Abstract
Introduction
Overactive Bladder Syndrome (OAB) significantly impacts quality of life, necessitating improved diagnostic tools and treatment monitoring. This study explores the potential of neurotrophins, nerve growth factor (NGF), and brain-derived neurotrophic factor (BDNF) as urinary biomarkers in patients with OAB undergoing mirabegron therapy, a β3-adrenergic agonist. This investigation is aimed at providing insights into the potential of neurotrophins to enhance OAB diagnosis and assess treatment efficacy.
Materials and Methods
Urinary NGF and BDNF levels were measured in 15 healthy controls and 30 patients with OAB. Patients were treated with mirabegron 50 mg once daily. Urinary NGF and BDNF levels were measured by enzyme-linked immunosorbent assay method and normalized by urinary creatinine levels (NGF/Cre and BDNF/Cre). The urinary NGF/Cre and BDNF/Cre levels were compared between controls and patients with OAB and subsequently at baseline and 3 months after mirabegron treatment. Treatment efficacy was assessed with the Indevus Urgency Severity Scale (IUSS) questionnaire.
Results
Urinary NGF/Cre and BDNF/Cre levels were significantly higher in patients with OAB than in the controls (p < 0.001 and p = 0.03 respectively). Moreover, NGF/Cre and BDNF/Cre levels significantly decreased post-mirabegron treatment (p < 0.001 and p = 0.005 respectively). Patients with improvement of OAB symptoms after treatment showed lower levels of NGF/Cre at the 3-month evaluation than those with no improvement (p = 0.05).
Conclusion
Although both NGF/Cre and BDNF/Cre levels were significantly decreased after mirabegron treatment, only NGF/Cre levels were associated with treatment response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Overactive bladder (OAB) syndrome is a urinary bladder disorder characterized by urinary urgency, usually with urinary frequency and nocturia, with or without urinary incontinence in the absence of urinary tract infection (UTI) or other proven disease [1]. The prevalence of OAB in females is 16.9% [2]. This condition significantly impacts the quality of life for affected individuals by causing depression, anxiety, poor quality of sleep, and social isolation [3]. OAB is a widely recognized term and is primarily diagnosed by the symptoms experienced by the patient. Moreover, diagnosis and treatment response are based on subjective tools such as history, self-reported symptoms, questionnaires, and voiding diaries rather than on specific clinical tests or objective markers [4]. Urgency is the main symptom used to diagnose OAB, and it is strongly associated with frequency, nocturia, and urge urinary incontinence. In certain cases, OAB is accompanied by involuntary contractions of the detrusor bladder muscle during the filling phase, a condition known as detrusor overactivity (DO). However, not all individuals with OAB exhibit DO. Conventional diagnostic methods only detect DO in roughly half of patients with OAB. Moreover, up to 50% of patients diagnosed with DO through urodynamic testing do not report clinical symptoms [5, 6]. This may indicate that we still need to fully understand the underlying mechanisms of this clinical condition.
The β3-adrenoreceptor is the predominant subtype among β-adrenoreceptors present in various bladder structures, including mucosa and detrusor smooth muscle. Mirabegron is a synthetic β-3 adrenergic agonist that binds to adrenoreceptors in the bladder and is widely used in the treatment of OAB, with an acceptable side effect profile [7]. This binding stimulates β3-adrenoreceptors and leads to the relaxation of the detrusor smooth muscle, improving bladder compliance and capacity [8].
Neurotrophins are proteins that belong to a large family of trophic factors. These proteins play a vital role in ensuring the survival of neurons and the development, maintenance, and regulation of synapses. Within the urinary bladder, neurotrophins are released by several cell types, including mast cells, urothelial cells, and smooth muscle cells. In the context of lower urinary tract disorders, the most extensively studied neurotrophins are nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF). Studies have demonstrated that NGF and BDNF play a significant role in the neural regulation of bladder storage, voiding processes, and urothelial regulation [9]. Moreover, some studies advocate that neurotrophins are implicated in the development of lower urinary tract dysfunctions, including OAB. In patients with OAB, it has been observed that the concentration of these neurotrophins in the urine is significantly higher than that in healthy individuals [10,11,12].
We tested the hypothesis that efficient treatment of OAB with adrenergic agonists might primarily influence adrenergic receptors within sensory pathways, leading to modifications in the production of urinary NGF and BDNF. This, in turn, could result in a reduction of the sensation of urgency during the filling phase. If it can be demonstrated that urinary levels of these neurotrophins decrease in patients with OAB who experience symptomatic improvement following treatment with mirabegron, it would support the existence of a connection between the production of neurotrophins and the activation of adrenergic receptors in OAB. Therefore, urinary NGF and BDNF levels could serve as an objective tool for evaluating the effectiveness of treatment.
Materials and Methods
This prospective study took place in the Urogynecology Unit of a University Hospital. The study received approval from the hospital's Ethical Committee (protocol number 115/13-02-2019), and all patients provided written informed consent. The principles of the Declaration of Helsinki were observed.
We included 30 patients with OAB symptoms who had not been treated with any medications for the last 3 months. Additionally, 15 healthy individuals with no lower urinary tract symptoms (LUTS) were included as a control group. Evaluation included history, physical examination, urinalysis, urine culture, and a 3-day voiding diary. All patients were screened for neurogenic bladder, UTIs, and upper urinary tract diseases at the time of enrolment. Control subjects were aged-matched hospital employees and patients from the outpatient gynecology clinic, and none had any LUTS.
At baseline, patients with OAB completed the Indevus Urgency Severity Scale (IUSS) questionnaire. The IUSS questionnaire was classified as 0—no sense of urgency, 1—occasional urgency, which was always tolerable, 2—urgency that could be tolerated for up to 5 min, 3—intolerable urgency, and 4—urgency usually accompanied by incontinence [13]. The same questionnaire was also given to the control group and all participants answered 0—no sense of urgency.
Exclusion criteria were age over 80 years, pregnancy, any neurological disease, active or recurrent UTI, significant pelvic organ prolapse, presence of hypertension, and any OAB treatment received in the last 3 months. The patients were treated with oral mirabegron 50 mg once daily. One month after the initiation of treatment, there was an over-the-phone evaluation for the tolerability of the medication, and if no adverse reactions had occurred, the medication was continued for 2 more months.
Patients were classified as responders when they had improvement in IUSS score by > 1 after mirabegron treatment; otherwise, the patients were considered nonresponders.
Urine samples were collected for the measurement of urine NGF and BDNF in the patients with OAB and controls. Regarding patients with OAB, urine samples were collected at baseline (before treatment) and 3 months after the initiation of the treatment with mirabegron. All samples were obtained with a full bladder. Right after the collection, the samples were transferred to the laboratory and centrifuged at 3,000 rpm for 5 min. The supernatant was separated into aliquots in 1.5-ml tubes and preserved in a freezer at -80 ºC until further processing. The remainder of the urine was used to determine the creatinine (Cre) level.
The NGF urine concentration was measured using an ELISA kit (Human NGF, PicoKine™ ELISA; Boster Biological Technology, Pleasanton, CA, USA) with a sensitivity < 1 pg/ml, detection range 15.6 to 1,000 pg/ml, intra-assay CV < 6.7%, and inter-assay CV < 6.8%. Similarly, BDNF was measured using an ELISA kit (Human BDNF, PicoKine™ ELISA) with a sensitivity < 15 pg/ml, detection range 31.2 to 2,000 pg/ml, intra-assay CV < 7.2%, and inter-assay CV < 8.7%. Assays were conducted following the manufacturer's provided instructions. The total urinary NGF and BDNF levels were subsequently adjusted by normalizing them to the urinary Cre concentration, resulting in the NGF/Cre and BDNF/Cre levels (pg/mg).
For statistical data analysis, parametric and nonparametric methods were used such as t test, Wilcoxon t test, Wilcoxon paired t test, Fisher’s exact test, Shapiro–Wilk normality test, and Levene's test. Statistical analysis was conducted using statistical package R (www.r-project.org, v3.6.2), whereas the level of statistical significance was defined at p ≤ 0.05.
Results
A total of 45 white women (30 patients with OAB and 15 healthy controls) were initially enrolled in the study. The mean age of the OAB group was 60 years (standard deviation, SD, 8.5; age range, 40–79 years), and of the control group, 54 years (SD, 6.2; age range, 45–65 years). There were no statistically significant differences in age, body mass index (BMI), smoking, and menopausal status between the two groups. A comprehensive summary of the characteristics of both the patient and the control group is shown in Table 1.
Of the initial 30 patients in the OAB group, 29 completed the 3-month reassessment following mirabegron treatment. One patient withdrew from the study after 1 month owing to adverse reactions, specifically recurrent headaches, and dizziness. The IUSS score between patients with OAB at baseline and at the 3-month evaluation differed significantly (p < 0.01).
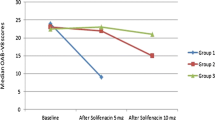
After normalization to Cre, a significant difference was observed in the baseline NGF/Cre and BDNF/Cre levels between the patients with OAB and the control group. More specifically, NGF/Cre levels for patients with OAB and controls were mean ± SD, 2.6 ± 0.92 and 0.38 ± 0.23 respectively (p < 0.001), whereas for BDNF/Cre the levels were median (IQR) 13.7 (20.57) and 6.6 (3.39) respectively (p = 0.03; Table 2). Following the 3-month treatment regimen with mirabegron in the OAB group, a statistically significant reduction in the mean urinary NGF/Cre and BDNF/Cre level was recorded (p < 0.001 and p = 0.005 respectively; Fig. 1, Table 2).
Among the OAB group, 19 patients (65.5%) exhibited symptomatic improvement (responders), whereas 10 patients (34.5%) did not experience such improvement (nonresponders), as determined by responses to the IUSS questionnaire. In the nonresponders subgroup, the levels of NGF/Cre and BDNF/Cre before and after treatment did not present a statistically significant difference (p = 0.052 and p = 0.878 respectively).
No statistically significant difference was observed between the responders group and the nonresponders group concerning age (60.05 ± 10.04 and 59.1 ± 5.56 respectively, p = 0.78), BMI (28.4 ± 6 and 25.9 ± 2.82 respectively, p = 0.53), smoking (26.3% and 10% respectively, p = 0.63), menopausal status (73.6% and 90% respectively, p = 0.63), and years with symptoms (2 ± 2.5 and 2.5 ± 1.75 respectively, p = 0.92). When comparing urinary neurotrophin levels in responders with those in nonresponders, the baseline levels of NGF/Cre did not exhibit a statistically significant differences (2.54 ± 0.93 and 2.66 ± 0.93 respectively, p = 0.76). No statistically significant difference was observed for baseline BDNF/Cre levels either (14.78 [25.5] and 14.54 [14.47] respectively, p = 0.24; Table 3).
However, the patients who showed improvement had lower levels of NGF/Cre at the 3-month evaluation than those who did not improve (p = 0.05). This finding was not evident in BDNF/Cre levels (p = 0.66; Table 3).
Discussion
The study findings indicated that individuals with OAB exhibit increased baseline urinary levels of NGF and BDNF in comparison with the control group. Following a 3-month course of mirabegron treatment, a significant decrease in neurotrophin levels was observed in the patient group. In patients who demonstrated a clinical improvement, there was a statistically significant reduction in NGF levels compared with those who did not. However, this difference was not significant for BDNF levels.
Based on very recent literature, there is a link between NGF/BDNF and OAB as neurotrophins could play a role in the connection between suburothelial sensory fibers and detrusor muscle excitability. These factors may act as intermediaries in the sensory threshold of urgency and influence the bidirectional communication between muscle or urothelium and nerves [14]. Additionally, bladder pathological conditions that may lead to OAB syndrome and are still unknown could cause increased production of neurotrophins from bladder smooth muscle cells or urothelium [12, 15, 16].
Increased secretion of NGF and BDNF by bladder muscle cells and urothelium leads to uptake of these neurotrophins by afferent nerves. This engrossment activates the trkA/p75 signaling pathway (NGF) and trkB/p75 (BDNF), causing alterations in the function of local sensory channels. Moreover, upon reaching the dorsal root ganglion (DRG) cells through transport, neurotrophins and/or activated signalosomes may induce changes in the functional properties of ion channels and receptors, thereby enhancing neuronal excitability. The heightened activity of afferent nerves triggers enhanced synaptic transmission and synaptic reorganization in the spinal cord, ultimately resulting in bladder overactivity [12, 16,17,18].
According to the aforementioned studies, in the present study, we investigated the hypothesis that if these neurotrophins are involved in the development of OAB, subsequently a reduction of their levels should be associated with the improvement of urgency symptoms.
The observation that baseline urinary NGF/Cre and BDNF/Cre levels are higher in patients with OAB than in healthy individuals is consistent with previous studies [19,20,21]. Inferentially, NGF and BDNF may have a significant impact on the sensitization of afferent neurons of the bladder, subsequently leading to the manifestation of symptoms associated with OAB.
Previous studies have investigated the levels of neurotrophins following various treatments for OAB. In a study by Antunes-Lopes et al., 24 patients with OAB were tested for urine NGF and BDNF levels at baseline, after a 3-month lifestyle intervention, and after a 3-month antimuscarinic treatment. It was found that following lifestyle intervention, the ratios of NGF/Cre and BDNF/Cre exhibited a decrease compared with baseline, which was statistically significant only for BDNF/Cre (p values of 0.318 and 0.033 respectively). Subsequent antimuscarinic treatment led to a further reduction in these ratios, which was statistically significant (p values of 0.008 and 0.001 for NGF and BDNF respectively). Furthermore, they found that the decrease in weekly urgency episodes related with the variation in BDNF/Cre (r = 0.607, p = 0.006), whereas no significant correlation was observed with the NGF/Cre ratio (r = 0.396, p = 0.094) [21].
Suh et al. investigated the viability of NGF as a biomarker to predict the effectiveness of antimuscarinic treatment and the likelihood of recurrence. They found that NGF/Cre levels tend to decrease among responders, encompassing both patients with recurrence and those without recurrence; however, this change was not statistically significant (p = 0.260). Notably, patients without recurrence demonstrated a significant decrease in urinary NGF/Cre levels at the end of the treatment (p = 0.047) [22].
In 2021, Sağır et al. assessed NGF levels in patients with OAB subjected to either antimuscarinic treatment or onabotulinum toxin-A (onaBoNT-A) injection. They found significant decreases in NGF levels at the 3rd and 6th month follow-ups in the antimuscarinic group (p = 0.003, p = 0.007 respectively), whereas the onaBoNT-A group showed nonsignificant change (p = 0.069) [23].
According to the studies mentioned above, neurotrophin levels mainly decreased after antimuscarinic treatment. A possible explanation is that antimuscarinics could affect the secretion of neurotrophins. This is supported by the expression of muscarinic receptors in both urothelial and detrusor smooth muscle cells, which can synthesize and release neurotrophins. Consequently, this action may elevate the threshold of bladder sensory fibers, contributing to a reduction in urgency episodes [10, 24].
To the best of our knowledge, this is the first published clinical study evaluating changes in NGF and BDNF levels after treatment with mirabegron, a β3-adrenergic agonist. This study demonstrated that the NGF/Cre and BDNF/Cre levels decreased significantly after mirabegron (a β3-adrenergic agonist) administration compared with baseline levels. β3-adrenoceptors are known to be abundant in the detrusor smooth muscle cells and urothelial cells. The decreased detrusor activity during bladder filling expected after adrenergic receptor activation may contribute to the subsequent reduction in urinary neurotrophin levels [19].
The limitations of the study include the relatively small sample and that the post-treatment evaluation relied only on the IUSS questionnaire.
In conclusion, NGF and BDNF may play an important role in the development of OAB symptoms in female patients and emerge as potential biomarkers for assessing therapeutic outcomes. Although both NGF/Cre and BDNF/Cre levels were significantly decreased after mirabegron treatment, only NGF/Cre levels were associated with treatment response. Further studies involving larger numbers of patients with OAB could provide insights into predicting the response to mirabegron treatment.
Despite the accumulating studies, neurotrophins are not yet utilized in clinical urology. The utilization of NGF and BDNF measurement to diagnose and assess treatment of OAB could serve as a more objective tool than existing patient-reported outcome measures. Moreover, understanding their role may give clues regarding the pathophysiology of the syndrome, and contribute to more targeted treatments in the future. To achieve this, more studies are needed to establish the association between neurotrophins and the therapeutic outcome.
Data Availability
The research data are available upon request.
Abbreviations
- BDNF:
-
Brain-derived neurotrophic factor
- BMI:
-
Body mass index
- Cre:
-
Creatinine
- DO:
-
Detrusor overactivity
- IUSS:
-
Indevus Urgency Severity Scale
- IQR:
-
Interquartile range
- LUTS:
-
Lower urinary tract symptoms
- NGF:
-
Nerve growth factor
- OAB:
-
Overactive bladder
- SD:
-
Standard deviation
- UTI:
-
Urinary tract infection
References
Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61(1):37–49.
Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–36.
Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26.
Drake MJ. Do we need a new definition of the overactive bladder syndrome? ICI-RS 2013. Neurourol Urodyn. 2014;33(5):622–4.
McGuire E. Bladder instability and stress incontinence. Neurourol Urodyn. 1988;7(6):563–7.
Bates CP, Bradley WE, Glen ES, et al. Third Report on the Standardisation of Terminology of Lower Urinary Tract Function: procedures related to the evaluation of micturition: pressure-flow relationships. Residual urine. Br J Urol. 1980;52(5):348–50.
Robinson D, Thiagamoorthy G, Cardozo L. A drug safety evaluation of mirabegron in the management of overactive bladder. Expert Opin Drug Saf. 2016;15(5):689–96.
Mirabegron. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Accessed 2 September 2023. Available from: http://www.ncbi.nlm.nih.gov/books/NBK547887/
Yoshida M, Masunaga K, Nagata T, Yono M, Homma Y. The forefront for novel therapeutic agents based on the pathophysiology of lower urinary tract dysfunction: pathophysiology and pharmacotherapy of overactive bladder. J Pharmacol Sci. 2010;112(2):128–34.
Cruz CD. Neurotrophins in bladder function: what do we know and where do we go from here? Neurourol Urodyn. 2014;33(1):39–45.
Ochodnicky P, Cruz CD, Yoshimura N, Cruz F. Neurotrophins as regulators of urinary bladder function. Nat Rev Urol. 2012;9(11):628–37.
Ochodnický P, Cruz CD, Yoshimura N, Michel MC. Nerve growth factor in bladder dysfunction: contributing factor, biomarker, and therapeutic target. Neurourol Urodyn. 2011;30(7):1227–41.
Nixon A, Colman S, Sabounjian L, et al. A validated patient reported measure of urinary urgency severity in overactive bladder for use in clinical trials. J Urol. 2005;174(2):604–7. https://doi.org/10.1097/01.ju.0000165461.38088.7b.
Steers WD. Pathophysiology of overactive bladder and urge urinary incontinence. Rev Urol. 2002;4(Suppl 4):S7–18.
Coelho A, Oliveira R, Antunes-Lopes T, Duarte Cruz C. Partners in crime: NGF and BDNF in visceral dysfunction. Curr Neuropharmacol. 2019;17(11):1021–38. https://doi.org/10.2174/1570159X17666190617095844.
Yuk SM, Shin JH, Song KH, Na YG, Lim JS, Sul CK. Expression of brain derived-neurotrophic factor and granulocyte-colony stimulating factor in the urothelium: relation with voiding function. BMC Urol. 2015;15:37. https://doi.org/10.1186/s12894-015-0036-3
Cheng C, Li Q, Lin G, Opara EC, Zhang Y. Neurobiological insights into lower urinary tract dysfunction: evaluating the role of brain-derived neurotrophic factor. Am J Clin Exp Urol. 2023;11(6):559–77
Kashyap MP, Pore SK, de Groat WC, Chermansky CJ, Yoshimura N, Tyagi P. BDNF overexpression in the bladder induces neuronal changes to mediate bladder overactivity. Am J Physiol Renal Physiol. 2018;315(1):F45–56.
Tsiapakidou S, Apostolidis A, Pantazis K, Grimbizis GF, Mikos T. The use of urinary biomarkers in the diagnosis of overactive bladder in female patients. A systematic review and meta-analysis. Int Urogynecol J. 2021;32(12):3143–55.
Liu HT, Chen CY, Kuo HC. Urinary nerve growth factor in women with overactive bladder syndrome. BJU Int. 2011;107(5):799–803.
Antunes-Lopes T, Pinto R, Barros SC, et al. Urinary neurotrophic factors in healthy individuals and patients with overactive bladder. J Urol. 2013;189(1)):359–65. https://doi.org/10.1016/j.juro.2012.08.187
Suh YS, Ko KJ, Kim TH, et al. Urinary nerve growth factor as a potential biomarker of treatment outcomes in overactive bladder patients. Int Neurourol J. 2017;21(4):270–81.
Sağır S, Bayrak Ö, Şen H, Kul S, Erturhan S, Seçkiner İ. Correlation between the NGF levels and questionnaire forms in patients receiving antimuscarinic treatment and those receiving onabotulinum toxin-A injection. Turk J Urol. 2021;47(3):223–8.
Birder LA, Wolf-Johnston AS, Sun Y, Chai TC. Alteration in TRPV1 and Muscarinic (M3) receptor expression and function in idiopathic overactive bladder urothelial cells. Acta Physiol (Oxf). 2013;207(1):123–9.
Funding
None declared.
Author information
Authors and Affiliations
Contributions
A.B.: conceptualization, methodology, formal analysis, investigation, data curation, resources, writing—original draft preparation, visualization; A.G.: formal analysis, data curation, writing—original draft preparation, visualization; D.R.: methodology, formal analysis, resources, writing—review and editing, supervision; A.M.: methodology, formal analysis, resources; E.D.: methodology, resources, writing—review and editing, supervision; P.B.: conceptualization, methodology, investigation, resources, writing—review and editing, visualization, supervision.
Corresponding author
Ethics declarations
Conflicts of Interest
None.
Additional information
Handling Editor: G. Alessandro Digesu
Editor in Chief: Maria A. Bortolini
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Beta, A., Giannouli, A., Rizos, D. et al. Nerve Growth Factor and Brain-Derived Neurotrophic Factor as Potential Biomarkers of Mirabegron Efficacy in Patients with Overactive Bladder Syndrome. Int Urogynecol J 35, 1317–1322 (2024). https://doi.org/10.1007/s00192-024-05809-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-024-05809-0




