date for OAB diagnosis [8, 9, 15, 17].
NGF is produced by bladder urothelium and smooth muscle in the urinary tract, and NGF levels typically increase in these tissues and in the urine of patients with interstitial cystitis, painful bladder syndrome, detrusor overactivity and OAB [8]. Urinary NGF levels were significantly elevated in patients with OAB dry and were even higher in patients with OAB wet compared to patients with interstitial cystitis, painful bladder syndrome and asymptomatic controls [9]. Another study measured urinary NGF and prostaglandin levels in female patients with OAB, finding that urinary NGF and prostaglandin levels were both significantly increased in OAB patients compared to non-OAB controls [13]; analogous results were found in male OAB patients [14]. NGF/Cr levels also increased in patients with interstitial cystitis/bladder pain syndrome and then decreased in patients who responded to treatment [19]. Similarly, urinary NGF/Cr levels were significantly higher in patients with bladder outlet obstruction (OOB) with OAB, but very low in OOB patients without OAB and normal controls, and successful medical treatment decreased the elevated urinary NGF levels to normal and relieved OAB symptoms [22]. Urinary NGF/Cr levels were elevated in patients with both idiopathic and neurogenic detrusor overactivity and significantly decreased in patients who responded to antimuscarinic or botulinum toxin-A treatment compared to nonresponders [23]. Finally, a study comparing possible biomarkers for bladder diseases found that NGF/Cr levels were significantly increased in female patients with interstitial cystitis and painful bladder syndrome compared to women with IBS and normal controls, but increased urinary prostaglandin (PGE2/Cr) levels were not found in all subgroups [15]. These results revealed that urinary NGF/Cr levels but not urinary PGE2/Cr levels are able to differentiate women with interstitial cystitis and painful bladder syndrome from those with frequency-urgency syndrome. However, as effective as NGF appears to be in diagnosing OAB, the sensitivity and specificity of urinary NGF/Cr levels in the diagnosis of OAB were reported to be only 75 and 65.5 %, respectively, which is not sufficient for reliable diagnosis [15]. In the present study, although we showed favorable results for NGF/Cr in identifying patients with OAB, the sensitivity, specificity and positive predictive values of BDNF/Cr were significantly higher than those of NGF/Cr in our OAB sample, suggesting its potential as a reliable biomarker for OAB diagnosis.
BDNF is the most ubiquitous neurotrophin in the human body and is synthesized in small- and medium-sized sensory neurons in dorsal root ganglia [24]. BDNF is also produced by nonneuronal cells in inflamed bladder tissue [25]. Synthesized BDNF is taken up by bladder afferents and undergoes anterograde transport to the central terminals of sensory afferents before being released into the spinal cord where it regulates neuronal function through phosphorylation of spinal TrkB receptors [26]. In a neuropathic pain model, prostaglandins (PGE2) overproduced in injured nerves induced the up-regulation of BDNF in dorsal root ganglion neurons [27]. Neurotrophins are considered to be mediators and modulators of pain; an NGF-dependent mechanism was shown to regulate the expression of BDNF in nociceptors, causing its release as a pain modulator when nociceptors were activated [28]. BGDF synthesis also has been shown to increase significantly in the urinary bladder after chronic cystitis or spinal cord injury [29, 30]. A recent study showed that BDNF sequestration improved bladder function in rats with chronic cystitis [31], demonstrating the effect of BDNF on bladder function in pathological conditions. Urinary BDNF concentration, as with NGF concentration, was noted to be elevated in bladder pain syndrome/interstitial cystitis patients and then significantly reduced after botulinum toxin treatment [32].
Our results are compatible with those of the first comprehensive study of BDNF in healthy volunteers, which showed consistently low levels of BDNF/Cr without regard to age, gender or time of urine sampling, while OAB patients had consistently high BDNF/Cr levels that correlated with symptom severity [11]. In the present study, BDFN/Cr levels were also consistently higher than those of NGF/Cr in the group with OAB. When urinary NGF/Cr levels increased 3.18-fold in OAB patients compared to baseline levels, urinary BDNF/Cr levels increased 11.58-fold. Among controls, urinary mean BDNF/Cr levels were 21.96-fold higher than NGF/Cr levels, and in OAB patients, urinary mean BDNF/Cr levels were 80-fold higher than NGF/Cr levels. In addition, BDNF levels were shown by OABSS to be markedly higher in patients with more severe symptoms. Together, these results along with the high sensitivity and specificity of BDNF/Cr analysis compared to NGF analysis in the OAB subject population suggest that BDNF may possibly be a more reliable diagnostic marker for OAB than NGF.
This study has several limitations, including that the sample is relatively small and from only one institution. Since cross-sectional study may also limit the reliability of urinary results for biomarkers due to possible sample-to-sample fluctuations in individual patients, we ensured that all urine samples in this study were collected in the morning, and total urinary NGF and BDNF levels were normalized by urinary creatinine concentrations (NGF/Cr level and BDNF/Cr level), and ratios of NGF/Cr and BDNF/Cr levels were used as normalized urinary NGF and BDNF levels. This study also focused on NGF and BDNF in OAB patients compared to normal controls without OAB, but patients with other lower urinary tract disorders were excluded, which limits estimations of marker specificity. Therefore, future prospective study must include patients with other lower urinary tract disorders so that BDNF/NGF specificity can be determined. Also, no other potential biomarkers were compared with NGF and BDNF. Although we can say definitively that NGF and BDNF are potential objective biomarkers for OAB, we cannot say definitively that they are superior to any other potential biomarkers or that they can replace current urodynamic measurements. NGF and BDNF are still relatively new prospects in the search for reliable biomarkers for OAB. Further study is needed to evaluate their diagnostic performance compared to other markers as well as imaging and urodynamic parameters, and to better understand their role in OAB.
Conclusions
Urinary BDNF levels are elevated in women with OAB and are significantly associated with symptom severity. No elevation is found in women without OAB. BDNF analysis has better sensitivity than NGF in detecting OAB, suggesting that BDNF may be a potential objective biomarker for OAB diagnosis.
References
Milsom I, Kaplan SA, Coyne KS et al (2012) Effect of bothersome overactive bladder symptoms on health-related quality of life, anxiety, depression, and treatment seeking in the United States: results from EpiLUTS. Urology 80:90
Stewart WF, Van Rooyen JB, Cundiff GW et al (2003) Prevalence and burden of overactive bladder in the United States. World J Urol 20:327
Abrams P, Cardozo L, Fall M et al (2002) The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 21:167
NIDDK (2013) Urodynamic testing. National institute of diabetes and digestive and kidney diseases. http://kidney.niddk.nih.gov/kudiseases/pubs/bcw_ez. Accessed 16 May
Rovner ES, Goudelocke CM (2010) Urodynamics in the evaluation of overactive bladder. Curr Urol Rep 11:343
Steers WD, Tuttle JB (2006) Mechanisms of disease: the role of nerve growth factor in the pathophysiology of bladder disorders. Nat Clin Pract Urol 3:101
Ochodnicky P, Cruz CD, Yoshimura N et al (2011) Nerve growth factor in bladder dysfunction: contributing factor, biomarker, and therapeutic target. Neurourol Urodyn 30:1227
Kuo HC, Liu HT, Chancellor MB (2010) Can urinary nerve growth factor be a biomarker for overactive bladder? Rev Urol 12:e69
Liu HT, Kuo HC (2008) Urinary nerve growth factor level could be a potential biomarker for diagnosis of overactive bladder. J Urol 179:2270
Antunes-Lopes T, Carvalho-Barros S, Cruz CD et al (2011) Biomarkers in overactive bladder: a new objective and noninvasive tool? Adv Urol 2011, Article ID 382431
Antunes-Lopes T, Pinto R, Carvalho-Barros S et al (2011) Urinary levels of brain-derived neurotrophic factor (BDNF) in women with overactive bladder (OAB) syndrome correlate with the severity of symptoms. Eur Urol Suppl 10:277
Kuo HC, Liu HT, Chancellor MB (2010) Urinary nerve growth factor is a better biomarker than detrusor wall thickness for the assessment of overactive bladder with incontinence. Neurourol Urodyn 29:482
Kim JC, Park EY, Seo SI et al (2006) Nerve growth factor and prostaglandins in the urine of female patients with overactive bladder. J Urol 175: 1773; discussion 1776
Kim JC, Park EY, Hong SH et al (2005) Changes of urinary nerve growth factor and prostaglandins in male patients with overactive bladder symptom. Int J Urol 12:875
Liu HT, Tyagi P, Chancellor MB et al (2010) Urinary nerve growth factor but not prostaglandin E2 increases in patients with interstitial cystitis/bladder pain syndrome and detrusor overactivity. BJU Int 106:1681
Ghoniem G, Faruqui N, Elmissary M et al (2011) Differential profile analysis of urinary cytokines in patients with overactive bladder. Int Urogynecol J 22:953
Bhide AA, Cartwright R, Khullar V et al (2013) Biomarkers in overactive bladder. Int Urogynecol J 12 Jan (Epub ahead of print.)
Homma Y, Yoshida M, Seki N et al (2006) Symptom assessment tool for overactive bladder syndrome–overactive bladder symptom score. Urology 68:318
Liu HT, Tyagi P, Chancellor MB et al (2009) Urinary nerve growth factor level is increased in patients with interstitial cystitis/bladder pain syndrome and decreased in responders to treatment. BJU Int 104:1476
Florkowski CM (2008) Sensitivity, specificity, receiver-operating characteristic (ROC) curves and likelihood ratios: communicating the performance of diagnostic tests. Lin Biochem Rev 29(Suppl 1):S83
Mandrekar JN (2010) Receiver operating characteristics curve in diagnostic test assessment. J Thorac Oncol 5:1315
Liu HT, Kuo HC (2008) Urinary nerve growth factor levels are increased in patients with bladder outlet obstruction with overactive bladder symptoms and reduced after successful medical treatment. Urology 72: 104; discussion 108
Liu HT, Chancellor MB, Kuo HC (2009) Urinary nerve growth factor levels are elevated in patients with detrusor overactivity and decreased in responders to detrusor botulinum toxin-A injection. Eur Urol 56:700
Obata K, Noguchi K (2006) BDNF in sensory neurons and chronic pain. Neurosci Res 55:1
Oddiah D, Anand P, McMahon SB et al (1998) Rapid increase of NGF, BDNF and NT-3 mRNAs in inflamed bladder. NeuroReport 9:1455
Di Luca M, Gardoni F, Finardi A et al (2001) NMDA receptor subunits are phosphorylated by activation of neurotrophin receptors in PSD of rat spinal cord. NeuroReport 12:1301
Cruz Duarte P, St-Jacques B, Ma W (2012) Prostaglandin E2 contributes to the synthesis of brain-derived neurotrophic factor in primary sensory neurons in ganglion explant cultures and in a neuropathic pain model. Exp Neurol 234:466
Pezet S, McMahon SB (2006) Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci 29:507
Qiao LY, Vizzard MA (2002) Cystitis-induced upregulation of tyrosine kinase (TrkA, TrkB) receptor expression and phosphorylation in rat micturition pathways. J Comp Neurol 454:200
Qiao LY, Vizzard MA (2005) Spinal cord injury-induced expression of TrkA, TrkB, phosphorylated CREB, and c-Jun in rat lumbosacral dorsal root ganglia. J Comp Neurol 482:142
Pinto R, Frias B, Allen S, Dawbarn D et al (2010) Sequestration of brain derived nerve factor by intravenous delivery of TrkB-Ig2 reduces bladder overactivity and noxious input in animals with chronic cystitis. Neuroscience 166:907
Pinto R, Lopes T, Frias B et al (2010) Trigonal injection of botulinum toxin A in patients with refractory bladder pain syndrome/interstitial cystitis. Eur Urol 58:360
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Long-wang Wang and Xiao-min Han contributed equally to this study.
Rights and permissions
About this article
Cite this article
Wang, Lw., Han, Xm., Chen, Ch. et al. Urinary brain-derived neurotrophic factor: a potential biomarker for objective diagnosis of overactive bladder. Int Urol Nephrol 46, 341–347 (2014). https://doi.org/10.1007/s11255-013-0540-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-013-0540-x