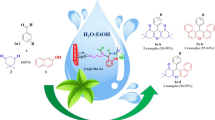
Abstract
In this study, a new heterogeneous nanocatalyst Cu@Zeo-Arg was prepared by functionalizing NaY nanozeolite with arginine and then by immobilizing copper metal ions on its surface. The formation of the synthesized nanocatalyst has been thoroughly investigated and confirmed by various analyses such as TGA, FT-IR, CHN, BET, XRD, SEM, ICP, DLS, and EDS mapping. The prepared nanocatalyst has special advantages such as easy separation, high stability, recyclability, and reusability. Also, the catalyst was recycled and reused for up to seven consecutive runs without a noticeable decrease in activity. The nanocatalyst activity was investigated in synthesizing 2-amino-4H-pyrans. These compounds were synthesized in good to high yields (72–95%) in ethanol as a green solvent at room temperature within 1 h. The structure of the products was identified and confirmed by measuring the melting points and their 1H NMR, 13C NMR, and FT-IR spectral data.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Today, the use of catalysts to reduce biological pollution and chemical waste has become very important and popular. Among them, heterogeneous catalysts are more widely used due to their special advantages such as recyclability, easy separation, and corrosion avoidance. However, these catalysts have suffered from some disadvantageous like low activity, low dispersibility, and homogeneity. Homogenization of heterogeneous catalysts including attaching different organic groups to the catalyst substrate can improve the catalyst activity, efficiency, and homogeneity properties [1,2,3,4,5].
Amino acids due to their unique structure properties have been widely applied as an organic ligand in the functionalization of solid surfaces. Among them, arginine (Arg) is a natural, non-toxic, available, and biologically active amino acid. This compound consists of three parts: a carboxyl group, an α-amino group, and a guanidine moiety that can be used for the modification of solid catalyst surfaces such as GO-Arg [6], gC3N4@L-Arg [7], Cu(II)@Fe3O4@SiO2-L-Arg [8], Fe3O4@SiO2@L-Arg[9], and Fe3O4 @L-Arg-CD-Cu(II) [10].
On the other hand, zeolites, as heterogeneous catalysts, have gained much attention due to their unique properties such as stability, non-toxic, readily available, high porosity, and high surface area [11,12,13,14,15]. There are many reports for preparation of modified zeolites such as Pd(0)/NaY [16], Fe3O4/SO3H NaY [17], USY zeolite [18], Pd-SBT@MCM-41 [19], Ag/HZSM-5 [20], PdNPs/SBA-NH2-LA [21], [Pd(NH3)4]-NaY [22], Au@HS-MCM41 [23], L-Proline-CuI-MCM-41[24], Au@NaY [25], SBA-Pr-SO3H [26], HPA-ZSM5 [27], Fe2O3@NaY [28], CuI-Zeo [29], Cu@ZeoSp-NN [30], Cu-Zeolite [31], ZS-DET [32], Cu@CBA-Ze [33], and NaY-Ugi [34].
As a result of the unique biological and chemical properties of 2-amino-4H-pyrans, they have been widely used in the preparation of various medicines, cosmetics, agricultural products, dyes, and pigments that display antibacterial [35], anti-allergy [5], anti-oxidant, anti-cancer [36], anti-malaria, and anti-Alzheimer’s properties [37]. As a result of these properties, the synthesis of these compounds has attracted much attention. However, various catalysts have been reported for the preparation of the 2-amino-4H-pyrans including SB-DABCO [38], [BMIm][Pro] [39], [P-DABCO]Cl [40], POPI [41], MZr4(PO4)6 [42], WEMFSA [43], Fe3O4/PEO/SO3H [44], Cs-EDTA-Cell [45], Basil seed [46], PPI [47], and [Cu(L’)(Imi)] [48].
Furthermore, the modification of solid catalysts with organic groups interests much more attention. Immobilization of transition metals onto the surface of the heterogeneous catalysts causes them to show different properties than when they are used as unsupported catalysts. Herein, we planned to present a highly efficient catalytic approach to synthesizing 2-amino-4H-pyranes. We describe a simple synthesis of Cu-arginine complex decorated in nano-NaY surface and also investigate its catalytic activity in green and one-pot three-component synthesis of 2-amino-4H-pyranes (Scheme 1). The final catalyst system has been divided into two phases, stationary and mobile in which solvent and reactants are considered as the mobile phase and the NaY zeolite, arginine, and complexed copper ions are ascribed to the stationary phase. Attached-arginine amino acid onto the NaY surface which plays as a flexible spacer role gives more homogeneity to the synthesized catalyst. Also, the presence of hetero-atoms in the arginine scaffold enhances its capability to coordinate with copper particles which causes an increase in the activity of the synthesized catalyst.
Result and discussion
Characterization of catalyst
To homogenize the NaY nanozeolite, it was modified by the arginine as an amino acid, and then the Cu@Zeo-Arg nanocatalyst was obtained by immobilizing copper ions on its surface (Scheme 2). The NaY zeolite due to the presence of hydroxyl groups on its surface suffers from low dispersity. Modification of the NaY surface with organic molecules can improve the dispersity and homogeneity of the catalyst in the reaction media. To this context, arginine as an easily available, natural, non-toxic, and biologically active amino acid, as well as including different functional groups with a long hydrocarbon chain has been used for modification of the NaY surface. Furthermore, arginine due to having guanidine, amine, and carboxyl moieties in its structure has a good ability of coordination with metal particles. As a result of this, arginine-supported NaY zeolite (Zeo-Arg) has a good ability for the chelation of copper ions to synthesize Cu@Zeo-Arg catalyst. Confirmation of the catalyst structure was investigated using various analysis techniques including FT-IR, CHN, FESEM, BET, XRD, ICP, EDS mapping, DLS, and TGA.
As depicted in Fig. 1, the FT-IR spectra of all samples including NaY nanozeolite (Zeo), arginine-functionalized nanozeolite (Zeo-Arg), copper-immobilized NaY zeolite (Cu@Zeo-Arg), and arginine (Arg) are exhibited. The spectra of Zeo, Zeo-Arg, and Cu@Zeo-Arg show distinct vibration bands at 3600–3250 cm−1, 1640 cm−1, and 1000–1030 cm−1 which related to the stretching vibrations of the hydroxy groups of the zeolite surface, bending vibration of hydroxy groups, and asymmetric and symmetric stretching vibrations of Si–O-Si bonds, respectively. These results demonstrate the structure of the zeolite did not significantly change during the modification processes [49].
The FT-IR spectrum of arginine (Arg) shows main peaks at 3345 and 3308 cm−1 (stretching vibration of the N–H), 3067 cm−1 (stretching vibration of the O–H), 2967 cm−1 (stretching vibration of C-H), 1680 cm−1 (stretching vibration of C = O), 1610 cm−1 (stretching vibration of C = N), and 1320–1420 cm−1 (bending vibrations of C-H) [50]. The FT-IR spectrum of Zeo-Arg as an arginine-supported NaY nanozeolite shows additional signals at 2967 cm−1 (stretching vibration of C-H), 1680 cm−1 (stretching vibration of C = O), 1610 cm−1 (stretching vibration of C = N), and 1320–1420 cm−1 (bending vibrations of C-H) confirming the successful modification of NaY surface in reaction with arginine [50]. As it can be seen in the FT-IR spectrum of copper–arginine complex immobilized on NaY (Cu@Zeo-Arg), observation of an additional peak at 509 cm−1 (stretching vibration of Cu–N) along with slight shifts at vibration stretching confirms the copper ions complexation on the Zeo surface [51, 52].
TGA diagrams for three samples of Zeo, Zeo-Arg, and Cu@Zeo-Arg are shown in Fig. 2. The samples were heated from 30 to 600 °C, at the rate of 10 °C/min. The TGA curves of all samples exhibit a weight loss at temperatures lower than 120°C related to the removal of surface-absorbed water molecules. The TGA diagram of Zeo-Arg shows another weight loss at 200–400 °C related to the removal of the arginine group (43%, 2.47 mmol/g) confirming the successful binding of arginine onto the Zeo surface. The TGA curve of the Cu@Zeo-Arg displays more stability than the Zeo-Arg catalyst (about 22%), which verifies the successful decoration of Cu(I) ions onto the Zeo-Arg surface.
In addition, the amount of attached arginine to the NaY zeolite surface was estimated using CHN analyses. As shown in Table 1, observation of carbon and nitrogen elements in the structure of Zeo-Arg confirms the successful modification of Zeo catalyst with arginine. According to N%, the amounts of the bonded arginine calculated about 2.31 mmol/g which is in good agreement with TGA results. Additionally, the ICP analysis was applied to determine copper loaded onto the Zeo-Arg surface at about 1.71 mmol/g.
As shown in Fig. 3, the size and type of holes were investigated using the BET and t-plot isotherm. According to the results, all samples are microporous and their isotherm is type 1. In addition, the data obtained from the BET and t-plot diagrams shown in Table 2 exhibit a decrease in the size of the pores during the modification process, which is related to the bonding of the organic part (Arg) to the NaY zeolite surface and the placement of copper particles in the zeolite pores.
In addition, X-ray diffraction patterns were used to investigate the crystal structure and the effect of chemical bonding on the surface of Zeo, arginine-modified NaY zeolite (Zeo-Arg), and copper–arginine complex immobilized onto the NaY zeolite (Cu@Zeo-Arg) (Fig. 4). Observing the most important peaks in all samples shows the maintenance of the crystal structure of nanozeolite during the modification process. As can be seen in the XRD patterns of the Cu@Zeo-Arg catalyst, the appearance of two additional signals in 2θ = 38.8°C and 35.5°C ascribed to copper(I) oxide phase confirms the decoration of copper (II) acetate on the surface of the nanocatalyst [53, 54]. Using the Debye–Scherrer equation, the crystallite size of Zeo, Zeo-Arg, and Cu@Zeo-Arg was estimated to be 19.15, 24.17, and 28.34, respectively.
The shape and size of the synthesized catalysts were investigated using a scanning electron microscope (SEM). The comparison of SEM images shows that the surface of the catalyst did not change significantly during the modification process with arginine and the immobilization of copper particles. Also, the average particle size obtained from the histogram of the diameter distribution for the Cu@Zeo-Arg is estimated at 83 nm (Fig. 5).
DLS analysis was used to determine the size and particle distribution. As illustrated in Fig. 6, the Cu@Zeo-Arg catalyst shows an average particle size of 108 nm confirming a narrow particle size distribution and polydispersity index (PDI) = 0.279.
The EDS mapping analyses of Zeo, Zeo-Arg, and Cu@Zeo-Arg samples are depicted in Figs. 7, 8, and 9. All elements in these figures have a uniform dispersion distribution on the surface of the sample. The presence of O, Si, and Al elements in the structure of all samples is related to the parent structure of the zeolite. Observation of N and C elements in Fig. 8 confirms the successful functionalization of the Zeo with arginine. Also, observation of the Cu element in Fig. 9 confirms the coordination of copper ions onto the surface of the Zeo-Arg.
The activity of the Cu@Zeo-Arg has been investigated in the synthesis of 2-amino-4H-pyranes from the one-pot three-component reaction between aromatic aldehydes 1a-n and active methylenes 2a-b with dimedone 3. Then, the reaction of dimedone (1 mmol), malononitrile (1 mmol), and 4-chlorobenzaldehyde (1 mmol) in ethanol (10 ml) was chosen as a model reaction.
As demonstrated in Table 3, initially the model reaction tested in the presence of Zeo, Zeo-Arg, and Cu@Zeo-Arg, as well as free-catalyst condition (entries 1–4). The results show that the model reaction produced only a trace of product 4j in the presence of Zeo catalyst and free-catalyst conditions. The yield of the reaction increased by 65% in the presence of Zeo-Arg catalyst which could be due to supported arginine on the Zeo surface. However, the reaction yield was significantly enhanced to 95% when 10 mg of Cu@Zeo-Arg catalyst was used. The model reaction examined in the presence of various amounts of Cu@Zeo-Arg demonstrating 10 mg of Cu@Zeo-Arg was chosen as the optimum amount and increasing the catalyst amounts did not change the reaction yield (entries 4–9). The various solvents were applied to investigate the solvent effect in the model reaction which shows the highest yield obtained when ethanol was used as a reaction solvent (entries 10–14). Increasing reaction temperature does not change the yield of 4j (entries 15 and 16). Additionally, the model reaction was investigated in the presence of Arg, Cu(OAc)2, and Cu-arginine complex (Cu(OAc)2-Arg) without support on the Zeo surface, which resulted in 95%, 42%, and 65% reaction yield, respectively (entries 17–19).
To illustrate the efficiency of the Cu@Zeo-Arg catalyst in the synthesis of pyrans, the synthesis of product 4j was chosen and its reaction conditions were compared with some previously reported procedures (Table 4). The model reaction in the presence of various catalytic systems such as DBSA (entry 1), Fe(ClO4)3/SiO2 (entry 2), β-CD (entry 3), IL-HSO4@SBA-15 (entry 4), Alum (entry 5), [B mim] Sac (entry 6), BaFe12O19@IM magnetic (entry 7), PC/AgNPs (entry 8), and WEMFSA (entry 9) suffer from some disadvantageous like lower yields (entries 1–6 and 9), higher temperature (entries 1 and 2, 4–8), high catalyst ratio (1,2 and 6), and longer reaction times (entries 1–5 and 9). However, the introduced catalytic system performed the model reaction at ambient temperature in EtOH to obtain the product 4j in 95% yield after 1 h (entry 10). The outcomes show that the Cu@ Zeo-Arg catalyst can be a suitable alternative catalyst for the synthesis of pyrans.
Under optimized reaction conditions obtained from Table 4, the generality of the optimized approach has been examined in a three-component reaction of aromatic aldehydes 1, active methylenes 2, and dimedone 3. The summarized results in Table 5 exhibit that aldehydes including both electron-donating and electron-withdrawing substituents resulted in corresponding pyrans 4a-4 × in good to excellent yields. The structure of synthesized compounds 4a-4 × was confirmed by comparing their physical data and NMR spectra with those previously reported in the literature.
As depicted in Scheme 3, a reasonable mechanism for synthesizing products 4a-4 × is shown. Initially, arylidenemalononitrile (A) resulted from the aldol condensation reaction of aldehyde 1 and malononitrile 3 in the presence of Cu@ZeoSpNN. Then, Michael's addition of dimedone 2 to intermediate A led to the adduct II. Finally, corresponding products 4a-4 × were obtained from the enolization and intramolecular nucleophilic cyclization of intermediate II [30].
Recycling and recovering the catalysts from the reaction media has always remained an important challenge. In the model reaction, the reusability and recovery of the Cu@Zeo-Arg have been investigated. The results show that the Cu@Zeo-Arg nanocatalyst is an efficient catalyst for the synthesis of 2-amino-4H-pyranes and can be easily recovered and reused after seven consecutive cycles, maintaining the structure without losing its catalytic activity (Fig. 10).
As shown in Figs. 11 and 12, the catalyst stability of the Cu@ZeoSpNN after seven successive runs was investigated with a comparison of their FT-IR, XRD, and SEM analyses.
Conclusion
In this study, simple synthesizing of the copper–arginine complex supported in the nano-NaY zeolite (Cu@Zeo-Arg) was described by functionalization of NaY nanozeolite with arginine and coordination with copper (I) ion. To confirm the catalyst structure, various analysis techniques including FT-IR, TGA, XRD, SEM, ICP, DLS, and elemental analysis were applied. The three-component reaction of aldehyde, active methylenes, and dimedone in the synthesis of pyrans was chosen due to the investigation of the catalytic activity of the Cu@Zeo-Arg catalyst. Simple separation from the reaction mixture, reusing seven consecutive times of the catalyst without loss in its reactivity, synthesis of pyrans at room temperature, higher yields, and short reaction times are the advantages of introduced catalytic systems. Activity, homogeneity, and copper coordination ability of the catalyst were improved due to the nature of the supported arginine (Arg) on the Zeo surface. The findings revealed that Cu@Zeo-Arg nanocatalyst could be beneficial in similar reactions. Finally, the introduced catalytic system could be simply replicated and be an excellent and efficient catalyst for the preparation of useful organic compounds under green reaction conditions.
Experimental
Materials and methods
NaY nanozeolite (Zeo) (Si/Al = 2.5) was purchased from Zeolist Corporation. Arginine and copper acetate were also purchased from Merck Chemical Company and were used without purification. The following techniques were used to confirm the structure of the samples: an electrothermal IA9100 (Essex, UK) to measure melting points, the Bruker-400 Avance III (Broker, Germany) with internal reference TMS and CDCl3 and DMSO-d6 solvents to determine 1H and 13C NMR spectra, the TGA device (Netzsch, SELB, German) for the thermal analysis of samples, FT-IR spectrometer (Bruker Tensor 27, Germany), dynamic dispersion of light (DLS) (Horiba SZ-100 (Horiba, Japan) to measuring particles size distribution, a Cu Ka (Philips PW-1830) source for X-ray powder diffractions at ambient temperature, Brunauer–Emmett–Teller (BET) (BEL Sorp Japan) to analyze the BET and t-plot, and the scanning electron microscope (SEM) on (Mira 3-XMU, TESCAN, Brno, Czech Republic) to study the morphology of the catalyst surface.
Catalyst preparation
Preparation of NaY-arg nanocatalyst
To a solution of 1 g of NaY nanozeolite (Zeo) in 50 mL of deionized water was added 2.60 g (15 mmol) of Arg and allowed to reflux for 48 h. Then the reaction mixture was cooled to room temperature, the catalyst was separated using a centrifuge, and the precipitate was washed with ethanol. The non-bounded arginine was removed using a Soxhlet extractor. The arginine-functionalized nanozeolite (Zeo-Arg) was collected in pure form and dried in a vacuum oven.
Copper-supported amine nano-NaY zeolite (Cu@Zeo-Arg)
0.5 g of copper acetate (1.72 mmol) was added to the solution of 0.5 g of Zeo-Arg in 25 mL of ethanol and stirred for 24 h at room temperature. To separate the catalyst, the reaction mixture was centrifuged, and the precipitate was thoroughly washed with ethanol and dried under a vacuum.
Synthesis of pyran derivatives
A mixture of benzaldehyde derivatives (1 mmol), dimedone (1 mmol), and active methylenes (malononitrile or ethyl cyanoacetate) (1 mmol) in ethanol solvent (10 ml) in the presence of 10 mg of Cu@Zeo-Arg nanocatalyst was stirred at room temperature for 1 h. Thin layer chromatography (TLC) was utilized to check the reaction progress. The catalyst was removed from the reaction mixture by centrifugation. After removing the solvent, the precipitate obtained was purified by recrystallization in hot ethanol. The structure of the products has been confirmed by checking their melting points and using FT-IR, 13CNMR, and 1HNMR spectra.
Spectral data for the synthesized 4h
2-Amino-4-(4-bromophenyl)-7, 7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (4h)
White powder, FT-IR (KBr): (νmax cm−1) 3319 and 3382 (N–H), 3057 (CSP2 -H), 2960 (CSP3 – H), 2191 (CNstretch), 1680 (C = O), 1520 (C = C), 1247 (CSP2 -O). 1H NMR (DMSO-d6): δ 6.95 (d, 2H, 3J = 8.0 Hz, CHAr), 6.90 (s, 2H, NH2), 6.63 (d, 2H, 3J = 8.0 Hz CHAr), 4.05 (s, 1H, CH), 2.46 and 2.52 (ABq, 2H, 2J = 16.0 Hz, CH2), 2.08 and 2.24 (ABq, 2H, 2J = 16.0 Hz, CH2), 1.04 (s, 3H, CH3) and 0.96 (s, 3H, CH3). 13C NMR (DMSO-d6): δ 27.21, 28.96, 32.24, 35.06, 39.98, 50.53, 59.40, 112.80, 113.74, 120.43, 128.19, 133.01, 149.69, 158.81, 162.31, 196.13.
Data availability and materials
The selected spectra data (Copies of the 1H NMR, and 13CNMR spectra) are included in the supplementary information file.
References
P. Gupta, S. Paul, Catal. Today 236, 153 (2014)
K. Nakajima, T. Yokoi, A. Katz, Mole. Catal. 496, 111197 (2020)
V. Polshettiwar, R.S. Varma, Green Chem. 12, 743 (2010)
A. Corma, H. Garcia, Adv. Synth. Catal. 348, 1391 (2006)
M. Sharma, M. Sharma, A. Hazarika, L. Satyanarayana, G.V. Karunakar, K.K. Bania, Mole. Catal. 432, 210 (2017)
S. Khabnadideh, E. Mirzaei, L. Amiri-Zirtol, J. Mol. Struct. 1261, 132934 (2022)
H. Ghafuri, Z. Tajik, N. Ghanbari, P. Hanifehnejad, Sci. Rep. 11, 19792 (2021)
M. Nikoorazm, P. Moradi, N. Noori, G. Azadi, J. Iran. Chem. Soc. 18, 467 (2021)
E. Courvoisier, P.A. Williams, G.K. Lim, C.E. Hughes, K.D. Harris, Chem. Commun. 48, 2761 (2012)
M.J. Nejad, A. Salamatmanesh, A. Heydari, J. Organomet. Chem. 911, 121128 (2020)
W. Hölderich, M. Hesse, F. Näumann, Angew. Chem. Int. Ed. Engl. 27, 226 (1988)
Y. Zhang, Y. Liu, J. Kong, P. Yang, Y. Tang, B. Liu, Small 2, 1170 (2006)
Q. Zhang, W. Huang, W. Cui, X. Dong, G. Liu, Y. Xu, Z. Liu, Mole. Catal. 545, 113189 (2023)
M.F. Paiva, E.F. de Freitas, J.O.C. de França, D. da Silva Valadares, S.C.L. Dias, J.A. Dias, Mol. Catal. 532, 112737 (2022)
S. Montalvo, L. Guerrero, R. Borja, E. Sánchez, Z. Milán, I. Cortés, M.A. De La La Rubia, Appl. Clay Sci. 58, 125 (2012)
L. Artok, H. Bulut, Tetrahedron Lett. 45, 3881 (2004)
M. Kalhor, Z. Zarnegar, RSC Adv. 9, 19333 (2019)
L.H. Alponti, M. Picinini, E.A. Urquieta-Gonzalez, A.G. Correa, J. Mol. Struct. 1227, 129430 (2021)
M. Nikoorazm, A. Ghorbani-Choghamarani, M. Ghobadi, S. Massahi, Appl. Organomet. Chem. 31, e3848 (2017)
M. Tajbakhsh, H. Alinezhad, M. Nasrollahzadeh, T.A. Kamali, J. Alloys Compd. 685, 258 (2016)
A. Narani, H.P.R. Kannapu, K. Natte, D.R. Burri, Mole. Catal. 497, 111200 (2020)
L. Djakovitch, P. Rollet, Adv. Synth. Catal. 346, 1782 (2004)
A. Feiz, A. Bazgir, Catal. Commun. 73, 88 (2016)
C. Luo, R. Zhao, M. Cai, Mole. Catal. 533, 112795 (2022)
L. Zhou, W. Yu, L. Wu, Z. Liu, H. Chen, X. Yang, Y. Su, J. Xu, Appl. Catal. A: Gen. 451, 137 (2013)
G.M. Ziarani, N.H. Nasab, M. Rahimifard, A.A. Soorki, J. Saudi Chem. Soc. 19, 676 (2015)
H. Shahbazi-Alavi, R. Teymuri, J. Safaei-Ghomi, Nanochem. Res. 6, 135 (2021)
B. Dutta, S. Jana, A. Bhattacharjee, P. Gütlich, S.I. Iijima, S. Koner, Inorg. Chim. Acta 363, 696 (2010)
M.J. Climent, A. Corma, S. Iborra, RSC Adv. 2, 16 (2012)
M. Azizi Amiri, G.F. Pasha, M. Tajbakhsh, S. Asghari, Appl. Organomet. Chem. 36, 6886 (2022)
V. Bénéteau, A. Olmos, T. Boningari, J. Sommer, P. Pale, Tetrahedron Lett. 51, 3673 (2010)
F. Babaei, S. Asghari, M. Tajbakhsh, Res. Chem. Intermed. 45, 4693 (2019)
H. Younesi, S. Asghari, G. Firouzzadeh Pasha, M. Tajbakhsh, Res. Chem. Intermed. 49, 5289 (2023)
H. Younesi, S. Asghari, G.F. Pasha, M. Tajbakhsh, Appl. Organomet. Chem. 37, 7127 (2023)
D. Kumar, V.B. Reddy, S. Sharad, U. Dube, S. Kapur, Eur. J. Med. Chem. 44, 3805 (2009)
M. Aghajani, S. Asghari, G.F. Pasha, M. Mohseni, Res. Chem. Intermed. 46, 1841 (2020)
Z.J. Yang, Q.T. Gong, Y. Wang, Y. Yu, Y.H. Liu, N. Wang, X.Q. Yu, Mole. Catal. 491, 110983 (2020)
A. Hasaninejad, M. Shekouhy, N. Golzar, A. Zare, M.M. Doroodmand, Appl. Catal. A 402, 11 (2011)
A.R. Hajipour, Z. Khorsandi, ChemistrySelect 2, 8976 (2017)
L.S. Huang, X. Hu, Y.Q. Yu, D.Z. Xu, ChemistrySelect 2, 11790 (2017)
M.G. Dekamin, M. Eslami, Green Chem. 16, 4914 (2014)
J. Safaei-Ghomi, A. Javidan, A. Ziarati, H. Shahbazi-Alavi, J. Nanopart. Res. 17, 1 (2015)
P.B. Hiremath, K. Kantharaju, ChemistrySelect 5, 1896 (2020)
A. Maleki, M. Azizi, Z. Emdadi, Green Chem. Lett. Rev. 11, 573 (2018)
N. Rostami, M.G. Dekamin, E. Valiey, H. Fanimoghadam, Sci. Rep. 12, 8642 (2022)
E. Kolvari, N. Koukabi, Z. Ozmaei, H. Khoshkho, F. Seidi, Curr. Res. Green Sustain. Chem. 5, 100327 (2022)
H. Kiyani, F. Ghorbani, J. Saudi Chem. Soc. 18, 689 (2014)
S.Y. Ebrahimipour, M. Khosravan, J. Castro, F.K. Nejad, M. Dusek, V. Eigner, Polyhedron 146, 73 (2018)
M. Kalhor, S. Banibairami, RSC Adv. 10, 41410 (2020)
A. Roda, F. Santos, Y.Z. Chua, A. Kumar, H.T. Do, A. Paiva, A.R.C. Duarte, C. Held, Phys. Chem. Chem. Phys. 23, 1706 (2021)
K.M. Parida, D. Rath, S.S. Dash, J. Mol. Catal. A: Cheml. 318, 85 (2010)
A. Ghorbani-Choghamarani, L. Shiri, G. Azadi, RSC Adv. 6, 32653 (2016)
L. Ren, L. Zhu, C. Yang, Y. Chen, Q. Sun, H. Zhang, C. Li, F. Nawaz, X. Meng, F.S. Xiao, Chem. Commun. 47, 9789 (2011)
K. Sivakumar, A. Santhanam, M. Natarajan, D. Velauthapillai, B. Rangasamy, Int. J. Appl. Ceram. Technol. 13, 1182 (2016)
E. Sheikhhosseini, D. Ghazanfari, V. Nezamabadi, Iran. J. Catal. 3, 197 (2013)
F.K. Behbahani, M. Naderi, Russ. J. Gen. Chem. 86, 2804 (2016)
J. Lu, X.W. Fu, G. Zhang, C. Wang, Res. Chem. Intermed. 42, 417 (2016)
S. Rostamnia, A. Hassankhani, H.G. Hossieni, B. Gholipour, H. Xin, J. Mol. Catal. A: Chem. 395, 463 (2014)
A.A. Mohammadi, M.R. Asghariganjeh, A. Hadadzahmatkesh, Arabian. J. Chem. 10, S2213 (2017)
H. Sharma, S. Srivastava, RSC Adv. 8, 38974 (2018)
S. Amirnejat, A. Nosrati, R. Peymanfar, S. Javanshir, Res. Chem. Intermed. 46, 3683 (2020)
S. Saneinezhad, L. Mohammadi, V. Zadsirjan, F.F. Bamoharram, M.M. Heravi, Sci. Rep. 10, 14540 (2020)
S. Chehab, Y. Merroun, T. Ghailane, R. Ghailane, S. Boukhris, B. Lakhrissi, A. Souizi, J. Iran. Chem. Soc. 18, 2665 (2021)
R.M. Mohareb, R.A. Ibrahim, E.M. Samir, J. Iran. Chem. Soc. 20, 2163 (2023)
N. Hazeri, M.T. Maghsoodlou, F. Mir, M. Kangani, H. Saravani, E. Molashahi, Chin. J. Catal. 35, 391 (2014)
M. Bakherad, A. Keivanloo, E. Moradian, A.H. Amin, R. Doosti, M. Armagha, J. Iran. Chem. Soc. 15, 2811 (2018)
H. Khodakarami, D. Habibi, Catal. Commun. 172, 106523 (2022)
H. Kiyani, F. Ghorbani, Res. Chem. Intermed. 41, 7847 (2015)
F. Matloubi Moghaddam, M. Daneshfar, H. Moghimi, Z. Daneshfar, Synth. Commun. 52, 974 (2022)
F. Shirini, N. Daneshvar, RSC adv. 6, 110190 (2016)
S. Chauhan, A. Mishra, P. Verma, V. Srivastava, Org. Prep. Proced. Int. 53, 441 (2021)
S. Banerjee, A. Saha, New J. Chem. 37, 4170 (2013)
J.M. Khurana, B. Nand, P. Saluja, J. Heterocycl. Chem. 51, 618 (2014)
M. Kangani, N. Hazeri, M.T. Maghsoodlou, J. Chin. Chem. Soc. 63, 896 (2016)
S.F. Hojati, N. MoeiniEghbali, S. Mohamadi, T. Ghorbani, Org. Prep. Proced. Int. 50, 408 (2018)
S. Rostamnia, A. Nuri, H. Xin, A. Pourjavadi, S.H. Hosseini, Tetrahedron Lett. 54, 3344 (2013)
Y. Pourshojaei, F. Zolala, K. Eskandari, M. Talebi, L. Morsali, M. Amiri, A. Khodadadi, R. Shamsimeymandi, E. Faghih-Mirzaei, A. Asadipour, J. Nanosci. Nanotechnol. 20, 3206 (2020)
R. Gupta, S. Layek, D.D. Pathak, Res. Chem. Intermed. 45, 1619 (2019)
F. Kalantari, A. Ramazani, M.R. Poor Heravi, H. Aghahosseini, K. Ślepokura, Inorg. Chem. 60, 15010 (2021)
F. Mohamadpour, Org. Prep. Proced. Int. 55, 345 (2023)
M.A. Nasseri, S.M. Sadeghzadeh, J. Iran. Chem. Soc. 10, 1047 (2013)
M. Nasr-Esfahani, T. Abdizadeh, J. Nanosci. Nanotechnol. 13, 5004 (2013)
J.K. Rajput, G. Kaur, Catal. Sci. Technol. 4, 142 (2014)
H. Faroughi Niya, N. Hazeri, M. Fatahpour, M.T. Maghsoodlou, Res. Chem. Intermed. 46, 3651 (2020)
Z.G. Zeng, L.Y. Wang, Y. Cao, Y.P. Luo, Res. Chem. Intermed. 38, 1751 (2012)
Acknowledgements
The authors acknowledge the Research Council of the University of Mazandaran
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All persons who meet authorship criteria are listed as follows, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
It is to specifically state that “No Competing interests are at stake and there is No Conflict of Interest” with other people or organizations that could inappropriately influence or bias the content of the paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khoshlahjeh, F., Asghari, S. & Firouzzadeh Pasha, G. Green one-pot synthesis of 2-amino-4H-pyranes catalyzed by copper–arginine complex decorated on nano-NaY zeolite. Res Chem Intermed 50, 1993–2014 (2024). https://doi.org/10.1007/s11164-024-05262-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-024-05262-0