Abstract
Tetrabutyl ammonium bromide (TBAB) catalyzed one-pot synthesis of 4H-chromene via a three-component cyclocondensation of aryl aldehydes, cyanoacetate and dimedone in green media–water. Then, 4H-chromene reacted with acyl chloride in acetonitrile at refluxing with catalytic amount of DMAP to yield the title compounds in good yields. The structures of all title compounds were confirmed by 1H NMR, MS and elementary analyses. The X-ray crystallography of compound 3n indicated that there were strong intermolecular hydrogen bonds.
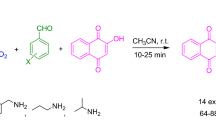
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heterocyclic compounds, especially pyran and benzopyran derivatives, have played an important role in medicines, pesticides, dyes, and other fine chemical industries. Pyran ring, because of its intrinsic activity, has become a very useful synthon for other heterocyclic compounds [1]. Benzopyran derivatives exhibit excellent physiological and pharmacological activity [2], such as anti-hypoplasia [3], anti-allergic effects [4], treatment of allergic bronchitis [5], and anti-cancer [6].
Furthermore, amides occupy an important place in medicines and pesticides due to their broad-spectrum, high-efficiency active features, for example, bactericidal [7], acaricidal [8], pesticidal [9], herbicidal [10], and other biological activities. In order to find bioactive compounds, we designed and synthesized a series of 2-amide-4H -benzo[b]pyran derivatives by assembling benzopyran and amide using particular methodology and obtaining the following results.
Results and discussion
The synthesis of 4H-benzo[b]pyran derivatives has been reported previously in the literature. Aryl aldehydes, alkyl nitriles, and dimedone have been synthesized in organic solvents (e.g., acetonitrile [1], ethanol [11, 12] and DMF [13]), ionic liquid [14], microwave [15], and so on. The main drawbacks of these methods stem from the requirement for organic solvents, drastic reaction conditions, high temperatures, long reaction times, or the complicated preparation of the catalysts. The methods of solvent-free grinding in the presence of a catalyst (e.g., triethylbenzylammonium chloride, TEBA [16]) and utilizing the reactants in a solid or molten state [17] have also been used for synthesis of these derivatives. Although these reactions were relatively novel, they had some limitations, such as the two-step reaction, very high temperature or requirement for a longer period of time. Recently, organic synthesis in water, such as the Claisen Rearrangement, Aldol Condensation, Diels–Alder Reaction, Michael Addition, and Knoevenagel Condensation, has been reported [18–23]. Three-component one-pot reaction in water could be important for bioactive compounds. Ziarani et al. [24]. have reported 2-amine-3-cyano-4H-chromenes, which were efficiently synthesized by the one-pot reaction of appropriate aromatic aldehydes, malononitrile and dimedone, in the presence of SiO2–Pr–SO3H as a solid acid catalyst. Due to the complicated preparation of the solid acid catalyst, it was difficult to apply this as the industrial process. Moreover, Bandgar et al. [25] have reported catalyst-free synthesis of those very similar products in one-pot reactions in aqueous media. The uncatalyzed reactions have been carried out smoothly at reflux in 3.5 h via three-component reactions of malononitrile, aryl aldehydes, and dimedone. But this method was not suitable for the three-component cyclocondensation involving ethyl cyanoacetate. In our research, the reactions needed a very long time, and during the procedures, clumps were very prone to form. It was difficult to accomplish the three-component reactions to substitute malononitrile with ethyl cyanoacetate under the same conditions. The reason may be that methylene in malononitrile was more active than in ethyl cyanoacetate. Because the electron withdrawing ability of cyano-group was stronger than the ester-group. Here, we have found an efficient, green, and convenient procedure to synthesize 2-amine-3-carboxylate-4H-benzo[b]pyran derivatives using phase-transfer catalyst in water. We chose tetrabutyl ammonium bromide (TBAB) as a catalyst, and aryl aldehydes, ethyl cyanoacetate, and dimedone reacted to give highly functionalized 4H-benzo[b]pyrans in water at 85 °C in excellent yields (Table 1). Furthermore, TBAB catalyzed to synthesize 2-amine-3-carboxylate-4H-chromenes in one-pot in water has not previously been reported (Scheme 1).
In order to synthesize compounds 3, we have studied the reaction conditions to use compound 1a and benzoyl chloride as a template reaction, and the results were shown in Table 2. It was obvious that the reaction would be accomplished comfortably in acetonitrile at refluxing conditions with a catalytic amount of DMAP. In other conditions, the reaction either did not carry on, or carried on very slowly, and yield was trace. At room temperature, no matter in what kind of solvent, no reaction carried on. The reason may be a strong impact from electron-withdrawing groups (e.g., its ortho-ester and benzene ring); the amino group of the pyran ring was not active enough. When the reaction was refluxing, the yields were different from nonpolar solvent to polar solvent, and the yields were very low. Under the condition of catalyst, the yields were higher than with no catalyst. So, it could be speculated that higher temperature, polar solvent, and catalyst were all useful to accomplish the title compounds synthesis. Additionally, an acid-binding agent such as triethylamine should be added, while the reaction system must also be kept dry.
All compounds 3 gave satisfactory elementary analyses and spectroscopic data (1H NMR and MS) consistent with their assigned structures. For example, in the 1H NMR spectra, compounds 3 revealed a singlet at δ 11.88–12.26 ppm, which was attributed to amide hydrogen protons. The MS spectra revealed that the molecular ion peaks and fragmentation peaks were in accordance with the synthesized structures of the title compounds 3, and the molecular ion peaks were observed for all the title compounds (Scheme 2).
The single-crystal structure of 3n was determined by X-ray crystallography as shown in Fig. 1. The crystal of 3n revealed an intermolecular hydrogen bond. The distance of N(1)–H(1)…O(5) was 2.64 Å, and the angle of N(1)–H(1)…O(5) was 136.4º.
Conclusion
In conclusion, we developed an easy and mild method to synthesize a new series of 2-amide-3-carboxylate-4H-benzo[b]pyrans in good yields. Further investigations on structural optimization and bioactivities are well under way.
Experimental
Melting points were measured on the ×6 micromelting-point apparatus (China) and are uncorrected. 1H NMR spectra were recorded on a Bruker AV 400 NMR spectrometer in CDCl3 with TMS as the internal reference. MS spectra were determined using a Finnigan Trace MS 2000 organic mass spectrometry, and signals were given in m/z. Elementary analyses were performed on a Vario EL III elementary analysis instrument. All chemical reagents were commercially available and treated with standard methods before use. Solvents were dried in a routine way and redistilled.
Preparation of the 2-amine-3-carboxylate-4H-chromenes (1)
To a stirred solution of cyanoacetate (2.2 mmol) in water (10 mL), a catalytic amount of TBAB, dimedone (5, 5-dimethyl-1, 3-cyclohexanedione, 2.2 mmol) and benzaldehyde (2 mmol) were added. The reaction mixture was stirred at 85 °C for 3–5 h according to thin layer chromatographic (TLC) analysis, and filtered off by suction. For further purification, samples were recrystallized from 95% ethanol to yield the compounds (1).
General procedure for the synthesis of the title compounds (3)
Compounds 1 (2 mmol), triethylamine (2.4 mmol) and catalytic amount of DMAP were added to dry acetonitrile (10 mL) in turn. Then, fresh acyl chlorides 2 (2.2 mmol) was slowly dropped into the mixture by strong stirring on an ice bath. Next, the reaction mixture was refluxed for 10–14 h according to TLC analysis. The mixture was poured into 100 mL of water, extracted with chloroform (30 mL × 3), the organic layer was dried with anhydrous magnesium sulfate, and the solvent was evaporated to give the crude product, which was purified by chromatography on silica using petroleum ether/ethyl acetate as the eluent to give the title compounds 3a–t.
Ethyl 2-acetamido-7,7-dimethyl-5-oxo-4-phenyl-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (3a) white solid; yield, 63.1%; mp 198–200 °C; 1H NMR (400 MHz, CDCl3), δ 0.97 (s, 3H, CH3), 1.13 (s, 3H, CH3), 1.19 (t, 3H, CH3, J = 7.12 Hz), 2.18 (s, 3H, CH3), 2.21 (q, 2H, CH2, J = 16.28 Hz), 2.60 (s, 2H, CH2), 4.08–4.13 (m, 2H, CH2), 4.85 (s, 1H, CH), 7.07–7.26 (m, 5H, ArH), 11.88 (s, 1H, NH); MS (EI): m/z (%) 382 ([M−1]+, 22), 368 (43), 241 (27), 227 (100), 199 (13), 171 (17), 102 (26), 83 (37). Anal. Calcd for C22H25NO5: C, 68.91; H, 6.57; N, 3.65. Found: C, 68.93; H, 6.48; N, 3.66.
Ethyl 2-benzamido-7,7-dimethyl-5-oxo-4-phenyl-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (3b). white solid; yield, 69.8%; mp 214–216 °C; 1H NMR (400 MHz, CDCl3), δ 0.98 (s, 3H, CH3), 1.13 (s, 3H, CH3), 1.21 (t, 3H, CH3, J = 7.12 Hz), 2.22 (q, 2H, CH2, J = 16.28 Hz), 2.64 (s, 2H, CH2), 4.10–4.16 (m, 2H, CH2), 4.83 (s, 1H, CH), 7.13–7.99 (m, 10H, ArH), 11.94 (s, 1H, NH); MS (EI): m/z (%) 445 ([M]+, 8), 368 (38), 322 (6), 294 (6), 105 (100), 77 (24). Anal. Calcd for C27H27NO5: C, 72.79; H, 6.11; N, 3.14. Found: C, 72.68; H, 6.09; N, 3.15.
Ethyl 2-(4-bromobenzamido)-7,7-dimethyl-5-oxo-4-phenyl-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (3c). white solid; yield, 60.0%; mp 276–278 °C; 1H NMR (400 MHz, CDCl3), δ 0.98 (s, 3H, CH3), 1.11 (s, 3H, CH3), 1.22 (t, 3H, CH3, J = 7.12 Hz), 2.23 (q, 2H, CH2, J = 16.28 Hz), 2.64 (s, 2H, CH2), 4.11–4.15 (m, 2H, CH2), 4.80 (s, 1H, CH), 7.22–8.03 (m, 9H, ArH), 12.16 (s, 1H, NH); MS (EI): m/z (%) 526 ([M+2]+, 18), 524 ([M])+, 42), 448 (16), 402 (29), 316 (25), 107 (100), 77 (36). Anal. Calcd for C27H26BrNO5: C, 61.84; H, 5.00; N, 2.67. Found: C, 61.67; H, 5.08; N, 2.68.
Ethyl 2-(4-methoxybenzamido)-7,7-dimethyl-5-oxo-4-phenyl-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (3d). white solid; yield, 71.3%; mp 245–247 °C; 1H NMR (400 MHz, CDCl3), δ 0.99 (s, 3H, CH3), 1.15 (s, 3H, CH3), 1.23 (t, 3H, CH3, J = 7.12 Hz), 2.25 (q, 2H, CH2, J = 16.28 Hz), 2.61 (s, 2H, CH2), 3.92 (s, 3H, CH3), 4.10–4.17 (m, 2H, CH2), 4.81 (s, 1H, CH), 7.11–7.91 (m, 9H, ArH), 11.98 (s, 1H, NH); MS (EI): m/z (%) 476 ([M+1]+, 15), 399 (43), 353 (17), 325 (13), 131 (22). Anal. Calcd for C28H29NO6: C, 70.72; H, 6.15; N, 2.95. Found: C, 70.74; H, 6.19; N, 2.77.
Ethyl 2-acetamido-7,7-dimethyl-4-(2-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (3e). white solid; yield, 57.4%; mp 170–172 °C; 1H NMR (400 MHz, CDCl3), δ 0.95 (s, 3H, CH3), 1.06 (s, 3H, CH3), 1.10 (t, 3H, CH3, J = 7.12 Hz), 2.18 (s, 3H, CH3), 2.20 (q, 2H, CH2, J = 16.28 Hz), 2.62 (s, 2H, CH2), 4.03–4.16 (m, 2H, CH2), 5.80 (s, 1H, CH), 7.25–7.46 (m, 4H, ArH), 11.99 (s,1H, NH); MS (EI): m/z (%) 428 ([M]+, 25), 385 (76), 370 (42), 341 (100), 295 (58), 230 (36), 128 (19). Anal. Calcd for C22H24N2O7: C, 61.67; H, 5.65; N, 6.54. Found: C, 61.64; H, 5.59; N, 6.65.
Ethyl 2-benzamido-7,7-dimethyl-4-(2-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (3f). white solid; yield, 51.9%; mp174–176 °C; 1H NMR (400 MHz, CDCl3), δ 0.95 (s, 3H, CH3), 1.07 (s, 3H, CH3), 1.09 (t, 3H, CH3, J = 7.12 Hz), 2.20 (q, 2H, CH2, J = 16.28 Hz), 2.61 (s, 2H, CH2), 4.02–4.18 (m, 2H, CH2), 5.84 (s, 1H, CH), 7.26–8.00 (m, 9H, ArH), 12.17 (s, 1H, NH); MS (EI): m/z (%) 490 ([M] +, 2), 473 (4), 369 (3), 225 (5), 105 (100). Anal. Calcd for C27H26N2O7: C, 66.11; H, 5.34; N, 5.71. Found: C, 66.23; H, 5.14; N, 5.76.
Ethyl 2-(4-bromobenzamido)-7,7-dimethyl-4-(2-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (3g). white solid; yield, 55.3%; mp 235–236 °C; 1H NMR (400 MHz, CDCl3), δ 0.97 (s, 3H, CH3), 1.08 (s, 3H, CH3), 1.10 (t, 3H, CH3, J = 7.12 Hz), 2.23 (q, 2H, CH2, J = 16.28 Hz), 2.61 (s, 2H, CH2), 4.02–4.19 (m, 2H, CH2), 5.88 (s, 1H, CH), 7.30–8.11 (m, 8H, ArH), 12.11 (s, 1H, NH); MS (EI): m/z (%) 571 ([M+2]+, 10), 569 ([M]+, 16), 497 (23), 480 (20), 356 (95), 125 (100), 77 (24). Anal. Calcd for C27H25BrN2O7: C, 56.95; H, 4.43; N, 4.92. Found: C, 56.86; H, 4.46; N, 4.84.
Ethyl 2-(4-methoxybenzamido)-7,7-dimethyl-4-(2-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (3h). white solid; yield, 60.2%; mp 220–222 °C; 1H NMR (400 MHz, CDCl3), δ 0.96 (s, 3H, CH3), 1.07 (s, 3H, CH3), 1.09 (t, 3H, CH3, J = 7.12 Hz), 2.21 (q, 2H, CH2, J = 16.28 Hz), 2.63 (s, 2H, CH2), 3.97 (s, 3H, CH3), 4.01–4.18 (m, 2H, CH2), 5.82 (s, 1H, CH), 7.27–8.07 (m, 8H, ArH), 12.19 (s, 1H, NH); MS (EI): m/z (%) 520 ([M]+, 18), 447 (57), 370 (23), 202 (32), 106 (100), 77 (21), 51 (10). Anal. Calcd for C28H28N2O8: C, 64.61; H, 5.42; N, 5.38. Found: C, 64.63; H, 5.37; N, 5.38.
Ethyl 2-acetamido-4-(3,4-dimethoxyphenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (3i). white solid; yield, 65.7%; mp162–164 °C; 1H NMR (400 MHz, CDCl3), δ 1.00 (s, 3H, CH3), 1.13 (s, 3H, CH3), 1.20 (t, 3H, CH3, J = 7.12 Hz), 2.15 (s, 3H, CH3), 2.20 (q, 2H, CH2, J = 16.28 Hz), 2.60 (s, 2H, CH2), 3.91 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 4.10–4.15 (m, 2H, CH2), 4.85 (s, 1H, CH), 7.11–8.30 (m, 3H, ArH), 12.20 (s, 1H, NH); MS (EI): m/z (%) 443 ([M]+, 26), 351 (84), 261 (100), 233 (19), 216 (15), 172 (10). Anal. Calcd for C24H29NO7: C, 65.00; H, 6.59; N, 3.16. Found: C, 64.87; H, 6.63; N, 3.26.
Ethyl 2-benzamido-4-(3,4-dimethoxyphenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (3j). white solid; yield, 61.4%; mp 188–189 °C; 1H NMR (400 MHz, CDCl3), δ 1.01 (s, 3H, CH3), 1.13 (s, 3H, CH3), 1.24 (t, 3H, CH3, J = 7.12 Hz), 2.25 (q, 2H, CH2, J = 16.28 Hz), 2.63 (s, 2H, CH2), 3.83 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 4.13–4.16 (m, 2H, CH2), 4.78 (s, 1H, CH), 7.16–8.12 (m, 8H, ArH), 12.23 (s, 1H, NH); MS (EI): m/z (%) 505 ([M]+, 18), 459 (5), 400 (13), 368 (23), 354 (15), 105 (100), 77 (24). Anal. Calcd for C29H31NO7: C, 68.90; H, 6.18; N, 2.77. Found: C, 68.95; H, 6.19; N, 2.88.
Ethyl 2-(4-bromobenzamido)-4-(3,4-dimethoxyphenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (3k). white solid; yield, 59.5%; mp 260–262 °C; 1H NMR (400 MHz, CDCl3), δ 1.01 (s, 3H, CH3), 1.14 (s, 3H, CH3), 1.21 (t, 3H, CH3, J = 7.12 Hz), 2.20 (q, 2H, CH2, J = 16.28 Hz), 2.62 (s, 2H, CH2), 3.91 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 4.12–4.14 (m, 2H, CH2), 4.89 (s, 1H, CH), 7.16–8.12 (m, 7H, ArH), 12.25 (s, 1H, NH); MS (EI): m/z (%) 586 ([M+2]+, 35), 584 ([M]+, 22), 512 (27), 354 (100), 292 (42), 262 (20), 166 (32). Anal. Calcd for C29H30BrNO7: C, 59.60; H, 5.17; N, 2.40. Found: C, 59.71; H, 5.09; N, 2.34.
Ethyl 4-(3,4-dimethoxyphenyl)-2-(4-methoxybenzamido)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (3l). white solid; yield, 75.3%; mp 244–246 °C; 1H NMR (400 MHz, CDCl3), δ 1.02 (s, 3H, CH3), 1.14 (s, 3H, CH3), 1.21 (t, 3H, CH3, J = 7.12 Hz), 2.20 (q, 2H, CH2, J = 16.28 Hz), 2.62 (s, 2H, CH2), 3.89 (s, 3H, CH3), 3.92 (s, 3H, OCH3), 3.97 (s, 3H, OCH3), 4.11–4.13 (m, 2H, CH2), 4.91 (s, 1H, CH), 7.16–8.19 (m, 7H, ArH), 12.26(s, 1H, NH); MS (EI): m/z (%) 535 ([M]+, 43), 462 (23), 353 (99), 291 (100), 261 (31), 177 (25), 77 (36). Anal. Calcd for C30H33NO8: C, 67.28; H, 6.21; N, 2.62. Found: C, 67.35; H, 6.26; N, 2.74.
Ethyl 2-acetamido-4-(furan-2-yl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (3m). white solid; yield, 71.6%; mp 145–146 °C; 1H NMR (400 MHz, CDCl3), δ 1.09 (s, 3H, CH3), 1.15 (s, 3H, CH3), 1.27 (t, 3H, CH3, J = 7.12 Hz), 2.29 (s, 3H, CH3), 2.71 (s, 2H, CH2), 4.18–4.23 (m, 2H, CH2), 5.05 (s, 1H, CH), 6.15–7.63 (m, 3H, ArH), 11.89 (s, 1H, NH); MS (EI): m/z (%) 375 ([M+2]+, 15), 329 (33), 270 (45), 228 (32), 182 (35), 103 (100), 77 (22). Anal. Calcd for C20H23NO6: C, 64.33; H, 6.21; N, 3.75. Found: C, 64.45; H, 6.03; N, 3.86.
Ethyl 2-benzamido-4-(furan-2-yl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (3n). white solid; yield, 67.3%; mp 161–163 °C; 1H NMR (400 MHz, CDCl3), δ 1.07 (s, 3H, CH3), 1.14 (s, 3H, CH3), 1.29 (t, 3H, CH3, J = 7.12 Hz), 2.31 (s, 2H, CH2), 2.63 (s, 2H, CH2), 4.19–4.25 (m, 2H, CH2), 5.04 (s, 1H, CH), 6.12–7.99 (m, 8H, ArH), 11.91 (s, 1H, NH); MS (EI): m/z (%) 435 ([M]+, 3), 389 (8), 362 (8), 330 (16), 284 (6), 105 (100), 77 (25). Anal. Calcd for C25H25NO6: C, 68.95; H, 5.79; N, 3.22. Found: C, 68.98; H, 5.78; N, 3.10.
Ethyl 2-(4-bromobenzamido)-4-(furan-2-yl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (3o). white solid; yield, 60.5%; mp 230–231 °C; 1H NMR (400 MHz, CDCl3), δ 1.08 (s, 3H, CH3), 1.16 (s, 3H, CH3), 1.28 (t, 3H, CH3, J = 7.12 Hz), 2.24 (s, 2H, CH2), 2.62 (s, 2H, CH2), 4.21–4.25 (m, 2H, CH2), 5.08 (s, 1H, CH), 6.11–8.03 (m, 7H, ArH), 11.95 (s, 1H, NH); MS (EI): m/z (%) 516 ([M+2]+, 5), 514 ([M]+, 16), 469 (12), 429 (25), 397 (22), 249 (100), 77 (21). Anal. Calcd for C25H24BrNO6: C, 58.38; H, 4.70; N, 2.72. Found: C, 58.25; H, 4.73; N, 2.75.
Ethyl 4-(furan-2-yl)-2-(4-methoxybenzamido)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (3p). white solid; yield, 73.0%; mp 202–204 °C; 1H NMR (400 MHz, CDCl3), δ 1.11 (s, 3H, CH3), 1.16 (s, 3H, CH3), 1.30 (t, 3H, CH3, J = 7.12 Hz), 2.35 (s, 2H, CH2), 2.65 (s, 2H, CH2), 4.20–4.24 (m, 2H, CH2), 5.11 (s, 1H, CH), 6.14–8.12 (m, 7H, ArH), 12.02 (s, 1H, NH); MS (EI): m/z (%) 466 ([M+1]+, 16), 420 (25), 318 (19), 286 (100), 240 (36), 77 (24). Anal. Calcd for C26H27NO7: C, 67.09; H, 5.85; N, 3.01. Found: C, 67.13; H, 5.88; N, 2.92.
Ethyl 2-acetamido-4-(2,4-dichlorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (3q). white solid; yield, 59.5%; mp 150–151 °C; 1H NMR (400 MHz, CDCl3), δ 1.01 (s, 3H, CH3), 1.13 (s, 3H, CH3), 1.21 (t, 3H, CH3, J = 7.12 Hz), 2.15 (s, 3H, CH3), 2.21 (q, 2H, CH2, J = 16.28 Hz), 2.55 (s, 2H, CH2), 4.08–4.15 (m, 2H, CH2), 4.81 (s, 1H, CH), 7.11–7.85 (m, 3H, ArH), 12.02 (s, 1H, NH); MS (EI): m/z (%) 453 ([M+1]+, 6), 283 (23), 237 (30), 207 (22), 101 (100), 77 (20). Anal. Calcd for C22H23Cl2NO5: C, 58.42; H, 5.13; N, 3.10. Found: C, 58.35; H, 5.15; N, 3.15.
Ethyl 2-benzamido-4-(2,4-dichlorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (3r). white solid; yield,57.1%; mp 231–233 °C; 1H NMR (400 MHz, CDCl3), δ 1.01 (s, 3H, CH3), 1.13 (s, 3H, CH3), 1.20 (t, 3H, CH3, J = 7.12 Hz), 2.21 (q, 2H, CH2, J = 16.28 Hz), 2.63 (s, 2H, CH2), 4.08–4.16 (m, 2H, CH2), 4.83 (s, 1H, CH), 7.14–7.99 (m, 8H, ArH), 12.05 (s, 1H, NH); MS (EI): m/z (%) 514 ([M]+, 4), 432 (5), 368 (24), 105 (100), 77 (23). Anal. Calcd for C27H25Cl2NO5: C, 63.04; H, 4.90; N, 2.72. Found: C, 63.17; H, 4.91; N, 2.74.
Ethyl 2-(4-bromobenzamido)-4-(2,4-dichlorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (3s). white solid; yield, 55.5%; mp 234–236 °C; 1H NMR (400 MHz, CDCl3), δ 1.02 (s, 3H, CH3), 1.18 (s, 3H, CH3), 1.22 (t, 3H, CH3, J = 7.12 Hz), 2.21 (q, 2H, CH2, J = 16.28 Hz), 2.60 (s, 2H, CH2), 4.10–4.16 (m, 2H, CH2), 4.83 (s, 1H, CH), 7.20–8.16 (m, 7H, ArH), 12.08 (s, H, NH); MS (EI): m/z (%) 595 ([M+2]+, 10), 593 ([M]+, 8), 513 (15), 467 (21), 334 (12), 185 (100), 77 (22). Anal. Calcd for C27H24BrCl2NO5: C, 54.66; H, 4.08; N, 2.36. Found: C, 54.70; H, 4.11; N, 2.41.
Ethyl 4-(2,4-dichlorophenyl)-2-(4-methoxybenzamido)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (3t). white solid; yield, 64.6%; mp 207–209 °C; 1H NMR (400 MHz, CDCl3), δ 1.02 (s, 3H, CH3), 1.19 (s, 3H, CH3), 1.23 (t, 3H, CH3, J = 7.12 Hz), 2.21 (q, 2H, CH2, J = 16.28 Hz), 2.61 (s, 2H, CH2), 3.95 (s, 3H, CH3), 4.10–4.17 (m, 2H, CH2), 4.83 (s, 1H, CH), 7.21–8.15 (m, 7H, ArH), 12.03 (s, H, NH); MS (EI): m/z (%) 545 ([M+1]+, 7), 463 (13), 361 (29), 212 (18), 140 (100), 77 (24). Anal. Calcd for C28H27Cl2NO6: C, 61.77; H, 5.00; N, 2.57. Found: C, 61.71; H, 5.02; N, 2.60.
X-ray analysis of compound 3n
The single crystal 3n was obtained by evaporating the solvent. An Orthorhombic crystal with dimensions of 0.16 × 0.12 × 0.10 mm was mounted on a Bruker SMART APEX CCD single crystal X-ray diffraction at 100(2)K with graphite-monochromated MoKα radiation (λ = 0.71073 Å). The crystal data for 3n: C25H25NO6; Triclinic, space group P−1, a = 14.838 Å, b = 14.838(2) Å, c = 19.744(3) Å, V = 4347.2(9) Å3, Z = 8, Dc = 1.331 mg/m3. A total of 31,275 reflections were measured in the range of 2.06°≤ θ ≤ 27.00°, of which 4,739 reflections (R int = 0.1203) were independent and 3,650 were observed with I>2σ (I). The structure was solved by direct methods and refined on F 2 by full-matrix least-squares procedure with Bruker SHELXTL-97 package [31]. All other calculations were performed with Bruker SAINT System and Bruker SMART programs [32]. Full-matrix least-squares refinement based on F 2 using the weight of 1/[σ 2(F 2o ) + (0.0602P)2 + 0.0000P] where P = (F 2o +2F 2c )/3 gave final values of R = 0.0428, ωR = 0.1076, and GOF(F) = 1.036 for 293 variables and 4,739 contributing reflections. Maximum shift/error 0.001, max/min residual electron density = 0.374/−0.281 e Å−3.
References
K. Singh, J. Singh, H. Singh, Tetrahedron 52, 14273–14280 (1996)
S. Hatokeyama, N. Ochi, H. Numata et al., J. Chem. Soc. Chem. Commun. 17, 1202–1204 (1988)
G.T. Brooks, A.P. Ottridge, R.C. Jennings et al., Pestic. Sci. 16, 571–588 (1985)
E.C. Witte, P. Neubert, A. Roesch, Ger. Offen. DE3427985, (1986)
N. Chand, W. Diamantis, R.D. Sofia, Brit. J. Pharmcol. 87, 443–448 (1986)
T. Hyana, H. Saimoto, Jpn Kokai Tokkyo Koho. JP 62181276, (1987)
V.B. Chavan, G.S. Sarate, N.S. Tankhiwale et al., Indian J. Anaesth. 42, 192–194 (1994)
Z.J. Liu, Z.R. Li, Chin. J. Org. Chem. 11, 433–436 (1991)
V.F. Paula, L.C.A. Barbosa, A.J. Demuner et al., Pest Manage. Sci. 56, 168–174 (2000)
J.S. Pizey, R.L. Wain, J. Sci. Food. Agric. 10, 577–584 (1959)
M. Suarez, E. Salfran, Y. Verdecia et al., Tetrahedron 58, 953–960 (2002)
Q.Y. Zhuang, N. Wu, D.Q. Shi et al., Chin. J. Org. Chem. 26, 1217–1220 (2006)
S.J. Tu, Y. Gao, C. Guo et al., Synth. Commun. 32, 2137–2141 (2002)
Z.Q. Jiang, S.J. Ji, J. Lu et al., Chin. J. Chem. 23, 1085–1089 (2005)
I. Devi, P.J. Bhuyan, Tetrahedron Lett. 45, 8625–8627 (2004)
L.C. Rong, X.Y. Li, H.Y. Wang et al., Synth. Commun. 36, 2363–2369 (2006)
G. Kaupp, M.R. Naimi-Zamal, J. Schmeyers, Tetrahedron 59, 3753–3760 (2003)
C.J. Li, Chem. Rev. 93, 2023–2035 (1993)
A. Lubineau, Chem. Ind. (London) 4, 123–126 (1996)
A. Meijer, S. Otto, J.B. Engberts, J. Org. Chem. 63, 8989–8994 (1998)
R. Ballini, G. Bosica, Tetrahedron Lett. 37, 8027–8030 (1996)
R. Ballini, G. Bosica, J. Org. Chem. 62, 425–427 (1997)
F. Bigi, S. Carloni, L. Ferrari et al., Tetrahedron Lett. 42, 5203–5205 (2001)
G.M. Ziarani, A. Abbasi, A. Badiei et al., J. Chem. 8, 293–299 (2011)
S.B. Bandgar, B.P. Bandga, B.L. Korbad et al., Aust. J. Chem. 60, 305–307 (2007)
X.S. Wang, D.Q. Shi, S.J. Tu et al., Synth. Commun. 33, 119–126 (2003)
Q.Y. Wang, Master’s degree thesis of Hubei University, China, (2009)
S.J. Tu, H. Wang, J.Q. Feng et al., Synth. Commun. 31, 2663–2666 (2001)
S.J. Tu, C.B. Miao, Y. Gao et al., Chin. J. Chem. 20, 703–706 (2002)
D.Q. Shi, S. Zhang, Q.Y. Zhuang, Chin. J. Org. Chem. 25, 1570–1574 (2005)
G.M. Sheldrick, SHELXS-97: program for crystal structure refinement; Göttingen, (1997)
SAINT and SMART; Bruker AXS: Madison, (2003)
Acknowledgments
We are grateful to financial support from the National NSFC (No.21162007), Project for Department of Education, Hainan Province (No.Hj2009-19) and Hainan University Postdoctoral Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeng, Zg., Wang, Ly., Cao, Y. et al. Synthesis of 2-amide-3-carboxylate-4-aryl-4H-chromene derivatives. Res Chem Intermed 38, 1751–1760 (2012). https://doi.org/10.1007/s11164-012-0500-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0500-6