Abstract
Introduction and hypothesis
Nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and other proteins are related to overactive bladder (OAB) syndrome, as their urinary concentrations are significantly different from those of the general non-OAB population. This review aims to systematically assess whether NGF, BDNF, and other urinary by-products can be used as potential biomarkers to manage women with OAB.
Methods
This was a systematic review and metanalysis that was conducted according to PRISMA guidelines. Studies were identified by electronic search of Medline, Scopus, ScienceDirect, Embase, and Cochrane Register until October 2020. The included studies investigated the correlation of OAB with NGF, BDNF, and other potential biomarkers in symptomatic women and their controls.
Results
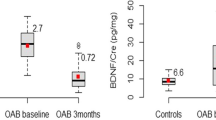
Twelve studies (581 female OAB patients and 394 female controls) were included. Urinary NGF, NGF/Cr, BDNF/Cr, ATP/Cr, and PGE2/Cr ratios were identified as potential biomarkers in female OAB patients. Results of the meta-analysis indicated that uNGF [standard mean difference (SMD) 1.45, 95% CI 0.53–2.36], NGF/Cr ratio (SMD 1.23, 95% CI 0.67–1.78), BDNF/Cr ratio (SMD 0.78, 95% CI 0.006–1.50), and BDNF/Cr ratio (RR 0.78, 95% CI 0.006–1.50) were increased in female OAB patients compared to healthy controls, whereas no difference was found for the PGE2/Cr and ATP/Cr ratios. Current data are inadequate to assess any other potential biomarkers, such as urinary MDA, ATP, and cytokines, in the management of OAB in female patients.
Conclusions
uNGF, NGF/Cr, and BDNF/Cr ratio could be used in the assessment of female OAB patients. Further studies are needed to specify OAB urinary titer levels in OAB subgroups and healthy women and their potential as diagnostic and management tools in OAB women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Overactive bladder (OAB) syndrome, according to the International Continence Society (ICS) and the International Urogynecological Association (IUGA), is a chronic clinical disorder of the urinary bladder that is defined as urinary urgency, usually accompanied by frequency and nocturia, with or without urgency urinary incontinence, in the absence of urinary tract infection or other obvious pathology [1,2,3]. The definition of the term biomarker is anything from simple cells to complicated tissues that can be easily measured and used as an indicator of normal or pathological biological conditions in diagnostic procedures or in evaluating the response to treatment as a prognostic index [4,5,6].
Although several experimental studies had initially reported an association between elevated urinary nerve growth factor (uNGF) levels in lower urinary tract disorders [7], more biomarkers that could be applicable in everyday clinical use for OAB patients have subsequently been studied. Currently, uNGF, urinary brain-derived neurotrophic factor (BDNF), and other substances have been investigated in patients with overactive bladder (OAB) [8,9,10].
NGF is a neurotrophin that regulates the survival and function of neurons and is released from smooth muscle cells and the urothelium [11]. There has been considerable interest in studying NGF in patients’ urine samples, and NGF is increased in OAB patients compared to healthy individuals [12]. However, NGF raised levels may not only be related to OAB; Chen et al. found that uNGF levels were also significantly increased in patients with detrusor underactivity or detrusor overactivity and inadequate contractility and marginally elevated in patients with detrusor overactivity (DO) [13].
Urinary BDNF is another studied biomarker, and its levels were found to be higher in OAB patients and correlated with symptom severity [9]. BDNF/creatinine ratio was persistently low in healthy volunteers, irrespective of gender or time of urine sampling. By contrast, the urinary BDNF/creatinine ratio was significantly higher in OAB patients than in controls [9]. Other reported biomarkers used in OAB assessment are C-reactive protein, cytokines, prostaglandins, adenosine triphosphate (ATP), and oxidate stress markers [14,15,16]. Studies in animals are pioneering the relevant research, as Takagi-Matsumoto et al.’s study in rats also showed that the urinary prostaglandin E2 (PGE2) is increased in OAB [17].
Current literature provides unclear information about the significance of biomarkers in the OAB female patient. Apart from pregnancy-induced changes, delivery, and menopause, bad urinary habits, recurrent urinary infections, and pelvic organ prolapse, among other minor clinical entities, are unique to or much more frequent in women: prostatic disease is usually the underlying pathology in men. Therefore, an evaluation of the potential OAB biomarkers’ performance in a strictly female population could indicate their possible use in clinical conditions unique in females.
This systematic review attempts to summarize the role of urinary NGF, BDNF, and other potential biomarkers in the diagnosis and management of OAB in women.
Materials and methods
This systematic review followed the Preferred Reported Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines [18]. A protocol was submitted and registered at the international prospective register of systematic reviews (PROSPERO) with registration number CRD42018095429 (http://www.crd.york.ac.uk/PROSPERO). Ethical approval was not required.
Study search
The primary studies were identified by electronic search of Medline (via Pubmed), Scopus, ScienceDirect, Embase (via Ovid), and the Cochrane Library (via Ovid) from inception till October 2020. The following MeSH and non-MeSH terms were used: ‘overactive bladder,’ ‘urinary incontinence,’ ‘pelvic organ prolapse,’ ‘urgency,’ ‘detrusor overactivity,’ ‘nocturia,’ and ‘bladder outlet obstruction’ and were combined with each one of the investigated biomarkers. The identified biomarkers which were used in the further literature search were: ‘nerve growth factor-NGF,’ ‘brain-derived neurotrophic factor-BDNF,’ ‘prostaglandin E2PGE2,’ ‘adenosine triphosphate-ATP,’ ‘cytokines,’ and ‘C-reactive protein-CRP.’ An additional search was also made using their abbreviations. We also manually searched the reference lists of all included articles and any relevant review articles. Articles published from database inception till September 2020 were included in the review.
Study selection
The PICO criteria were applied for the selection of studies.
-
1.
Participants: adult women with idiopathic OAB symptoms (urinary frequency, urgency, urgency urinary incontinence (UUI), and duration of symptoms > 3 months;
-
2.
Intervention: the measurement of a biomarker in the urine during initial diagnosis or after treatment;
-
3.
Comparators: healthy women or female patients with lower urinary tract symptoms (LUTS) but no-OAB symptoms; and
-
4.
Outcomes: the concentrations of the biomarkers in the study subgroups.
We excluded studies not written in English, case reports and/or case series reporting on less than ten patients, and all the gray literature (conference abstracts, letters to editors, pre-prints of articles).
One of the authors (ST) performed a systematic search of the literature to map the different diagnostic tests and their characteristics. Two reviewers (ST, TM) independently reviewed and documented all the identified studies by title and/or abstract. The identified studies were divided into three groups: ‘include,’ ‘exclude,’ or ‘unsure.’ Those classified as ‘include’ or ‘unsure’ were marked, and their full-text versions were retrieved for a definitive assessment of eligibility. At that stage, only those titles classified by both reviewing authors as ‘exclude’ were excluded. The articles that were finally considered eligible for further assessment were selected after the consensus of the two reviewers (ST, TM). We used Mendeley software as reference management to eliminate any duplicates.
Eligibility criteria
Inclusion criteria of the OAB patients were: (1) studies reporting on pure OAB populations suffering from urgency and/or urge urinary incontinence, and/or urodynamically proven DO and/or no improvement following initial antimuscarinic treatment; (2) adequate description of the laboratory process for the measurement of the biomarkers. Inclusion criteria of controls were: (1) recruited subjects were free of OAB symptoms, LUTS, or any other pelvic pathology; (2) adequate description of the laboratory process to measure the biomarkers. Exclusion criteria were: (1) studies where there was no clear separate report of the results for the female group; (2) studies dealing with other LUTS, such as urinary tract infection, interstitial cystitis, or cancer.
Data extraction and data items
Data were independently extracted by two reviewers (ST and TM) using a predefined form for data extraction, which was previously piloted. The variables assessed were study author, year and location of the study, study design, sample size, presenting symptoms, method of assessment of the patients, biomarker studied, biomarker measurement procedure, and biomarker levels. Any discrepancies between the reviewers were resolved by discussion. Outcomes were defined according to the use of the biomarker and its measurement procedure.
Quality assessment
As there were no randomized controlled trials (RCTs) found and the Cochrane Risk of Bias assessment tool could not be used [19], ST and TM independently assessed the methodological quality of the included studies based on a modified version of the Newcastle-Ottawa Scale (NOS) [20] checklists for observational studies, a risk of bias assessment tool that is recommended by the Cochrane Collaboration [21]. This evaluation is important as low-quality studies are highly likely to present biased results. Quality of the reporting’s results was collected in a specific data collection form. Any disagreement was resolved by discussion. In both cohort and case-control studies, NOS consists of three categories with eight parameters, and the total maximum score of these is nine. We considered a study that scored ≥ 7 as a high-quality study since a standard criterion for evaluation has not yet been established.
Data synthesis
All data were collected with Microsoft Office Excel, and the search results were combined for meta-analysis with Revman Software (version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011). For the analysis, the studies were combined into groups according to the studied biomarker as either a diagnostic tool or a treatment outcome predictor in female OAB patients. The continuous data were expressed as standardized mean differences (standard mean difference, SMDs) with 95% confidence intervals (CIs). Τhe method given by the Tiejun Tong group was used to calculate the closest approximation of mean and standard deviation (SD) from median and interquartile range (IQR) [22, 23]. Also, the use of the I2 index was employed to indicate heterogeneity between studies that could not be attributed to chance, with I2 ≥ 50% indicating significant heterogeneity. The overall effect was also calculated. Statistical significance was set at a P-level of 0.05. For each comparison, random-effects model analyses were conducted with continuous data (mean difference).
Results
Study selection
The initial study search identified 988 studies. Of these, 12 studies were included in the qualitative synthesis and the quantitative analysis: 975 women were included in these studies (581 women with OAB and 394 controls). Figure 1 shows the flow diagram of the literature identification and selection process.
Study characteristics
All the studies included in the review were observational and prospective: one was a cross-sectional study [24], two were cohort studies [7, 25], and nine were case controls [9, 10, 26,27,28,29,30,31,32]. No relevant randomized clinical trials were found. Table 1 summarizes the characteristics of the included studies. The studies were geographically diverse, originating from China, South Korea, Taiwan, the USA, Canada, UK, and Portugal. Eight of the studies used uNGF and/or BDNF to investigate the diagnosis and pathogenesis of OAB [10, 24, 26, 27, 29,30,31,32] and four to evaluate the response to OAB therapy [7, 9, 28, 33]. In 11 reports, the urinary NGF/Cr ratio was studied as a potential biomarker in OAB; in four of these studies, the urinary NGF/Cr ratio was measured before and after antimuscarinic treatment. There was only one study where urinary NGF was used without normalization against the concentration of the urinary creatinine [31, 32]. Urinary BDNF/Cr ratio was measured in four studies [9, 10, 24, 27], three as a biomarker in diagnosing OAB [10, 24, 27] and one in correlation with the response to treatment [8]. The ATP/Cr was measured in two studies, evaluating its relationship with the pathogenesis of OAB [26, 29]. The PGE2/Cr was studied in two studies, in diagnosis and regarding treatment response [26, 30]. Other biomarkers, such as HB-EGF/Cr ratio, MDA, and cytokines, were each reported in only one study [14,15,16]. Follow-up time varied between 18 and 72 weeks. All studies had a full physical examination during the selection process, and the majority used a 3-day bladder diary and several standardized questionnaires.
Qualitative assessment of the studies
Cohort studies scored seven stars each, indicating a moderate quality (7.33). Antunes-Lopes et al. reached the highest score of nine among the case-control studies, indicating high quality [9, 10, 26,27,28,29,30,31,32]. (Supplementary Tables 1 and 2).
Synthesis of the results
The uNGF levels ranged from 5.66 ± 9.1 to 66.8 ± 94.9 and from 0.48 ± 0.84 to 2.45 ± 6.57 in 280 OAB patients and 142 controls, respectively; differences were statistically significant in two out of three studies [7, 21, 32]. The uNGF/Cr ratio levels ranged from 0.21 ± 0.23 to 488.59 ± 591.80 and from 0.07 ± 0.21 to 188.30 ± 290.20 in 762 OAB patients and 481 controls, respectively. There were significantly increased uNGF/Cr concentrations in 7 out of 11 of these studies [9, 10, 24,25,26,27,28,29,30,31,32].
The urinary BDNF/Cr ratio was differently measured in the four studies and ranged from 4 ± 4.1 to 1627.25 ± 1119.95 and from 1.66 ± 0.58 to 859.58 ± 835.35 in 188 OAB patients and 115 controls, respectively. In three out of four studies, BDNF/Cr ratio levels were significantly higher in female OAB patients [9, 10, 27]. However, in one study, healthy controls had higher concentrations compared to the female OAB group [24].
The urinary PGE2/Cr ratio was studied in two studies and ranged from 7.2 ± 1.7 to 475.9 ± 526.7 and from 1.6 ± 0.4 to 237.2 ± 91.7 in 50 OAB patients and 35 controls, respectively [26, 30]. Although the PGE2/Cr ratio levels were higher in female OAB patients in both studies, only one study achieved statistical significance [30].
ATP/Cr ranged from 13.2 ± 8.8 to 27.5 ± 8.3 and from 7.2 ± 1.7 to 9.4 ± 8.01 in 54 OAB patients and 56 healthy controls, respectively [26, 29]. In both studies, the ATP/Cr ratio levels were higher in female OAB patients, but one study demonstrated statistical significance [29] (see Table 1).
Meta-analysis results
Sufficiently similar studies were available for separate meta-analyses for the outcome of uNGF, NGF/Cr, and BDNF/Cr with statistically significant results. Meta-analysis results are shown in Table 2. Forest plots for the meta-analyses are shown in Fig. 2.
The results of the meta-analysis indicated that uNGF (SMDs 1.45, 95% CI 0.53–2.36) [7, 31, 32], NGF/Cr ratio (SMDs 1.23, 95% CI 0.67–1.78) [9, 10, 24,25,26,27,28,29,30,31,32], and BDNF/Cr ratio (SMDs 0.78, 95% CI 0.006–1.50) [9, 10, 24, 27] were statistically increased in the female OAB patients compared to healthy controls (Fig. 2a, b, and c). The between-study heterogeneity in each performed meta-analysis was low, as demonstrated by low I2 values. There was no statistical difference in the PGE2/Cr ratio and ATP/Cr ratio in female OAB patients compared to controls (Fig. 2d and e) [26, 29, 30]. Current data are inadequate to assess any other potential biomarkers, such as urinary MDA, ATP, cytokines, serum PGE2, and C-reactive protein, in the management of OAB in female patients [14,15,16, 35, 36].
Conflict of interest and sponsorship source
Ethics approval was obtained in all studies. Only three studies declared their conflicts of interest and commercial relationships [7, 9, 24]. Five studies were sponsored or funded, and in two, the role of the financial support in study design and execution was explained [9, 24, 25, 28, 29].
Discussion
Our meta-analysis findings show that uNGF, NGF/Cr ratio, and BDNF/Cr are significantly higher in female OAB patients compared to healthy controls; on the other hand, ATP/Cr and PGE2/Cr ratio were not significantly different between these two groups, but only two studies were available for analysis. These results indicate that uNGF, NGF/Cr ratio, and BDNF/Cr ratio could be used as potential biomarkers in the diagnostic approach to female OAB patients along with all the other existing diagnostic OAB modalities (i.e., LUTS history, bladder diary, standardized questionnaires, urodynamics).
This study tried to quantitatively review the existing literature on the role of biomarkers in female OAB patients. Our findings are in full agreement with the published literature. Previous studies in mixed male and female OAB patient populations also showed that urinary NGF levels, uNGF/Cr ratio, and BDNF/Cr ratio are increased at initial diagnosis and decrease after appropriate OAB treatment [12]. The levels of these potential biomarkers were also found to correlate with the severity of OAB symptoms and lower urinary tract dysfunction associated with other conditions such as bladder pain syndrome [37]. The normalization of biomarker levels in OAB patients after therapeutic interventions could signify their potential application in the management strategy of OAB patients. These observations can be explained by the active role of NGF and BDNF in bladder physiology. Both are elicited by the urothelium and the detrusor muscle and are involved in mechanisms for the regulation of nerve cells of the bladder. In OAB, the function of the afferent and efferent neurons is impaired, leading to the generation of urgency symptoms. Increased release of both NGF and BDNF accompanies the symptomatology of OAB, supporting their role in bladder pathophysiology [35].
The next issue that this study examines is why anybody should expect different findings in the concentrations of potential biomarkers in female OAB patients compared to men with OAB. Current knowledge does not support that different OAB pathophysiology is associated with differences in response to treatment and/or in the clinical course of the disease in patients of different sex. In the EPIC study, the incidence of OAB in men has been reported to be 10.8% and in women 12.8% [38]. However, the underlying potential mechanisms relating to the development of OAB symptoms may be quite different in female patients. Pregnancy and delivery-induced pelvic floor changes as well as the abrupt arrest of estrogen production at menopause are exclusively gender-dependent prerequisite factors for OAB. Moreover, bad urinary habits, recurrent urinary tract infections (UTI), and pelvic organ prolapse are pre-existing conditions that are much more frequent in females with OAB compared to men with OAB.
Urinary habits appear to have a bidirectional relationship with OAB symptoms. It is not unusual for many healthy women to exercise pre-emptively voiding when the opportunity arises or initiate voiding by abdominal straining in ‘convenient’ circumstances. It is well known that the voiding habits of women may play a role in LUTS development and progression, whereas, on the other hand, women modify their voiding behaviors to adapt to LUTS [39].
Recurrent UTIs are related to OAB symptoms because they cause voiding dysfunction patterns with large post-void residual (PVR) of urine, increased voiding pressure, and impaired urothelial regeneration. Moreover, because of the bladder intracellular bacterial colonization, the altered diversity of urinary microbiota, and the possible decreased host immunity in women with recurrent UTI, the bladder barrier function is impaired, the urothelium shows increased apoptosis, and the sensory activity of the bladder is increased. Therefore, women with OAB symptomatology may subsequently develop recurrent UTIs [40].
Finally, pelvic organ prolapse can lead to OAB symptoms because it primarily causes bladder outlet obstruction, a condition related to denervation of the obstructed bladder, changes in detrusor muscles, and alterations in spinal micturition reflexes [41].
Further research should enlighten the relationships between these pathophysiological processes, the role of the biomarkers in the development of these symptoms, and the potential use of them in the initial diagnostic steps of female OAB patients as well as during their treatment as prognostic markers.
NGF as biomarker
In our analysis of published results, uNGF measurements range from 5.7 ± 9.1 to 66.8 ± 94.9 pg/mg in female OAB patients and from 0.5 ± 0.8 to 2.4 ± 6.6 pg/mg in healthy control groups. Levels of uNGF were significantly higher in the OAB group. Similarly, the meta-analysis of Qu et al. showed that patients with OAB symptoms had a higher uNGF level than healthy volunteers (MD = 31.74, 95% CI = 9.48–54.00, P = 0.005) [35]. In the same study, the patients with OAB-wet symptoms had a higher uNGF or uNGF/Cr level than patients with OAB-dry symptoms. Liu et al. were the first team to report a clear difference of NGF and uNGF/Cr levels between a group of 26 female OAB patients and 31 healthy controls [31]. Cho et al. confirmed these results showing in their prospective case-control study that the uNGF/Cr ratio was significantly increased in the OAB patients [30]. Antunes-Lopes et al. found lower values of sensitivity and specificity for NGF/Cr ratio (> 200 pg/mg), with an area under the curve in receiver-operator characteristic (ROC) analysis of 0.68 [42]. Interestingly, in OAB patients with a high urinary concentration of NGF, Birder et al. did not find similar increases in bladder biopsies obtained from the same group of patients [36]. Similarly, Mossa et al. studied the role of uNGF/Cr, PGE2/Cr, and ATP/Cr in a prospective case-control study: no difference was found between OAB patients and healthy controls, a finding which is challenging to the usefulness of these biomarkers in the diagnostic approach to OAB [26].
Another challenge to the clinical application of uNGF/Cr as a biomarker is the wide range of values of the absolute measurement levels. Caution has been raised about the validity of the NFG measurements with the used assay kit [45]. Several reports have questioned the specificity of the NGF kits due to concerns that the precursor of NGF can interfere with NGF detection and result in false-positive results [46]. The extreme differences in average values of uNGF/Cr make its use problematic in the current clinical practice. Therefore, the clinical explanation of any uNGF/Cr measurements should be in the context of a comparison between different clinical groups. Moreover, there were a few studies where normalization with urinary creatinine was not performed; this made the synthesis and the between-study comparison more difficult. Lastly, the study participants were poorly aged-matched between the two groups, a fact which weakens the included studies’ results and further hampers the interpretation of our findings.
BDNF as a biomarker
According to our results, BDNF/Cr measurements range from 4.0 ± 4.1 to 1627 ± 1119 pg/mg in the female OAB patients and from 4.7 ± 5.4 to 859 ± 835 pg/mg in the healthy control groups. The BDNF/Cr ratio appears to be significantly higher in the OAB group. Wang et al. studied 90 women with urgency/frequency symptoms and reported that BDNF has higher sensitivity than NGF in detecting OAB patients without other lower urinary tract disorders. The ratio of uBDNF/Cr in this study was higher in the OAB female patients compared to healthy controls [10]. Antunes-Lopes et al. performed a controlled study on 37 female treatment-naive OAB patients and found that the baseline BDNF/Cr levels were significantly increased in the OAB group. Moreover, there was a significant decrease in urinary BDNF/Cr ratio after the 3-month lifestyle intervention and/or 3-month anticholinergic treatment compared to baseline levels [9]. Another study from Tian et al. confirmed the above findings, reporting significantly increased BDNF/Cr levels in a group of 30 female OAB patients compared to healthy controls [27]. However, Pennycuff et al. studied 38 female OAB patients and found no difference in BDNF/Cr levels between the study group and an age-matched control group of postmenopausal women. This study described that neurotrophins increase along with the age of the patient, not being related to any underlying pathology [24]. A ROC analysis has been performed in the studies mentioned above, evaluating the biomarkers’ diagnostic value, where the AUC for NGF ranged between 0.69 and 0.79 and for BDNF between 0.67 and 0.95. Both neurotrophins showed mostly a ‘poor’ to ‘fair’ diagnostic performance, except for the study by Wang et al., who showed high sensitivity and specificity for BDNF and reported the highest AUC (0.95) [14].
Little is known about the role of BDNF in bladder function, in both normal and pathological conditions, and the available studies are mostly based on experimental non-human or in vitro studies. Other studies not included in our review have similar findings. Pinto et al. studied uBDNF levels in bladder pain syndrome/interstitial cystitis patients who were treated with intravesical botulinum toxin (BoNT-A) injection. The initially high uBDNF levels decreased significantly after the BoNT-A injection, supporting a potential correlation between uBDNF change and LUTS improvement [13].
These promising results are weakened by the variety of kits used in the various studies, the small sample sizes, and the small number of relevant studies (only 4). BDNF’s diagnostic performance in women with OAB thus needs to be demonstrated in further studies [10, 14, 42].
Other biomarkers
Apart from NGF and BDNF, several other potential biomarkers (other neurotrophic factors, cytokines, CRP, and prostaglandins) have been studied to evaluate their use in the management of OAB patients [15, 16, 43]. Bladder urothelium and smooth muscle seem to respond to chemical and inflammatory stimuli by secreting various other substances, such as ATP, nitric oxide, and acetylcholine [44]. These factors’ release may interact with the detrusor muscle eliciting intramolecular alterations that may ultimately decrease the detrusor contractility threshold.
Inflammatory compounds have been implicated in OAB pathogenesis and severity. Ghoniem et al. have hypothesized that pro-inflammatory cytokines and chemokines are released in excess from the bladder mucosa, promoting bladder overactivity in OAB patients based on their findings that cytokines are differently expressed in OAB. The association of chemokines in other inflammatory diseases, such as rheumatoid arthritis, has now been studied in chronic pelvic pain patients, and it is thought that their fluctuation may be linked to OAB [15].
Hegele et al. reported significant PGE2 levels in OAB patients’ serum compared to healthy controls; in particular, PGE2 levels were increased in the OAB-wet compared to the OAB-dry group. Furthermore, they demonstrated a correlation of serum PGE2 levels with the severity of OAB, supporting its potential use at follow-up after therapy [34]. NGF, BDNF, and PGE2 seem to have essential roles in developing OAB symptoms in women [7, 10, 25, 28, 30]. Further studies with many female OAB patients should allow clinicians to associate those compounds with OAB better.
There is intense ongoing research about health conditions related to the potential increase of the concentrations of urinary mediators. As early as 2009, Liu et al. published a series of 143 individuals with idiopathic detrusor overactivity and 100 individuals with neurogenic detrusor overactivity and found that uNGF could serve as a sensitive biomarker for the diagnosis of both conditions [46]. Yamauchi et al. investigated the potential role of PGE2, NGF, and substance P in patients with suprapontine diseases and OAB symptomatology and found that urinary PGE2 was putatively elevated in these subjects [47]. Interestingly, it appears that uNGF/Cr may serve as a guide for therapy and improve outcomes in the treatment of children with non-monosymptomatic nocturnal enuresis [48]. Thus, a number of LUT-independent conditions are related to the presence of increased mediators in the urine. As this study was a review, attention was paid to the use of strict inclusion/exclusion criteria, so as not to measure results from studies that included individuals with non-idiopathic OAB symptoms.
Strengths and limitations
This is the first comprehensive systematic literature review, to our knowledge, which studies the use of biomarkers in female OAB patients. All 12 studies included in this review were prospective in design but without a long-term follow-up to increase the studies’ validity. Our review highlights the need for additional high-quality clinical studies of biomarkers in OAB patients, particularly women. Our findings also highlight the lack of a precise clarification of the studied subjects, as female and male patients with OAB may have different outcomes. Gender-specific differences may exist in OAB symptoms, related bother, and quality of life and could be evaluated in correlation with the use of urine biomarkers [49].
The robustness of the results of this review is limited by the quality of included studies, as no randomized trial of biomarkers in OAB women was found. Observational research involves a large possibility of selection bias and has low statistical strength. Moreover, our analysis showed high heterogeneity between studies, and the numbers of patients studied were small, which may reduce the overall reliability of results.
Conclusions
Our analysis suggests that uNGF, NGF/Cr ratio, and BDNF/Cr ratio levels could be potential biomarkers for assessing OAB in women. However, there are still limitations, including lack of randomization in most published studies, controversy concerning the available kits, and insufficient data on the sensitivity and specificity of these biomarkers. Further studies are warranted to provide more robust data on the association of these urinary biomarkers with various management options in OAB patients. Gender-specific research and research on various OAB etiologies could highlight further diagnostic and management aspects of urine biomarkers in OAB.
References
Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Wein A, et al. Standardisation Sub-Committee of the International Continence Society. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61(1):37–49. https://doi.org/10.1016/s0090-4295(02)02243-4.
Haylen BT, Freeman RM, Swift SE, Cosson M, Davila GW, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS)joint terminology and classification of the complications related directly to the insertion of prostheses (meshes, implants, tapes) & grafts in female pelvic floor surgery. Int Urogynecol J. 2011;22:3–15. https://doi.org/10.1007/s00192-010-1324-9.
Drake MJ. Do we need a new definition of the overactive bladder syndrome? ICI-RS 2013. Neurourol Urodyn. 2014;33(5):622–4. https://doi.org/10.1002/nau.22609.
Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010;5(6):463–6. https://doi.org/10.1097/COH.0b013e32833ed177.
Bhide AA, Cartwright R, Khullar V, Digesu GA. Biomarkers in overactive bladder. Int Urogynecol J. 2013;24(7):1065–72. https://doi.org/10.1007/s00192-012-2027-1 Erratum in: Int Urogynecol J 2013;24(10):1775–6.
Cruz CD. Neurotrophins in bladder function: what do we know and where do we go from here? Neurourol Urodyn. 2014;33(1):39–45. https://doi.org/10.1002/nau.22438.
Vijaya G, Cartwright R, Derpapas A, Gallo P, Fernando R, Khullar V. Changes in nerve growth factor level and symptom severity following antibiotic treatment for refractory overactive bladder. Int Urogynecol J. 2013;24(9):1523–8. https://doi.org/10.1007/s00192-012-2038-y.
Kuo HC. Potential biomarkers utilized to define and manage overactive bladder syndrome. Low Urin Tract Symptoms. 2012;4(Suppl 1):32–41. https://doi.org/10.1111/j.1757-5672.2011.00131.x.
Antunes-Lopes, Antunes-Lopes T, Pinto R, Barros SC, Botelho F, Silva CM, et al. Urinary neurotrophic factors in healthy individuals and patients with overactive bladder. J Urol. 2013;189(1):359–65. https://doi.org/10.1016/j.juro.2012.08.187.
Wang LW, Han XM, Chen CH, Ma Y, Hai B. Urinary brain-derived neurotrophic factor: a potential biomarker for objective diagnosis of overactive bladder. Int Urol Nephrol. 2014;46(2):341–7. https://doi.org/10.1007/s11255-013-0540-x.
Ochodnický P, Cruz CD, Yoshimura N, Michel MC. Nerve growth factor in bladder dysfunction: contributing factor, biomarker, and therapeutic target. Neurourol Urodyn. 2011;30(7):1227–41. https://doi.org/10.1002/nau.21022.
Seth JH, Sahai A, Khan MS, van der Aa F, de Ridder D, Panicker JN, et al. Nerve growth factor (NGF): a potential urinary biomarker for overactive bladder syndrome (OAB)? BJU Int. 2013;111(3):372–80. https://doi.org/10.1111/j.1464-410X.2012.11672.x.
Chen SF, Jiang YH, Kuo HC. Urinary biomarkers in patients with detrusor underactivity with and without bladder function recovery. Int Urol Nephrol. 2017;49(10):1763–70. https://doi.org/10.1007/s11255-017-1666-z.
Rada MP, Ciortea R, Măluţan AM, Doumouchtsis SK, Bucuri CE, Clim A, et al. The profile of urinary biomarkers in overactive bladder. Neurourol Urodyn. 2020;39(8):2305–13. https://doi.org/10.1002/nau.24487.
Ghoniem G, Faruqui N, Elmissiry M, Mahdy A, Abdelwahab H, Oommen M, Abdel-Mageed AB. Differential profile analysis of urinary cytokines in patients with overactive bladder. Int Urogynecol J. 2011 Aug;22(8):953–961. https://doi.org/10.1007/s00192-011-1401-8. Erratum in: Int Urogynecol J. 2011 Aug;22(8):1069.
Chung SD, Liu HT, Lin H, Kuo HC. Elevation of serum c-reactive protein in patients with OAB and IC/BPS implies chronic inflammation in the urinary bladder. Neurourol Urodyn. 2011;30(3):417–20. https://doi.org/10.1002/nau.20938.
Takagi-Matsumoto H, Ng B, Tsukimi Y, Tajimi M. Effects of NSAIDs on bladder function in normal and cystitis rats: a comparison study of aspirin, indomethacin, and ketoprofen. J Pharmacol Sci. 2004;95(4):458–65. https://doi.org/10.1254/jphs.fp0040098.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–94. https://doi.org/10.7326/0003-4819-151-4-200908180-00136.
Viswanathan M, Ansari MT, Berkman ND, Chang S, Hartling L, McPheeters M, Santaguida PL, Shamliyan T, Singh K, Tsertsvadze A, Treadwell JR (2008) Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions. 2012 Mar 8. In: Methods Guide for Effectiveness and Comparative Effectiveness Reviews [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US)
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [assessed 03 Feb 2021]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Higgins J, Green S (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. http://www.cochrane-handbook.org
Shi J, Luo D, Weng H, Zeng XT, Lin L, Chu H, et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods. 2020;11(5):641–54. https://doi.org/10.1002/jrsm.1429.
Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–805. https://doi.org/10.1177/0962280216669183.
Pennycuff JF, Schutte SC, Hudson CO, Karp DR, Malykhina AP, Northington GM. Urinary neurotrophic peptides in postmenopausal women with and without overactive bladder. Neurourol Urodyn. 2017;36(3):740–4. https://doi.org/10.1002/nau.23011.
Suh YS, Ko KJ, Kim TH, Lee HS, Sung HH, Cho WJ, et al. Urinary nerve growth factor as a potential biomarker of treatment outcomes in overactive bladder patients. Int Neurourol J. 2017;21(4):270–81. https://doi.org/10.5213/inj.1732794.397.
Mossa AH, Cammisotto PG, Shamout S, Campeau L. Imbalance of nerve growth factor metabolism in aging women with overactive bladder syndrome. World J Urol. 2020. https://doi.org/10.1007/s00345-020-03422-6.
Tian XJ, Liu C, Liu K, Tang SY. Urinary biomarkers of overactive bladder. Chin Med J. 2019;132(9):1104–6. https://doi.org/10.1097/CM9.0000000000000214.
Kim SR, Moon YJ, Kim SK, Bai SW. NGF and HB-EGF: potential biomarkers that reflect the effects of fesoterodine in patients with overactive bladder syndrome. Yonsei Med J. 2015;56(1):204–11. https://doi.org/10.3349/ymj.2015.56.1.204.
Silva-Ramos M, Silva I, Oliveira O, Ferreira S, Reis MJ, Oliveira JC, et al. Urinary ATP may be a dynamic biomarker of detrusor overactivity in women with overactive bladder syndrome. PLoS One. 2013;8(5):e64696. https://doi.org/10.1371/journal.pone.0064696.
Cho KJ, Kim HS, Koh JS, Kim JC. Changes in urinary nerve growth factor and prostaglandin E2 in women with overactive bladder after anticholinergics. Int Urogynecol J. 2013;24(2):325–30. https://doi.org/10.1007/s00192-012-1854-4.
Liu HT, Kuo HC. Urinary nerve growth factor level could be a potential biomarker for diagnosis of overactive bladder. J Urol. 2008;179(6):2270–4. https://doi.org/10.1016/j.juro.2008.01.146.
Liu HT, Chen CY, Kuo HC. Urinary nerve growth factor in women with overactive bladder syndrome. BJU Int. 2011;107(5):799–803. https://doi.org/10.1111/j.1464-410X.2010.09585.x.
Suh YS, Ko KJ, Kim TH, Lee HS, Sung HH, Cho WJ, et al. Potential biomarkers for diagnosis of overactive bladder patients: urinary nerve growth factor, prostaglandin E2, and adenosine triphosphate. Int Neurourol J. 2017;21(3):171–7. https://doi.org/10.5213/inj.1732728.364.
Hegele A, Knippschild S, Frohme C, Hänze J, Olbert P, Hofmann R. Changes in prostaglandin E2 in patients with idiopathic overactive bladder syndrome after botulinum toxin type a treatment: is there a clinical benefit? BMC Urol. 2014;14:85. https://doi.org/10.1186/1471-2490-14-85.
Qu HC, Yan S, Zhang XL, Zhu XW, Liu YL, Wang P. Urinary nerve growth factor levels could be a biomarker for overactive bladder symptom: a meta-analysis. Genet Mol Res. 2014;13(4):8609–19. https://doi.org/10.4238/2014.April.14.14.
Birder LA, Wolf-Johnston A, Griffiths D, Resnick NM. Role of urothelial nerve growth factor in human bladder function. Neurourol Urodyn. 2007;26(3):405–9. https://doi.org/10.1002/nau.20372.
Magalhaes TF, Baracat EC, Doumouchtsis SK, Haddad JM. Biomarkers in the diagnosis and symptom assessment of patients with bladder pain syndrome: a systematic review. Int Urogynecol J. 2019;30(11):1785–94. https://doi.org/10.1007/s00192-019-04075-9.
Irwin DE, Milsom I, Hunskaar S, Reilly K, Kopp Z, Herschorn S, Coyne K, Kelleher C, Hampel C, Artibani W, Abrams P. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol 2006;50(6):1306–1314; discussion 1314-5. https://doi.org/10.1016/j.eururo.2006.09.019.
Palmer MH, Wu JM, Marquez CS, Rupp B, Conover MM, Newman DK. A secret club: focus groups about women’s toileting behaviors. BMC Womens Health. 2019 Mar 7;19(1):44. https://doi.org/10.1186/s12905-019-0740-3.
Ke QS, Lee CL, Kuo HC. Recurrent urinary tract infection in women and overactive bladder - is there a relationship? Tzu Chi Med J. 2020;33(1):13–21. https://doi.org/10.4103/tcmj.tcmj_38_20.
de Boer TA, Salvatore S, Cardozo L, Chapple C, Kelleher C, van Kerrebroeck P, et al. Pelvic organ prolapse and overactive bladder. Neurourol Urodyn. 2010;29(1):30–9. https://doi.org/10.1002/nau.20858.
Antunes-Lopes T, Carvalho-Barros S, Cruz CD, Cruz F, Martins-Silva C. Biomarkers in overactive bladder: a new objective and noninvasive tool? Adv Urol. 2011;2011:382431. https://doi.org/10.1155/2011/382431.
Cheng Y, Mansfield KJ, Allen W, Chess-Williams R, Burcher E, Moore KH. ATP during early bladder stretch is important for urgency in detrusor overactivity patients. Biomed Res Int. 2014;2014:204604. https://doi.org/10.1155/2014/204604.
Birder L, Andersson KE. Urothelial signaling. Physiol Rev. 2013;93(2):653–80. https://doi.org/10.1152/physrev.00030.2012.
Malerba F, Paoletti F, Cattaneo A. NGF and proNGF reciprocal interference in immunoassays: open questions, criticalities, and ways forward. Front Mol Neurosci. 2016;9:63. https://doi.org/10.3389/fnmol.2016.00063.
Liu HT, Chancellor MB, Kuo HC. Urinary nerve growth factor levels are elevated in patients with detrusor overactivity and decreased in responders to detrusor botulinum toxin-a injection. Eur Urol. 2009;56(4):700–6. https://doi.org/10.1016/j.eururo.2008.04.037.
Telli O, Samancı C, Sarıcı H, Hascicek AM, Kabar M, Eroglu M. Can urinary nerve growth factor and bladder wall thickness correlation in children have a potential role to predict the outcome of non-monosymptomatic nocturnal enuresis? J Pediatr Urol. 2015 Oct;11(5):265.e1–5. https://doi.org/10.1016/j.jpurol.2015.03.018.
Yamauchi H, Akino H, Ito H, Aoki Y, Nomura T, Yokoyama O. Urinary prostaglandin E2 was increased in patients with suprapontine brain diseases, and associated with overactive bladder syndrome. Urology. 2010 Nov;76(5):1267.e13–9. https://doi.org/10.1016/j.urology.2010.06.012.
Eapen RS, Radomski SB. Gender differences in overactive bladder. Can J Urol. 2016;23(Suppl 1):2–9.
Author information
Authors and Affiliations
Contributions
S Tsiapakidou: Protocol, Project development, Data collection and analysis, Manuscript writing.
A Apostolidis: Protocol, Project development, Manuscript editing.
K Pantazis: Protocol, Data analysis, Manuscript editing.
G Grimbizis: Protocol, Project development, Manuscript editing.
T Mikos: Protocol, Project development, Data collection and analysis, Manuscript editing.
Corresponding author
Ethics declarations
Financial disclaimers/conflict of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tsiapakidou, S., Apostolidis, A., Pantazis, K. et al. The use of urinary biomarkers in the diagnosis of overactive bladder in female patients. A systematic review and meta-analysis. Int Urogynecol J 32, 3143–3155 (2021). https://doi.org/10.1007/s00192-021-04945-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-021-04945-1