Abstract
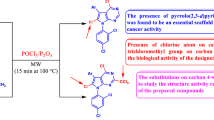
This work aimed to produce novel heterocyclic compounds such as chromene, thieno[3,2-f]chromene, chromeno[5,6-d]thiazole and quinoline derivatives. The newly synthesized heterocyclic compounds were evaluated against cancer cell lines aiming to get new anticancer agents. Dimedone underwent different multi-component reactions to produce fused thiophene, thiazole, coumarin, pyran and pyridine derivatives. Some reactions were catalyzed by effective magnetically separable nanocatalyst. The anti-proliferative activity of the newly synthesized compounds toward six cancer cell was studied. In addition, inhibitions of the most active compounds the thieno[3,2-f]chromene derivatives 16a–f toward cancer cell lines classified according to the disease were also studied. Moreover, the newly synthesized compounds were screened for their anticancer potentials against hepatocellular carcinoma HepG2 and cervical carcinoma HeLa cell lines. Anti-proliferative evaluations, inhibitions were performed for all of the synthesized compounds where the varieties of substituent through the aryl ring and the heterocyclic ring afforded compounds with high activities. Inhibitions toward cancer cell lines classified according to the disease together with inhibitions toward HepG2 and cervical carcinoma HeLa cell lines were measured. Molecular docking of compounds 15c and 18c was performed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The multi-component reactions (MCRs) was considered as an elegant and rapid way to synthesize structurally diverse bioactive heterocyclic compounds in a single synthetic operation from simple reagents through. In the field of drug discovery and medicinal chemistry, multi-component reactions were the most applicable reactions. Due to the advantages of multi-component reactions like high atom-economy, simplification of reagents, high yields of products and high selectivity of products many researches were directed through their applications in recent years [1,2,3,4]. One of the most important classes of compounds produced via the multi-component reactions was the 4H-benzo[b]pyran derivatives. Such group of compounds exhibited numerous pharmacological and biological activities [5]. They showed pronounced biological activities among which diuretic, anti-allergic, antibacterial, anticoagulant, anticancer and anti-anaphylactic activities, as well as their use in therapy [6,7,8,9]. There are different methods for the synthesis of 4H-benzo[b]pyran derivatives depending on the catalyst used through the reaction of aromatic aldehydes, malononitrile (or ethyl cyanoacetate) and dimedone. Some reactions were achieved using tetra-alkyl ammonium salts, acidic salts, tricarboxylic acid salts, organo-metallic catalyst, acidic ionic liquids and some silica salts [10,11,12,13,14,15,16,17,18,19,20,21,22]. The yield of products were varied from catalyst to another as some of them gave high yield and others showed lower yields, in additions, some of these catalysts with necessity of use toxic solvents especially during the work-up procedures [23]. To overcome such difficulties, other alternating and clean procedures were adopted for the synthesis of 4H-benzo[b]pyran derivatives. According to WHO Cancer is a generic term for a large group of diseases that can affect any part of the body. Other terms used are malignant tumors and neoplasm’s. One defining feature of cancer is the rapid creation of abnormal cells that grow beyond their usual boundaries, and which can then invade adjoining parts of the body and spread to other organs; the latter process is referred to as metastasis. Widespread and metastases are the primary cause of death from cancer. Cancer is a leading cause of death worldwide, accounting for nearly 10 million deaths in 2020 (1). The most common in 2020 (in terms of new cases of cancer) were breast, lung, colon, prostate, skin and stomach [24,25,26]. As the result of the large spread of cancer and the anticancer activities of 4H-benzo[b]pyran derivatives we were concerned with the synthesis of such compounds [27,28,29]. Moreover, the 4H-pyran derivatives were known to be good anti-cancer agents this appeared in many reports [30,31,32]. Due to the large applications of 4H-pyran derivatives we were involved through a comprehensive program for the synthesis of such group of compounds. As a continuation of that work and in view of our ongoing efforts to explore newer reactions for synthesis of heterocyclic compounds, in this work, in regard we report here different muti-component reactions of dimedone with different active ethylene compounds and 1,3-dicarbonyl compounds. The products underwent further heterocyclization reactions to give annulated products. Apart from that, some of the inhibitors identified were subjected to molecular docking study using Saccharomyces cerevisiae isomaltase crystal structure obtained from the protein data bank. This in silico analysis can help to visualize the binding mode and interaction between the inhibitors and the proteins that cause the inhibitory activity [33,34,35]. The computer-aided QSAR modeling, molecular docking simulation and ADMET predictions were employed to develop a validated QSAR model through different statistical parameters, elucidating molecular interactions between the 3D structure of the receptor and ligands with their binding modes and also to predict the Pharmacokinetics and ADMET properties of the compounds as inhibitors of serotonin transporter (SERT) [36]. Consequently, the information obtained from this study could be used as a reliable framework and rational template for structural modifications/adjustments of the compounds in developing potential inhibitors of the serotonin transporter (SERT) as novel antidepressant agents with the improved inhibitory potency. The antitumor evaluations of the newly synthesized products were measured in the aim of producing anticancer agents.
Results and discussion
The reaction sequences for the synthesized compounds were demonstrated through Schemes 1, 2, 3 and 4 starting with dimedone. The multi-component reaction of dimedone (1) with either benzaldehyde (2a), 4-methoxybenzaldehyde (2b) or 4-chlorobenzaldehyde (2c) and either maononitrile (3a) or ethyl cyanoacetate (3b) gave the 7,8-dihydro-4H-chromen-5(6H)-one derivatives 4a–f, respectively. Compounds 4a, 4c or 4a reacted with elemental sulfur and either of malononitrile (3a) or ethyl cyanoacetate (3b) in 1,4-dioxane containing tiethylamine to give the thieno[3,2-f]chromene-8-carbonitrile derivatives 5a–f, respectively (Scheme 1). The reaction took place through Gewald’s thiophene synthesis [37,38,39]. The structures of the latter products were based on their respective analytical and spectral data. Thus, the 1H NMR spectrum of compound 5a showed the presence of two NH2 groups at δ 4.87, 5.22 ppm (D2O exchangeable) and a singlet at δ 2.40 ppm indicating one CH2 group. In addition, the 13C NMR spectrum revealed the presence of two signals at δ 116.8 and 117.0 due to the presence of two CN groups and signals at δ 136.0, 138.2, 139.8, 140.1, 142.8, 143.2, 143.8 and 145.8 due to the presence of the thiophene and pyran carbons.
On the other hand, the multi-component reactions of dimedone (1), the 3-methyl-1H-pyrazol-5(4H)-one (6) and either benzaldehyde (2a), 4-methoxybenzaldehyde (2b) or 4-chlorobenzaldehyde (2c) in ethanol containing a catalytic amount of triethylamine gave the tetrahydrochromeno[2,3-c]pyrazol-5(1H)-one derivatives 7a–c, respectively. In addition, the multi-component reactions of dimedone (1), cyanothioacetamide (8) and either benzaldehyde (2a), 4-methoxybenzaldehyde (2b) or 4-chlorobenzaldehyde (2c) in 1,4-dioxane containing a catalytic amount of triethylamine gave the hexahydroquinoline-3-carbonitrile derivatives 9a–c, respectively (Scheme 2).
In continuation of such series of reactions, the multi-component reactions of dimedone (1), with either ethyl benzoylacetate (10a) or ethyl acetoacetate (10b) and salicylaldehyde (11) in ethanol containing a catalytic amount of triethylamine gave the dihydrochromeno[3,4-c]chromene 12a and 12b, respectively. Structures of the latter products were based on their respective analytical data. Thus, the 1H NMR spectrum of 12a showed a singlet at δ 6.52 ppm indicating CH of pyran, and 13C NMR spectrum revealed the presence of a signal at δ 166.5, 168.4 due to the presence of two C=O groups and signals at δ 139.3, 140.2, 142.8 and 145.2 due to the pyran carbons.
On the other hand, the multi-component reactions of dimedone (1), with either of malononitrile (3a) or ethyl cyanoacetate (3b) and salicylaldehyde (11) gave the same dihydrochromeno[3,4-c]chromene (13). Formation of the same product can be explained through the intermediate formation of the imino product in case of the reaction with malononitrile followed by hydrolysis of the C=NH group into C=O. On the other extreme, in case of ethyl cyanoacetate ethanol elimination took place to give directly the C=O.
The multi-component reaction of dimedone (1), with either benzaldehyde (2a), 4-methoxybenzaldehyde (2b) or 4-chlorobenzaldehyde (2c) and ethyl benzoylacetate (10a) in ethanol containing a catalytic amount of triethylamine yielded the pyran derivatives 14a–c. However, carrying the same reaction but using ammonium acetate instead of triethylamine gave the pyridine derivatives 15a–c, respectively (Scheme 3).
Compounds 14a–c were capable for the Gewald’s thiophene synthesis due to the presence of cyclohexenone moiety. Thus, either compounds 14a, 14b or 14c reacted with elemental sulfure and either malononitrile (3a) or ethyl cyanoacetate (3b) to produce the thieno[3,2-f]chromene-8-carboxylate derivatives 16a–f, respectively. The analytical and spectral data of the latter products were in agreement with their respective structures. Thus, the 1H NMR spectrum of 16a as an example) showed the presence of one NH2 group at δ 5.18 ppm (D2O exchangeable) and a singlet at δ 6.50 ppm indicating pyran CH, and the 13C NMR spectrum showed the presence of a signal at δ 116.8 due to the presence of CN group and signals at δ 136.3, 138.8, 139.4, 141.4, 142.5, 143.0, 143.2, 144.5 due to the thiophene and pyran carbons. On the other hand, the reaction of either of compounds 14a–c with elemental sulfur and phenylisothiocyanate gave the 2,4,5,9-tetrahydro-1H-chromeno[5,6-d]thiazole derivatives 18a–c, respectively. Either of the latter compounds 18a–c reacted with two fold of hydrazine hydrate or phenylhydrazine to give the 2-hydrazono-chromeno[5,6-d]thiazole-8-carbohydrazide derivatives 20a–f, respectively (Scheme 4). Structures of compounds 18a–c and 20a–f were based on analytical and spectral data (see experimental section). It is of a great value to mention that the multi-component reactions producing compounds 4a–f; 7a–c; 9a–c; 12a,b; 13 and 14a–c took place by two methods. The first method was the regular solvent method using a catalytic amount of triethylamine and the second method was the solvent free using by nanoparticle-immobilized ionic liquid [40]. Table 1 showed a comparison between yields of the two methods where the second method in all cases of higher yields than the first method.
Ionic liquids immobilized on MNPs in multicomponent reactions
Recent studies represent that magnetic nanoparticles (MNPs) are excellent supports for ILs owing to their good stability, easily preparation and functionalization, high surface special features have made MNPs a convenient alternative to catalyst supports. As an example, a magnetically Fe3O4@SiO2 nanoparticle-immobilized ionic liquid (MNPs@SiO2-IL) was prepared by Azgomi and Mokhtary [41]. The Fe3O4@MCM‐41‐SO3H@[HMIm][HSO4] efficiently catalyzed the one‐pot three‐component condensation reactions for the synthesis of 4a–f; 7a–c; 9a–c; 12a,b; 13 and 14a–c (Schemes 1, 2 and 3). The yields and purity of obtained compounds were much better than the same compounds obtained using ethanol and triethylamine. Table 1 showed a comparison between the reactions catalyzed by Et3N and those catalyzed by nanoparticle-immobilized ionic liquid. Furthermore, the catalyst was able to be good distributed in the reaction media, simply retrived from the reaction mixture by using a magnet, and reused for several times with no significant loss in activity. This procedure offered several advantages including mild reaction conditions, cleaner reaction, and satisfactory yields of products, as well as a simple experimental and isolated procedure, which make it a useful and attractive protocol for the synthesis of these compounds.
Biology assay
Cell proliferation assay
The six cancer cell lines namely A549, HT-29, MKN-45, U87MG, SMMC-7721 and H460 were used for the valuation of the newly synthesized compounds using Foretinib as the positive control [42]. The in-vitro assay was carried out using standard MTT procedure. IC50’s (inhibitory concentrations 50%) were measured for each compound and determined as the result of the average of three determinations.
The mean values of three independent experiments, expressed as IC50 values, were presented in Table 2. Most of the synthesized compounds exhibited potent anti-proliferative activity with IC50 values less than 30 µM. Generally, the variations of substituent’s within the aryl and the heterocycle ring being attached have a notable influence on the anti-proliferative activity.
Structure activity relationship
It was clear from Table 1 that compounds 4e, 4f, 5e, 5f, 7c, 9c, 13, 14c, 15c, 16e, 16f, 18c, 20c and 20f were the most cytotoxic compounds against the six cancer cell lines. Considering the pyran derivatives 4a–f, it was clear that compounds 4e and 4f were the most cytotoxic compounds exhibiting IC50 < 1.0. Considering the 5,9-dihydro-4H-thieno[3,2-f]chromene derivatives 5a–f, it was clear that compound 5a showed the lowest activities among the tested compounds while compound 5c eexhibited moderate inhibitions toward the six cancer cell lines. In addition compounds 5e and 5f exhibited the highest inhibitions toward the six cancer cell lines. Considering the tetrahydrochromeno[2,3-c]pyrazole derivatives 7a–c, interestingly compound 7a (X = H) exhibited moderate inhibitions while compound 7c (X = Cl) exhibited high inhibitions toward the six cancer cell lines. For the hexahydroquinoline derivatives 9a–c, compound 9c (X = Cl) exhibited the highest inhibitions toward the six cancer cell lines among the three compounds. Both of the two compounds 12a and 12b exhibited from moderate to high inhibitions this is due to the presence of the chromeno[3,4-c]chromene nucleus within the structure of both compounds. However compound 12a exhibited high inhibitions than compound 12b due to the presence of the phenyl substituent. Similarly, for the 3,4-dihydrochromeno[3,4-c]chromene derivative 13 exhibited high inhibitions toward the six cancer cell lines. Considering the pyran derivatives 14a–c and the pyridine derivatives 15a–c where compound 14a and 15a showed relatively high inhibitions than 14b and 15b since the latter compounds have an electron donating substituent (X = OCH3) in their structures. In addition, compounds 14c (X = Cl) and 15c (X = Cl) exhibited the highest cytotoxicity among the six compounds, this was attributed to the presence of the electronegative substituent Cl group in their structures. On the other hand for the thieno[3,2-f]chromene derivatives 16a–f, compound 16c although it contains an electron donating substituent (X = OCH3) showed moderate activities toward the six cancer cell lines. Moreover, compounds 16e and 16f exhibited the highest inhibitions among the six compounds. This was attributed to the presence of the thiophene moiety together of the electron withdrawn substituent the Cl group in their structures. However, for the thiazole derivatives 18a–c and 19a–f compounds 18b and 19c although they contain an electron donating group, they showed high cytotoxicities toward the six cancer cell lines. This was attributed to the conjugation of the methoxy group with the aryl group. In addition, compounds 18c, 19e and 19f were the most cytotoxic among the nine compounds and this was attributed to the presence of the electron withdrawn substituent Cl group within their structures.
Mechanism of action
It is clear from Table 1 that compounds 4e, 7c and 20f were the most cytotoxic compounds among the tested compounds towards the six cancer cell lines. From the structure point of view, it is clear that the high activity of compound 4e was attributed to the presence of the 4-chlorophenyl moiety beside the CO and CN groups. Similarly, the high activity of compound 7c was due to not only 4-chlorophenyl but also the carbonyl and pyrazole moieties. Moreover, the reactivity of 20f was attributed to the 4-chlorophenyl together to the hydrazone and hydrazide moieties. A large number of points of contact are favorable from a pharmacodynamic perspective since it enables a more specific and unique drug-receptor interaction, concomitantly decreasing the likelihood of toxicity. However, a large number of points of contact are unfavorable from a pharmacokinetic perspective, since the resulting increased polarity of the drug molecule tends to decrease the pharmacological half-life and also to decrease the ability of the drug to diffuse across membranes during its distribution throughout the body. In general, most neuroactive drugs have 2–4 points of contact, while most non-neuroactive drugs have 3–6 points of contact. Through our study, it is clear that most of the synthesized molecules with two carbonyl groups (toxicophores) together with the heteroatom sites that enables 2–4 points of contact. A relationship between chemical structure or chemical properties and biological action, SAR, is in the nature of things and undeniable, not with standing the fact that it is not always easily recognized. Firstly, the functional group approachs: This takes into account the significance of particular groups in the molecule for particular aspects, part processes, in the biological action. Examples are groups described aspharmacophores or toxicophores [43, 44]. Secondly, the integral approach: In this case the overall properties of the molecules count. When these two approaches were applicable through our synthesized molecule increases their applicability as good anticancer agents.
In vitro evaluation of the anticancer activity
A panel of approximately seventeen tumor cell lines at tenfold dilutions of five concentrations (100 µM, 10 µM, 1.0 µM, 0.1 µM and 0.01 µM) [45, 46] were used for testing the thieno[3,2-f]chromene derivatives 16a–f (Table 3) + . The percentage of growth was evaluated spectrophotometrically versus controls not treated with test agents after 48-h exposure and using SRB protein assay to estimate cell viability or growth. Dose–response parameters were calculated for each cell line: GI50-molarconcentration of the compound that inhibits 50% net cell growth. The tested compounds showed inhibition activity (GI50 < 5 µM) against the selected cancer cell lines that are classified into groups according to the type of disease, the data were shown through Table 3. Throughout compounds 16a–f, there are three factors, the substituent at C-3 of the thiophene ring and the substituent at the 4-position of the aryl group. It is clear from Table 2 that all tested compounds exhibited high inhibitions toward the cell lines categorized according to the type of the disease. Compound 16a (X = H, R′ = CN) exhibited high inhibition toward HOP-62, MDA-MB-435 and UACC-62 cell lines with IG50’s 0.42, 0.41 and 0.88 µM, respectively. On the other hand, compound 16b (X = H, R’ = COOEt) showed high inhibitions toward HOP-62, HCT-15, UACC-62, OVCAR-3 cell lines with IG50’s 0.31, 0.59, 0.39 and 0.25 µM, respectively. Compound 16c (X = OCH3, R′ = CN) showed high inhibitions toward RPMI-8262, HOP-62, HCT-15, KM12, UACC-62 and 786-0 cancer cell lines with IG50’s 0.85, 0.29, 0.32, 0.35, 0.27 and 0.42 µM, respectively. Moreover, compound 16d (X = OCH3, R′ = COOEt) exhibited high inhibitions toward HI-60 (TB), HOP-62, HOP-62, NCI-H460, HCT-116, HCT-15, KM12, SF-295, UACC-62, OVCAR-3 and 786-0 cell lines with IG50’s 0.47, 0.37, 0.22, 0.73, 0.40 and 0.31 µM, respectively. Interestingly, compounds 16e (X = Cl, R′ = CN) and 16f (X = Cl, R′ = COOEt) exhibited high inhibitions toward all cancer cell lines except compound 16e showed moderate inhibitions toward NCI-H460 and MDA-MB-435 cell lines with IG50’s 2.31 and 1.52 µM, respectively.
Cytotoxic activity
Hepatocellular carcinoma HepG2 and cervical carcinoma HeLa were used for screening of the newly synthesized compounds. The cytotoxicity of the compounds was determined using MTT assay and Doxorubicin as a positive control [47,48,49,50,51]. In general, it can be seen that all synthesized compounds exhibited cytotoxic activities against both tested cancer cell lines. Moreover, it can be seen that both cells reacted in a dose-dependent manner toward the applied concentrations. Additionally, both tested cell lines varied in their response toward different synthesized compounds. Furthermore, based on the IC50 values (Table 4) obtained for the tested compounds, it can be seen that cytotoxic activities ranged from very strong to non-cytotoxic. Most of the tested compounds exhibited high cytotoxicity except compounds 4c, 4d, 5a, 5c, 7a, 9b, 12b, 14a and 16a. The cytotoxic compounds exhibited higher inhibitions than the reference doxorubicin. For compounds 4a–c and 5a–f it was surprisingly that compound 4d and 5d exhibited the high cytotoxicity although they contain the electron donating OCH3 group. On the other hand, compounds 4e, 4f, 5e and 5f (X = Cl) exhibited the highest inhibitions among the twelve compounds. Considering compounds 7a–c and 9a–c it was clear that compounds 7c and 9c exhibited the highest inhibitions toward and HeLa cell lines. The 3,4-dihydrochromeno[3,4-c]chromene derivative 13 exhibited high inhibitions against HepG2 and HeLa cell lines. For compounds 14a–c and 15a–c it was obvious compounds 14b, 14c, 15a and 15c showed highest inhibitions with IC50’s 3.25, 1.68, 2.18 and 0.49 µM, respectively, against HepG2 cell line and IC50’s 2.39, 3.14, 3.26 and 0.58 µM, respectively, against the HeLa cell line. For the thieno[3,2-f]chromene 16a–f, compounds 16d, 16e and 16f exhibited the highest inhibitions among the six compounds. Interestingly, the three compounds of the chromeno[5,6-d]thiazole derivatives 18a–c and 20a–f exhibited high inhibitions toward the HepG2 and HeLa cell lines.
In silico study for compounds 15c and 18c
EGFR
Docking study of compounds 15c and 18c was prepared by using AutoDock suite 4.2.6 softwar. Due to the structural similarities between the synthesized compounds and reference drugs presented in both EGFR and PIM-1 enzymes that may give promising molecular docking or at least give hint about expecting the antitumor activity of compounds we studied the molecular docking results of compounds 15c and 18c. The molecular docking study was done at first at ATP binding site of EGFR and PIM-1 kinases to surmise if these compounds have similar binding mode to the EGFR PIM-1 kinase inhibitors (Figs. 1, 2).
Many anticancer agents were designed to inhibit the tumor cells by diminishing the replication and transcription of DNA.EGFR (epidermal growth factor receptor) is a cellular trans-membrane tyrosine kinase significantly, secreted in elevated levels in many types of human tumors e.g. ovarian, colon, breast, renal and prostate [52, 53]. Irregular EGFR signaling probably play substantial role in the pathogenesis of cancer, and thus, the mechanisms of EGFR-mediated oncogenic signaling are of concern. The amplification of EGFR gene and over expression are a predominantly attractive feature of glioblastoma (GBM), which found in nearly 40% of tumors. In adults, GBM is most widespread primary malignant tumor of the central nervous system (CNS) [54]. The EGFR coding network donates an attractive objective for therapeutic intervention, and massive effort is converged on the trials to deactivating or blocking the receptor in different cancer types by using antibodies, tyrosine kinase inhibitors (TKIs) or even vaccines [55].
The docking results revealed good binding ability with highly energetically stable score of compounds 15c and 18c for EGFR. Molecular docking results of the compounds 15c and 18c into the ATP binding site of EGFR kinases were demonstrated. The targets compounds were docked into ATP of EGFR (1m17, from PDB: code 1M17 [56, 57] receptor active site pocket, which contain Erlotinib as co-crystallized ligand (Figs. 1, 2). A two hydrogen bonds showed clearly, and they expounded as, N-1 of the quinazoline ring binds to the hydrophobic pocket of N-terminal domain of Epidermal growth factor receptor tyrosine kinase (EGFR-TK) with NH Of Met-769 (backbone interaction) and C-2 of the quinazoline ring binds to C=O of Gln-767 (backbone interaction) via hydrog-en bonding. That interactions showed how quinazoline ring bindings explicating inhibitory effect EGFR. Similarly, 15c and 18c docking results showed that compounds 15c with two hydrogen bond with good bond length 3.22 and with very good harmony to the reference drug (RMSD = 1.32 and energy score of -6.60) (Figs. 3, 4). Whereas, compound 18c gave one hydrogen bond supported with one aromatic interaction shown in (Table 5) (Figs. 5, 6).
PIM1 receptor
PIM1 crystal structure complex with its inhibitor uploaded from Protein Data Bank (PDB ID code: 2OBJ) [58,59,60]. Like EGFR, targets 15c and 18c were docked into the pocket of receptor active site shown in Figs. 3, 4, 5 and 6. Docking profiles do not differ from the practical data and revealed excellent RMSD &binding scores. Compound 18c binds to the active amino acid residue with H-bonding and hydrophobic (aromatic) interaction typically as the co-crystalline ligand (6-(5-BROMO-2-HYDROXYPHENYL)-2-OXO-4-PHENYL-1,2-DIHYDRO-PYRIDINE-3-CARBONITRILE or VRV as named in PDB) as in Figs. 5 and 6. Compound 18c showed two hydrogen bonds with 0.91 RMSD value confirming the superimposition fitness of 18c interior the active site pocket similarly to VRVco-crystallized ligand. Whereas, VRV itself displayed two interactions one of them is strong hydrogen bonding between Lys 67 and C=O with bond length of 4.0 angstrom and the other is aromatic interaction with Val52.
Docking procedure of EGFR kinase and PIM-1 kinase
The reference (co-crystallized) compounds in both 1M17 & 2OBJ were labeled as colored structure. The binding sites were recognized automatically from surfaces and maps options and docking proceeded directly after ligand and protein preparation. Default settings were done to perform molecular docking using MOE-Dock options through “Rotate Bonds” selection to permit flexible ligand-rigid receptor docking. The scoring function fitted to be London G with a replacement of Triangle matcher. 30Conformers of the highest score ligand were retained. The top five scoring of ligand-receptor docking was then viewed by 2D and 3D ligand-receptor interactions.
Enzyme inhibition
Based on the data obtained from the antitumor results listed in Tables 2 and 3 and molecular docking study consequences, compounds 15c and 18c were chosen to be in-vitro tested against both EGFR and Pim-1 enzymes and the equivalent Table 5 especially, these two compounds were chosen to study their enzyme inhibitions using ELISA-based EGFR-TK and Pim kinase kits. Based on the data of Table 5 compounds 15c and 18c showed high inhibitions toward EGFR and Pim-1 enzymes and such inhibitions were close to the references used Erlotinib and Qurecitin (Table 6).
Experimental
Dry solvents were used through this work and all Melting points of the synthesized compounds were recorded on Buchi melting point apparatus D-545. The IR spectra (KBr discs) were recorded on Bruker Vector 22 instrument. 13C NMR and 1H NMR spectra were measured on Bruker DPX300 instrument in DMSO-d6 with TMS as internal standard. Mass spectra were measured using EIMS (Shimadzu) and ESI-esquire 3000 Bruker Daltonics instrument. Elemental analyses were measured using the Micro-analytical Data center at Cairo University. All reactions was monitored by TLC on 2 × 5 cm pre-coated silica gel 60 F254 plates of thickness of 0.25 mm (Merck) for getting complete reactions.
General procedure for the synthesis of the 7,8-dihydro-4H-chromen-5-one derivatives 4a–c
Method (A): Each of either benzaldehyde (1.06 g, 0.01 mol), 4-methoxybenzaldehyde (1.36 g, 0.01 mol) or chlorobenzaldehyde (1.40 g, 0.01 mol) and either malononitrile or ethyl cyanoacetate were added to a solution of dimedone (1.40 g, 0.01 mol) in ethanol (40 mL) containing triethylamine (0.50 mL). The reaction mixture was heated under reflux for 3 h then left to cool and the formed solid product, in each case was collected by filtration.
Method (B): Each of either benzaldehyde (1.06 g, 0.01 mol), 4-methoxybenzaldehyde (1.36 g, 0.01 mol) or chlorobenzaldehyde (1.40 g, 0.01 mol) and either malononitrile or ethyl cyanoacetate were added to dimedone (1.40 g, 0.01 mol). To the reaction mixture the Fe3O4@MCM-41-SO3H@[HMIm][HSO4] catalyst was added. The whole reaction mixture was reacted in a test tube with a glass bar at 110 °C under solvent-free condition for the appropriate time. When the reaction was completed, checked by TLC, the reaction mixture was dissolved in ethanol (5 mL) and the catalyst was isolated by applying the magnetic field. Then, the filtrate was concentrated on a rotary evaporator under reduced.pressure and the solid crude product created was washed with water and recrystallized from ethanol to afford pure products.
2-Amino-7,7-dimethyl-5-oxo-4-phenyl-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (4a)
Yellow crystals from 1,4-dioxane, yield (1.99 g, 68%), m.p.195–197 °C. IR (KBr) ν max (cm−1): 3480–3320 (NH2), 3050 (CH-aromatic), 2953 (CH-aliphatic), 2220 (CN), 1703 (CO), 1580 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.09, 1.03 (2s, 6H, 2CH3), 2.27, 2.39 (2s, 4H, 2CH2), 4.80 (s, 2H, D2O exchangeable, NH2), 6.50 (s, 1H, CH- pyran), 7.26–7.40 (m, 5H, C6H5). 13C NMR (DMSO-d6, 75 MHz): δ 24.3 (2CH3), 36.3, 56.2 (2CH2), 24.5 (2CH3), 90.8 (pyran C-4), 116.8 (CN), 120.4, 121.8, 123.3, 124.2 (C6H5), 139.6, 140.3, 142.6, 145.8 (pyran C-2, C-3, C-5, C-6), 168.3 (C=O). Analysis Calculated for: C18H18N2O2 (294.35): C, 73.45; H, 6.16; N, 9.52. Found: C, 73.58; H, 6.22; N, 9.69%. EIMS: m/z 294 [M]+ (40%).
Ethyl 2-amino-7,7-dimethyl-5-oxo-4-phenyl-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (4b)
Yellow crystals from 1,4-dioxane, yield (2.04 g, 60%), m.p. 145–147 °C. IR (KBr) ν max (cm−1): 3490–3342 (NH2), 3050 (CH-aromatic), 2953 (CH-aliphatic), 1703, 1690 (2CO), 1580 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.08, 1.04 (2s, 6H, 2CH3), 1.12 (t, 3H, J = 6.88 Hz, CH3), 2.27, 2.38 (2s, 4H, 2CH2), 4.20 (q, 2H, J = 6.88 Hz, CH2), 4.83 (s, 2H, D2O exchangeable, NH2), 6.52 (s, 1H, CH- pyran), 7.25–7.42 (m, 5H, C6H5). 13C NMR (DMSO-d6, 75 MHz): δ 16.1 (OCH2CH3), 24.4 (2CH3), 36.1, 56.5 (2CH2), 24.6 (2CH3), 52.3 (OCH2CH3), 90.7 (pyran C-4), 120.1, 120.9, 122.5, 124.9 (C6H5), 139.3, 140.2, 142.5, 144.3 (pyran C-2, C-3, C-5, C-6), 165.3, 168.1 (2C=O). Analysis Calculated for: C20H23NO4 (341.40): C, 70.36; H, 6.79; N, 4.10. Found: C, 70.58; H, 6.72; N, 4.37%. EIMS: m/z 341 [M]+ (40%).
2-Amino-4-(4-methoxyphenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (4c)
Yellow crystals from 1,4-dioxane, yield (2.36 g, 73%, m.p. 219–221 °C. IR (KBr) ν max (cm−1): 3468–3320 (NH2), 3050 (CH-aromatic), 2953 (CH-aliphatic), 2220 (CN), 1702 (CO), 1582 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.07, 1.04 (2s, 6H, 2CH3), 2.29, 2.32 (2s, 4H, 2CH2), 3.67 (s, 3H, OCH3), 4.82 (s, 2H, D2O exchangeable, NH2), 6.53 (s, 1H, CH- pyran), 7.23–7.42 (m, 4H, C6H4). 13C NMR (DMSO-d6, 75 MHz): δ 24.6 (2CH3), 36.1, 56.0 (2CH2), 24.3 (2CH3), 50.2 (OCH3), 90.8 (pyran C-4), 116.8 (CN), 120.1, 122.6, 123.8, 125.6 (C6H4), 139.4, 140.1, 143.1, 144.5 (pyran C-2, C-3, C-5, C-6), 168.6 (C=O). Analysis Calculated for: C19H20N2O3 (324.37): C, 70.35; H, 6.21; N, 8.64%. Found: C, 70.51; H, 6.32; N, 8.70%. EIMS: m/z 324 [M]+ (32%).
Ethyl 2-amino-4-(4-methoxyphenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (4d)
Pale yellow crystals from 1,4-dioxane, yield 64%, m.p. 145–147 °C. IR (KBr) ν max (cm−1): 3479–3322 (NH2), 3050 (CH-aromatic), 2950 (CH-aliphatic), 1702, 1690 (2CO), 1580 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.09, 1.06 (2s, 6H, 2CH3), 1.12 (t, 3H, J = 7.11 Hz, CH3), 2.27, 2.38 (2s, 4H, 2CH2), 3.70 (s, 3H, OCH3), 4.22 (q, 2H, J = 7.11 Hz, CH2), 4.83 (s, 2H, D2O exchangeable, NH2), 6.50 (s, 1H, CH- pyran), 7.21–7.48 (m, 4H, C6H4). 13C NMR (DMSO-d6, 75 MHz): δ 16.2 (OCH2CH3), 24.6 (2CH3), 36.8, 56.3 (2CH2), 24.8 (2CH3), 52.1 (OCH2CH3), 90.6 (pyran C-4), 120.2, 120.4, 122.8, 124.2 (C6H5), 139.1, 140.6, 142.8, 143.1 (pyran C-2, C-3, C-5, C-6), 165.5, 168.7 (C=O). Analysis Calculated for: C21H25NO5 (371.43): C, 67.91; H, 6.78; N, 3.77%. Found: C, 67.83; H, 6.59; N, 3.80%. EIMS: m/z 371 [M]+ (24%).
2-Amino-4-(4-chlorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (4e)
Yellow crystals from 1,4-dioxane, yield (2.39 g, 73%, m.p. 210–213 °C. IR (KBr) ν max (cm−1): 3469–3372 (NH2), 3050 (CH-aromatic), 2955 (CH-aliphatic), 2220 (CN), 1703 (CO), 1580 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.08, 1.03 (2s, 6H, 2CH3), 2.29, 2.36 (2s, 4H, 2CH2), 4.81 (s, 2H, D2O exchangeable, NH2), 6.53 (s, 1H, CH- pyran), 7.21–7.48 (m, 4H, C6H4). 13C NMR (DMSO-d6, 75 MHz): δ 24.6 (2CH3), 36.2, 56.0 (2CH2), 24.8 (2CH3), 90.6 (pyran C-4), 116.7 (CN), 120.2, 121.6, 122.9, 125.6 (C6H4), 139.3, 140.1, 143.2, 144.0 (pyran C-2, C-3, C-5, C-6), 168.6 (C=O). Analysis Calculated for: C18H17ClN2O2 (328.79): C, 65.75; H, 5.21; N, 8.52. Found: C, 65.90; H, 5.41; N, 8.73%. EIMS: m/z 328 [M]+ (46%).
Ethyl 2-amino-4-(4-chlorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (4f)
Yellow crystals from ethanol, yield (2.70 g, 72%), m.p. 133–135 °C. IR (KBr) ν max (cm−1): 3487–3361 (NH2), 3050 (CH-aromatic), 2953 (CH-aliphatic), 1702, 1688 (2CO), 1583 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.08, 1.05 (2s, 6H, 2CH3), 1.12 (t, 3H, J = 7.02 Hz, CH3), 2.24, 2.39 (2s, 4H, 2CH2), 4.20 (q, 2H, J = 7.02 Hz, CH2), 4.85 (s, 2H, D2O exchangeable, NH2), 6.50 (s, 1H, CH- pyran), 7.23–7.52 (m, 4H, C6H4). 13C NMR (DMSO-d6, 75 MHz): δ 16.2 (OCH2CH3), 24.6 (2CH3), 36.2, 56.3 (2CH2), 24.8 (2CH3), 52.3 (OCH2CH3), 90.6 (pyran C-4), 120.5, 122.9, 123.8, 126.2 (C6H4), 139.5, 140.6, 143.2, 144.1 (pyran C-2, C-3, C-5, C-6), 165.6, 168.7 (C=O). Analysis Calculated for: C20H22ClNO4 (375.85: C, 63.91; H, 5.90; N, 3.72%. Found: C, 64.27; H, 6.11; N, 3.59%. EIMS: m/z 375 [M]+ (32%).
General procedure for the synthesis of the thieno[3,2-f]chromene derivatives 5a–f
Each of either malononitrile (0.66 g, 0.01 mol) or ethyl cyanoacetate (1.07 g, 0.01 mol) were added to a solution of either compounds 4a (2.94 g, 0.01 mol), 4c (3.24 g, 0.01 mol) or 4e (3.28 g, 0.01 mol) in 1,4-dioxane (50 mL) containing triethylamine (0.50 mL). The reaction mixture was heated under reflux for 2 h then poured onto ice/water containing a few drops of hydrochloric acid and the precipitated solid product was collected by filtration.
2,7-Diamino-4,4-dimethyl-9-phenyl-5,9-dihydro-4H-thieno[3,2-f]-chromene-1,8-dicarbonitrile (5a)
Orange crystals from ethanol, yield (2.24 g, 60%), m.p. 185–187 °C. IR (KBr) ν max (cm−1): 3497–3340 (2NH2), 3050 (CH-aromatic), 2953 (CH-aliphatic), 2223, 2220 (2CN), 1580 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.08, 1.03 (2s, 6H, 2CH3), 2.40 (s, 2H, CH2), 4.87, 5.22 (2s, 4H, D2O exchangeable, 2NH2), 6.52 (s, 1H, CH- pyran), 7.23–7.42 (m, 5H, C6H5). 13C NMR (DMSO-d6, 75 MHz): δ 24.6 (2CH3), 56.0 (CH2), 24.5 (2CH3), 90.5 (pyran C-4), 116.8, 117.0 (2CN), 120.2, 121.4, 124.1, 125.6 (C6H5), 136.0, 138.2, 139.8, 140.1, 142.8, 143.2, 143.8, 145.8 (thiophene C, pyran C-2, C-3, C-5, C-6). Analysis Calculated for C21H18N4OS (374.46): C, 67.36; H, 4.85; N, 14.56; S, 8.56%. Found: C, 67.53; H, 5.61; N, 15.23; S, 8.73%. EIMS: m/z 374 [M]+ (28%).
Ethyl 2,7-diamino-8-cyano-4,4-dimethyl-9-phenyl-5,9-dihydro-4H-thieno-[3,2-f]hromene-1-carboxylate (5b)
Orange crystals from ethanol, yield (2.86 g, 68%, m.p. 215–217 °C. IR (KBr) ν max (cm−1): 3488–3318 (2NH2), 3050 (CH-aromatic), 2953 (CH-aliphatic), 2220 (CN), 1580 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.07, 1.04 (2s, 6H, 2CH3), 1.12 (t, 3H, J = 5.92 Hz, CH3), 2.49 (s, 2H, CH2), 4.20 (q, 2H, J = 5.92 Hz, CH2), 4.85, 5.29 (2s, 4H, D2O exchangeable, 2NH2), 6.51 (s, 1H, CH- pyran), 7.25–7.40 (m, 5H, C6H5). 13C NMR (DMSO-d6, 75 MHz): δ 16.2 (OCH2CH3), 24.6 (2CH3), 52.3 (OCH2CH3), 56.3 (CH2), 24.6 (2CH3), 90.8 (pyran C-4), 116.9 (CN), 120.3, 122.7, 124.8, 126.1 (C6H5), 136.4, 137.8, 139.2, 140.6, 142.3, 143.9, 143.3, 144.5 (thiophene C, pyran C-2, C-3, C-5, C-6). Analysis Calculated for C23H23N3O3S (421.51): C, 65.54; H, 5.50; N, 9.97; S, 7.61. Found: C, 65.36; H, 5.72; N, 10.13; S, 7.80%. EIMS: m/z 421 [M]+ (36%).
2,7-Diamino-9-(4-methoxyphenyl)-4,4-dimethyl-5,9-dihydro-4H-thieno-[3,2-f]chromene-1,8-dicarbonitrile (5c)
Orange crystals from ethanol, yield (2.50 g, 62%), m.p.187–189 °C. IR (KBr) ν max (cm−1): 3488–3362 (2NH2), 3050 (CH-aromatic), 2953 (CH-aliphatic), 2222, 2220 (2CN), 1580 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.07, 1.03 (2s, 6H, 2CH3), 2.42 (2s, 4H, 2CH2), 4.84, 5.25 (2s, 4H, D2O exchangeable, 2NH2), 6.58 (s, 1H, CH- pyran), 7.22–7.46 (m, 5H, C6H5). 13C NMR (DMSO-d6, 75 MHz): δ 24.6 (2CH3), 50.4 (OCH3), 56.3 (CH2), 24.8 (2CH3), 90.6 (pyran C-4), 116.7, 117.1 (2CN), 120.4, 120.8, 123.6, 126.4 (C6H4), 136.3, 137.6, 139.5, 140.6, 142.3, 143.6, 143.5, 144.2 (thiophene C, pyran C-2, C-3, C-5, C-6). Analysis Calculated for C22H20N4O2S (404.48): C, 65.33; H, 4.98; N, 13.85; S, 7.93%. Found: C, 65.40; H, 5.19; N, 14.02; S, 8.04%. EIMS: m/z 404 [M]+ (22%).
Ethyl 2,7-diamino-8-cyano-9-(4-methoxyphenyl)-4,4-dimethyl-5,9-dihydro-4H-thieno[3,2-f]chromene-1-carboxylate (5d)
Orange crystals from ethanol, yield (3.47 g, 77%), m.p. 187–189 °C. IR (KBr) ν max (cm−1): 3464–3358 (2NH2), 3050 (CH-aromatic), 2953 (CH-aliphatic), 2220 (CN), 1582 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.08, 1.03 (2s, 6H, 2CH3), 1.12 (t, 3H, J = 6.26 Hz, CH3), 2.47 (s, 2H, CH2), 3.71 (s, 3H, OCH3), 4.21 (q, 2H, J = 6.26 Hz, CH2), 4.84, 5.26 (2s, 4H, D2O exchangeable, 2NH2), 6.53 (s, 1H, CH- pyran), 7.22–7.45 (m, 4H, C6H4). 13C NMR (DMSO-d6, 75 MHz): δ 16.1 (OCH2CH3), 24.6 (2CH3), 50.4 (OCH3), 52.3 (OCH2CH3), 56.6 (CH2), 24.3 (2CH3), 90.8 pyran C-4), 116.8 (CN), 120.1, 122.4, 123.9, 125.2 (C6H4), 136.2, 137.6, 138.3, 139.2, 142.6, 143.3, 143.6, 144.1 (thiophene C, pyran C-2, C-3, C-5, C-6). Analysis Calculated for C24H25N3O4S (451.54): C, 63.84; H, 5.58; N, 9.31; S, 7.10%. Found: C, 65.03; H, 5.68; N, 9.44; S, 7.29%. EIMS: m/z 451 [M]+ (26%).
2,7-Diamino-9-(4-chlorophenyl)-4,4-dimethyl-5,9-dihydro-4H-thien[3,2-f]chromene-1,8-dicarbonitrile (5e)
Orange crystals from ethanol, yield (2.69 g, 66%), m.p. 185–188 °C. IR (KBr) ν max (cm−1): 3486–3343 (2NH2), 3050 (CH-aromatic), 2952 (CH-aliphatic), 2222, 2220 (2CN), 1580 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.07, 1.03 (2s, 6H, 2CH3), 2.41 (s, 2H, CH2), 4.89, 5.21 (2s, 4H, D2O exchangeable, 2NH2), 6.50 (s, 1H, CH- pyran), 7.21–7.43 (m, 4H, C6H4). 13C NMR (DMSO-d6, 75 MHz): δ 56.2 (CH2), 24.7 (2CH3), 90.8 (pyran C-4), 116.7, 117.1 (2CN), 120.4, 122.7, 124.6, 126.2 (C6H4), 136.2, 137.7, 139.3, 140.6, 142.7, 143.8, 144.2, 145.5 (thiophene C, pyran C-2, C-3, C-5, C-6). Analysis Calculated for C21H17ClN4OS (408.90): C, 61.68; H, 4.19; N, 13.70; S, 7.84%. Found: C, 61.73; H, 4.35; N, 13.83; S, 9.12%. EIMS: m/z 408 [M]+ (22%).
Ethyl 2,7-diamino-8-cyano-9-(4-methoxyphenyl)-4,4-dimethyl-5,9-dihydro-4H-thieno[3,2-f]chromene-1-carboxylate (5f)
Orange crystals from ethanol, yield (3.50, 77%), m.p. 197–180 °C. IR (KBr) ν max (cm−1): 3464–3358 (2NH2), 3050 (CH-aromatic), 2953 (CH-aliphatic), 2220 (CN), 1687 (CO), 1582 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.08, 1.03 (2s, 6H, 2CH3), 1.12 (t, 3H, J = 6.26 Hz, CH3), 2.47 (s, 2H, CH2), 4.21 (q, 2H, J = 6.26 Hz, CH2), 4.84, 5.26 (2s, 4H, D2O exchangeable, 2NH2), 6.53 (s, 1H, CH- pyran), 7.22–7.45 (m, 4H, C6H4). 13C NMR (DMSO-d6, 75 MHz): δ 16.1 (OCH2CH3), 52.3 (OCH2CH3), 56.6 (CH2), 24.3 (2CH3), 90.6 (pyran C-4), 116.8 (CN), 120.1, 122.4, 123.9, 125.2 (C6H4), 136.2, 137.6, 138.3, 139.2, 142.6, 143.3, 143.6, 144.1 (thiophene C, pyran C-2, C-3, C-5, C-6). Analysis Calculated for C23H22ClN3O3S (455.96): C, 60.59; H, 4.86; N, 9.22; S, 7.03%. Found: C, 60.26; H, 5.02; N, 9.53; S, 7.26%. EIMS: m/z 455 [M]+ (26%).
General procedure for the synthesis of the tetrahydrochromeno[2,3-c]pyrazol-5(1H)-one derivatives 7a–c
Method (A): To a solution of compounds 1 (1.40 g, 0.01 mol) in absolute ethanol (50 mL) containing triethylamine (0.50 mL) each of either benzaldehyde (1.06 g, 0.01 mol), 4-methoxybenzaldehyde (1.36 g, 0.01 mol) or chlorobenzaldehyde (1.40 g, 0.01 mol) and the 3-methyl-1H-pyrazol-5(4H)-one (0.98 g, 0.01) were added. The reaction mixture was heated under reflux for 2 h then poured onto ice/water containing a few drops of hydrochloric acid and the precipitated solid product was collected by filtration.
Method (B): Each of either benzaldehyde (1.06 g, 0.01 mol), 4-methoxybenzaldehyde (1.36 g, 0.01 mol) or chlorobenzaldehyde (1.40 g, 0.01 mol) and either malononitrile or ethyl cyanoacetate were added to 3-methyl-1H-pyrazol-5(4H)-one (0.98 g, 0.01). To the reaction mixture the Fe3O4@MCM-41-SO3H@[HMIm][HSO4] catalyst (0.50 g) was added and the whole mixture was reacted in a test tube with a glass bar at 110 °C under solvent-free condition for the appropriate time. When the reaction was completed, checked by TLC, the reaction mixture was dissolved in ethanol (5 mL) and the catalyst was isolated by applying the magnetic field. Then, the filtrate was concentrated on a rotary evaporator under reduced pressure and the solid crude product created was washed with water and recrystallized from ethanol to afford pure products.
3,7,7-Trimethyl-4-phenyl-4,6,7,8-tetrahydrochromeno[2,3-c]pyrazol-5(1H)-one (7a)
Yellow crystals from 1,4-dioxane, yield (2.21 g, 72%), m.p. 163–167 °C. IR (KBr) ν max (cm−1): 3494–3346 (NH), 3050 (CH-aromatic), 2953 (CH-aliphatic), 1702 (CO), 1580 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.08, 1.03 (2s, 6H, 2CH3), 2.23, 2.36 (2s, 4H, 2CH2), 2.68 (s, 3H, CH3), 6.52 (s, 1H, CH- pyran), 7.24–7.43 (m, 5H, C6H5), 8.31 (s, 1H, D2O exchangeable, NH). 13C NMR (DMSO-d6, 75 MHz): δ 24.4 (2CH3), 36.1, 56.4 (2CH2), 24.7 (2CH3), 90.6 (pyran C-4), 116.6 (CN), 120.2, 121.5, 122.8, 123.8 (C6H5), 138.2, 139.3, 140.3, 141.8, 142.4, 143.5 (pyrazole C-4, C-5, pyran C-2, C-3, C-5, C-6), 168.6 (C=O). Analysis Calculated for: C19H20N2O2 (308.37): C, 74.00; H, 6.54; N, 9.08%. Found: C, 73.92; H, 6.39; N, 9.17%. EIMS: m/z 308 [M]+ (36%).
4-(4-Methoxyphenyl)-3,7,7-trimethyl-4,6,7,8-tetrahydrochromeno[2,3-c]pyrazol-5(1H)-one (7b)
Yellow crystals from 1,4-dioxane, yield (3.56 g, 76%), m.p.124–126 °C. IR (KBr) ν max (cm−1): 3483–3351 (NH), 3050 (CH-aromatic), 2955 (CH-aliphatic), 1702 (CO), 1581 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.07, 1.03 (2s, 6H, 2CH3), 2.24, 2.39 (2s, 4H, 2CH2), 2.66 (s, 3H, CH3), 3.66 (s, 3H, OCH3), 6.54 (s, 1H, CH- pyran), 7.24–7.48 (m, 4H, C6H4), 8.31 (s, 1H, D2O exchangeable, NH). 13C NMR (DMSO-d6, 75 MHz): δ 36.3, 56.2 (2CH2), 24.8 (2CH3), 36.8 (CH3), 50.4 (OCH3), 90.6 (pyran C-4), 116.8 (CN), 120.6, 121.8, 122.4, 125.2 (C6H4), 138.1, 139.5, 141.7, 142.3, 143.1, 143.9 (pyrazole C-4, C-5, pyran C-2, C-3, C-5, C-6), 168.8 (C=O). Analysis Calculated for:C20H22N2O3 (338.40): C, 70.99; H, 6.55; N, 8.28%. Found: C, 70.73; H, 6.33; N, 8.40%. EIMS: m/z 338 [M]+ (28%).
4-(4-Chlorophenyl)-3,7,7-trimethyl-4,6,7,8-tetrahydrochromeno[2,3-c]pyrazol-5(1H)-one (7c)
Yellow crystals from 1,4-dioxane, yield (2.25 g, 66%, m.p. 123–126 °C. IR (KBr) ν max (cm−1): 3463–3325 (NH), 3050 (CH-aromatic), 2953 (CH-aliphatic), 1701 (CO), 1583 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.08, 1.02 (2s, 6H, 2CH3), 2.22, 2.38 (2s, 4H, 2CH2), 2.66 (s, 3H, CH3), 6.54 (s, 1H, CH- pyran), 7.24–7.49 (m, 4H, C6H4), 8.30 (s, 1H, D2O exchangeable, NH). 13C NMR (DMSO-d6, 75 MHz): δ = 36.4, 56.2 (2CH2), 24.8 (2CH3), 36.7 (CH3), 50.8 (pyran C-4), 116.9 (CN), 120.5, 122.7, 123.2, 124.5 (C6H5), 138.5, 139.2, 140.8, 141.6, 142.2, 143.8 (pyrazole C-4, C-5, pyran C-2, C-3, C-5, C-6), 168.8 (C=O). Analysis Calculated for: C19H19ClN2O2 (342.82): C, 66.57; H, 5.59; N, 8.17. Found: C, 66.39; H, 5.42; N, 8.38%. EIMS: m/z 342 [M]+ (40%).
General procedure of the hexahydroquinoline-3-carbonitrile derivatives 9a–c
Method (A): To a stirred solution of compounds 1 (1.40 g, 0.01 mol) in 1,4-dioxane (50 mL) containing triethylamine (0.50 mL) each of either benzaldehyde (1.06 g, 0.01 mol), 4-methoxybenzaldehyde (1.36 g, 0.01 mol) or 4-chlorobenzaldehyde (1.40 g, 0.01 mol) and cyanothioacetamide (1.00 g, 0.01) were added. The reaction mixture was heated under reflux for 3 h then poured onto ice/water containing a few drops of hydrochloric acid and the precipitated solid product was collected by filtration.
Method (B): Each of either benzaldehyde (1.06 g, 0.01 mol), 4-methoxybenzaldehyde (1.36 g, 0.01 mol) or chlorobenzaldehyde (1.40 g, 0.01 mol) and either cyanothioacetamide (1.00 g, 0.01) were added to compounds 1 (1.40 g, 0.01 mol). To the reaction mixture the Fe3O4@MCM-41-SO3H@[HMIm][HSO4] catalyst was added. The whole reaction mixture was reacted in a test tube with a glass bar at 110 °C under solvent-free condition for the appropriate time. When the reaction was completed, checked by TLC, the reaction mixture was dissolved in ethanol (5 mL) and the catalyst was isolated by applying the magnetic field. Then, the filtrate was concentrated on a rotary evaporator under reduced pressure and the solid crude product created was washed with water and recrystallized from ethanol to afford pure products.
7,7-Dimethyl-5-oxo-4-phenyl-2-thioxo-1,2,5,6,7,8-hexahydroquinoline-3-carbonitrile (9a)
Yellow crystals from ethanol, yield (2.09 g, 68%), m.p. 223–226 °C. IR (KBr) ν max (cm−1): 3479–3331 (NH), 3050 (CH-aromatic), 2950 (CH-aliphatic), 2220 (CN), 1703 (CO), 1582 (C=C), 1206 (C=S). 1H NMR (DMSO-d6, 300 MHz): δ = 1.08, 1.04 (2s, 6H, 2CH3), 2.25, 2.38 (2s, 4H, 2CH2), 7.26–7.41 (m, 5H, C6H5), 8.38 (s, 1H, D2O exchangeable, NH). 13C NMR (DMSO-d6, 75 MHz): δ 24.8 (2CH3), 36.4, 56.3 (2CH2), 94.2 (pyridine C-4), 116.9 (CN), 120.7, 122.8, 124.3, 125.6 (C6H5), 138.6, 139.8, 142.9, 143.2 (pyridine C-2, C-3, C-5, C-6), 168.4 (C=O), 180.3 (C=S). Analysis Calculated for: C18H16N2OS (308.40): C, 70.10; H, 5.23; N, 9.08; S, 10.40. Found: C, 70.38; H, 5.42; N, 9.25; S, 10.29%. EIMS: m/z 308 [M]+ (22%).
4-(4-Methoxyphenyl)-7,7-dimethyl-5-oxo-2-thioxo-1,2,5,6,7,8-hexahydro-quinoline-3-carbonitrile (9b)
Pale yellow crystals from ethanol, yield (2.64 g, 73%), m.p. 180–182 °C. IR (KBr) ν max (cm−1): 3458–3329 (NH), 3050 (CH-aromatic), 2950 (CH-aliphatic), 2220 (CN), 1702 (CO), 1586 (C=C), 1208 (C=S). 1H NMR (DMSO-d6, 300 MHz): δ = 1.08, 1.02 (2s, 6H, 2CH3), 2.23, 2.37 (2s, 4H, 2CH2), 3.66 (s, 3H, OCH3), 7.21–7.48 (m, 4H, C6H4), 8.38 (s, 1H, D2O exchangeable, NH). 13C NMR (DMSO-d6, 75 MHz): δ 36.3, 56.5 (2CH2), 24.8 (2CH3), 50.8 (OCH3), 94.5 (pyridine C-4), 116.8 (CN), 120.2, 122.4, 124.3, 126.9 (C6H5), 138.6, 139.6, 140.8, 143.2 (pyridine C-2, C-3, C-5, C-6), 168.2 (C=O), 180.5 (C=S). Analysis Calculated for:C19H18N2O2S (338.42): C, 67.43; H, 5.36; N, 8.28; S, 9.47%. Found: C, 67.58; H, 5.29; N, 8.36; S, 9.52%. EIMS: m/z 338 [M]+ (18%).
4-(4-Chlorophenyl)-7,7-dimethyl-5-oxo-2-thioxo-1,2,5,6,7,8-hexahydro-quinoline-3-carbonitrile (9c)
Yellow crystals from ethanol, yield (1.98 g, 58%), m.p. 166–168 °C. IR (KBr) ν max (cm−1): 3493–3326 (NH), 3050 (CH-aromatic), 2950 (CH-aliphatic), 2220 (CN), 1701 (CO), 1582 (C=C), 1205 (C=S). 1H NMR (DMSO-d6, 300 MHz): δ = 1.07, 1.05 (2s, 6H, 2CH3), 2.26, 2.37 (2s, 4H, 2CH2), 7.21–7.48 (m, 4H, C6H4), 8.37 (s, 1H, D2O exchangeable, NH). 13C NMR (DMSO-d6, 75 MHz): δ 36.2, 56.5 (2CH2), 24.6 (2CH3), 116.9 (CN), 120.5, 122.6, 124.6, 126.2 (C6H4), 138.8, 139.5, 142.3, 143.6 (pyridine C-3, C-4, C-5, C-6), 168.6 (C=O), 180.5 (C=S). Analysis Calculated for: C18H15ClN2OS (342.84): C, 63.06; H, 4.41; N, 8.17; S, 9.35%. Found: C, 63.32; H, 4.46; N, 8.25; S, 9.60%. EIMS: m/z 342 [M]+ (28%).
General procedure for the synthesis of the dihydrochromeno[3,4-c]chromene derivatives 12a,b
Method (A): To a solution of compounds 1 (1.40 g, 0.01 mol) in absolute ethanol (50 mL) containing triethylamine (0.50 mL) each of either ethyl benzoylacetate (1.92 g, 0.01 mol) or ethyl acetoacetate (1.30 g, 0.01 mol) and salicylaldehyde (1.22 g, 0.01) were added. The reaction mixture was heated under reflux for 3 h then poured onto ice/water containing a few drops of hydrochloric acid and the precipitated solid product was collected by filtration.
Method (B) Each of either ethyl benzoylacetate (1.92 g, 0.01 mol) or ethyl acetoacetate (1.30 g, 0.01 mol) and salicylaldehyde (1.22 g, 0.01) were added to compounds 1 (1.40 g, 0.01 mol). To the reaction mixture, the Fe3O4@MCM-41-SO3H@[HMIm][HSO4] catalyst was added. The whole reaction mixture was reacted in a test tube with a glass bar at 110 °C under solvent-free condition for the appropriate time. When the reaction was completed, checked by TLC, the reaction mixture was dissolved in ethanol (5 mL) and the catalyst was isolated by applying the magnetic field. Then, the filtrate was concentrated on a rotary evaporator under reduced pressure and the solid crude product created was washed with water and recrystallized from ethanol to afford pure products.
3,3-Dimethyl-6-phenyl-3,4-dihydrochromeno[3,4-c]chromene-1,7-(2H,12bH)-dione (12a)
Yellow crystals from 1,4-dioxane, yield (2.04 g, 55%), m.p. 186–188 °C. IR (KBr) ν max (cm−1): 3055 (CH-aromatic), 2950 (CH-aliphatic), 1703–1690 (2CO), 1580 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.09, 1.03 (2s, 6H, 2CH3), 2.27, 2.41 (2s, 4H, 2CH2), 6.52 (s, 1H, CH- pyran), 7.26–7.42 (m, 9H, C6H5, C6H4). 13C NMR (DMSO-d6, 75 MHz): δ 36.5, 56.0 (2CH2), 24.8 (2CH3), 90.6 (pyran C-4), 120.6, 120.9, 121.8, 122.3, 125.2, 126.1, 128.3 (C6H5, C6H4), 139.3, 140.2, 142.8, 145.2 (pyran C-2, C-3, C-5, C-6), 166.5, 168.4 (2C=O). Analysis Calculated for: C24H20O4 (372.41): C, 77.40; H, 5.41%. Found: C, 77.26; H, 5.60%. EIMS: m/z 372 [M]+ (38%).
3,3,6-Trimethyl-3,4-dihydrochromeno[3,4-c]chromene-1,7(2H,12bH)-dione (12b)
Yellow crystals from ethanol/DMF, yield (2.10 g, 68%), m.p. 208–211 °C. IR (KBr) ν max (cm−1): 3055 (CH-aromatic), 2950 (CH-aliphatic), 1701–1690 (2CO), 1582 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.08, 1.04 (2s, 6H, 2CH3), 2.28, 2.43 (2s, 4H, 2CH2), 2.69 (s, 3H, CH3), 6.55 (s, 1H, CH- pyran), 7.24–7.45 (m, 4H, C6H4). 13C NMR (DMSO-d6, 75 MHz): δ 24.8 (CH3), 36.53, 56.2 (2CH2), 24.7 (2CH3), 28.4 (CH3), 120.5, 123.2, 125.2, 128.5 (C6H4), 139.4, 140.5, 142.3, 144.6 (pyran C-2, C-3, C-5, C-6), 166.3, 168.2 (2C=O). Analysis Calculated for: C19H18O4 (310.34): C, 73.53; H, 5.85. Found: C, 73.70; H, 5.92%. EIMS: m/z 310 [M]+ (26%).
6-Amino-3,3-dimethyl-3,4-dihydrochromeno[3,4-c]chromene-1,7-(2H,12bH)-dione (13)
Mrthod (A): Equimolar amounts of compounds 1 (1.40 g, 0.01 mol) in absolute ethanol (50 mL) containing triethylamine (0.50 mL) and either malononitrile (0.66 g, 0.01 mol) or ethyl cyanoacetate (1.07 g, 0.01 mol) and salicylaldehyde (1.22 g, 0.01) were mixed. The reaction mixture was heated under reflux for 3 h then poured onto ice/water containing a few drops of hydrochloric acid and the precipitated solid product was collected by filtration.
Method B: Each of either malononitrile (0.66 g, 0.01 mol) or ethyl cyanoacetate (1.07 g, 0.01 mol) and salicylaldehyde (1.22 g, 0.01) were added to compounds 1 (1.40 g, 0.01 mol). To the reaction mixture, the Fe3O4@MCM-41-SO3H@[HMIm][HSO4] catalyst was added. The whole reaction mixture was reacted in a test tube with a glass bar at 110 °C under solvent-free condition for the appropriate time. When the reaction was completed, checked by TLC, the reaction mixture was dissolved in ethanol (5 mL) and the catalyst was isolated by applying the magnetic field. Then, the filtrate was concentrated on a rotary evaporator under reduced pressure and the solid crude product created was washed with water and recrystallized from ethanol to afford pure products.
Pale yellow crystals from 1,4-dioxane, yield (217 g, 70%), m.p. 196–198 °C. IR (KBr) ν max (cm−1): 3055 (CH-aromatic), 2950 (CH-aliphatic), 1703–1700 (2CO), 1580 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.08, 1.04 (2s, 6H, 2CH3), 2.28, 2.43 (2s, 4H, 2CH2), 4.93 (s, 2H, D2O exchangeable, NH2), 6.54 (s, 1H, CH- pyran), 7.26–7.42 (m, 4H, C6H4). 13C NMR (DMSO-d6, 75 MHz): δ 24.8 (CH3), 36.53, 56.2 (2CH2), 24.7 (2CH3), 90.7 (pyran C-4), 120.3, 122.7, 124.5, 127.3 (C6H4), 139.9, 141.3, 142.8, 143.4 (pyran C-2, C-3, C-5, C-6), 165.8, 168.6 (2C=O). Analysis Calculated for: C18H17NO4 (311.33): C, 69.44; H, 5.50; N, 4.50%. Found: C, 69.60; H, 5.73; N, 4.69%. EIMS: m/z 311 [M]+ (32%).
General procedure for the synthesis of the pyran derivatives 14a–c
Method (A): Each of either benzaldehyde (1.06 g, 0.01 mol), 4-methoxybenzaldehyde (1.36 g, 0.01 mol) or 4-chlorobenzaldehyde (1.40 g, 0.01 mol) and ethyl benzoylacetate (1.92 g, 0.01) were added to a solution of compounds 1 (1.40 g, 0.01 mol) in absolute ethanol (50 mL) containing triethylamine (0.50 mL). The reaction mixture was heated under reflux for 5 h then poured onto ice/water containing a few drops of hydrochloric acid and the precipitated solid product was collected by filtration.
Method (B) Each of either benzaldehyde (1.06 g, 0.01 mol), 4-methoxybenzaldehyde (1.36 g, 0.01 mol) or 4-chlorobenzaldehyde (1.40 g, 0.01 mol) and ethyl benzoylacetate (1.92 g, 0.01) were added to a solution of compounds 1 (1.40 g, 0.01 mol). To the reaction mixture, the Fe3O4@MCM-41-SO3H@[HMIm][HSO4] catalyst was added. The whole reaction mixture was reacted in a test tube with a glass bar at 110 °C under solvent-free condition for the appropriate time. When the reaction was completed, checked by TLC, the reaction mixture was dissolved in ethanol (5 mL) and the catalyst was isolated by applying the magnetic field. Then, the filtrate was concentrated on a rotary evaporator under reduced pressure and the solid crude product created was washed with water and recrystallized from ethanol to afford pure products.
Ethyl 7,7-dimethyl-5-oxo-2,4-diphenyl-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (14a)
Yellow crystals from ethanol, yield (257 g, 64%), m.p. 195–197 °C. IR (KBr) ν max (cm−1): 3050 (CH-aromatic), 2953 (CH-aliphatic), 1705, 1690 (2CO), 1580 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.08, 1.03 (2s, 6H, 2CH3), 1.12 (t, 3H, J = 7.13 Hz, CH3), 2.25, 2.39 (2s, 4H, 2CH2), 4.22 (q, 2H, J = 7.13 Hz, CH2), 6.52 (s, 1H, CH- pyran), 7.26–7.46 (m, 10H, 2C6H5). 13C NMR (DMSO-d6, 75 MHz): δ 16.3 (OCH2CH3), 36.3, 56.7 (2CH2), 24.8 (2CH3), 52.1 (OCH2CH3), 90.6 (pyran C-4), 120.1, 120.6, 121.4, 121.8, 122.5, 123.6, 124.0, 124.8 (2C6H5), 139.4, 140.2, 142.8, 143.8 (pyran C-2, C-3, C-5, C-6), 165.6, 168.7 (2C=O). Analysis Calculated for: C26H26O4 (402.48): C, 77.59; H, 6.51%. Found: C, 77.80; H, 6.39%. EIMS: m/z 402 [M]+ (36%).
Ethyl 4-(4-methoxyphenyl)-7,7-dimethyl-5-oxo-2-phenyl-5,6,7,8-tetra-hydro-4H-chromene-3-carboxylate (14b)
Yellow crystals from ethanol, yield (2.80 g, 65%), m.p. 253–256 °C. IR (KBr) ν max (cm−1): 3050 (CH-aromatic), 2955 (CH-aliphatic), 1705, 1692 (2CO), 1580 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.08, 1.02 (2s, 6H, 2CH3), 1.13 (t, 3H, J = 7.42 Hz, CH3), 2.23, 2.37 (2s, 4H, 2CH2), 3.67 (s, 3H, OCH3), 4.22 (q, 2H, J = 7.42 Hz, CH2), 6.50 (s, 1H, CH- pyran), 7.24–7.49 (m, 9H, C6H5, C6H4). 13C NMR (DMSO-d6, 75 MHz): δ 16.2 (OCH2CH3), 36.6, 56.4 (2CH2), 24.7 (2CH3), 50.8 (OCH3), 52.2 (OCH2CH3), 90.8 (pyran C-4), 120.2, 120.4, 121.6, 121.9, 122.2, 123.8, 124.0, 125.6 (C6H5, C6H4), 139.2, 140.8, 142.4, 143.6 (pyran C-2, C-3, C-5, C-6), 165.3, 168.9 (2C=O). Analysis Calculated for: C27H28O5 (432.51): C, 74.98; H, 6.53%. Found: C, 75.26; H, 6.32%. EIMS: m/z 432 [M]+ (25%).+
Ethyl 4-(4-chlorophenyl)-7,7-dimethyl-5-oxo-2-phenyl-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (14c)
Yellow crystals from 1,4-dioxane, yield (3.05 g, 70%), m.p. 239–242 °C. IR (KBr) ν max (cm−1): 3053 (CH-aromatic), 2953 (CH-aliphatic), 1703, 1689 (2CO), 1580 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.08, 1.04 (2s, 6H, 2CH3), 1.12 (t, 3H, J = 6.40 Hz, CH3), 2.25, 2.39 (2s, 4H, 2CH2), 4.22 (q, 2H, J = 6.40 Hz, CH2), 6.53 (s, 1H, CH- pyran), 7.24–7.52 (m, 9H, C6H5, C6H4). 13C NMR (DMSO-d6, 75 MHz): δ 16.2 (OCH2CH3), 36.2, 56.8 (2CH2), 24.8 (2CH3), 52.3 (OCH2CH3), 90.6 (pyran C-4), 120.4, 120.9, 121.6, 121.5, 122.7, 124.8, 125.6, 126.4 (C6H5, C6H4), 139.2, 140.5, 142.3, 144.7 (pyran C-2, C-3, C-5, C-6), 165.3, 168.9 (2C=O). Analysis Calculated for: C26H25ClO4 (436.93): C, 71.47; H, 5.77%. Found: C, 71.24; H, 5.92%. EIMS: m/z 436 [M]+ (42%).
General procedure for the synthesis of the pyridine derivatives 15a–c
To a solution of compounds 1 (1.40 g, 0.01 mol) in absolute ethanol (50 mL) containing ammonium acetate (1.0 g mL) each of either benzaldehyde (1.06 g, 0.01 mol), 4-methoxybenzaldehyde (1.36 g, 0.01 mol) or 4-chlorobenzaldehyde (1.40 g, 0.01 mol) and ethyl benzoylacetate (1.92 g, 0.01) were added. The reaction mixture was heated under reflux for 5 h then poured onto ice/water containing a few drops of hydrochloric acid and the precipitated solid product was collected by filtration.
Ethyl 7,7-dimethyl-5-oxo-2,4-diphenyl-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (15a)
Yellow crystals from 1,4-dioxane, yield (3.12 g, 78%), m.p. 202–205 °C. IR (KBr) ν max (cm−1): 3570–3329 (NH), 3050 (CH-aromatic), 2950 (CH-aliphatic), 1703, 1690 (2CO), 1580 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.07, 1.03 (2s, 6H, 2CH3), 1.12 (t, 3H, J = 5.80 Hz, CH3), 2.22, 2.38 (2s, 4H, 2CH2), 4.22 (q, 2H, J = 5.80 Hz, CH2), 6.50 (s, 1H, CH- pyridine), 7.28–7.40 (m, 10H, 2C6H5), 8.38 (s, 1H, D2O exchangeable, NH). 13C NMR (DMSO-d6, 75 MHz): δ 16.2 (OCH2CH3), 36.4, 56.5 (2CH2), 24.7 (2CH3), 52.2 (OCH2CH3), 94.2 (pyridine C-4), 119.8, 120.4, 121.6, 121.9, 122.3, 122.6, 124.3, 125.2 (2C6H5), 139.2, 141.1, 143.4, 144.2 (pyridine C-2, C-3, C-5, C-6), 165.4, 168.9 (2C=O). Analysis Calculated for: C26H27NO3 (401.50): C, 77.78; H, 6.78; N, 3.49%. Found: C, 77.85; H, 6.53; N, 3.62%. EIMS: m/z 401 [M]+ (28%).
Ethyl 4-(4-methoxyphenyl)-7,7-dimethyl-5-oxo-2-phenyl-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate (15b)
Yellow crystals from 1,4-dioxane, yield (3.01 g,70%), m.p. 266–268 °C. IR (KBr) ν max (cm−1): 3570–3352 (NH), 3050 (CH-aromatic), 2955 (CH-aliphatic), 1703, 1690 (2CO), 1580 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.08, 1.03 (2s, 6H, 2CH3), 1.12 (t, 3H, J = 7.29 Hz, CH3), 2.23, 2.37 (2s, 4H, 2CH2), 3.67 (s, 3H, OCH3), 4.22 (q, 2H, J = 7.29 Hz, CH2), 6.50 (s, 1H, CH- pyridine), 7.24–7.49 (m, 9H, C6H5, C6H4), 8.28 (s, 1H, NH).. 13C NMR (DMSO-d6, 75 MHz): δ 16.2 (OCH2CH3), 36.6, 56.4 (2CH2), 24.8 (2CH3), 50.7 (OCH3), 52.1 (OCH2CH3), 95.2 (pyridine C-4), 120.1, 120.6, 121.8, 121.3, 122.7, 123.3, 124.2, 126.9 (C6H5, C6H4), 139.3, 140.2, 141.8, 144.2 (pyridine C-2, C-3, C-5, C-6), 165.3, 168.9 (2C=O). Analysis Calculated for: C27H29NO4 (431.52): C, 75.15; H, 6.77; N, 3.25. Found: C, 75.28; H, 6.49; N, 3.50%. EIMS: m/z 431 [M]+ (32%).
Ethyl 4-(4-chlorophenyl)-7,7-dimethyl-5-oxo-2-phenyl-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate (15c)
Yellow crystals from 1,4-dioxane, yield (2.87 g, 66%), m.p. 168–170 °C. IR (KBr) ν max (cm−1): 3472–3327 (NH), 3055 (CH-aromatic), 2950 (CH-aliphatic), 1702, 1689 (2CO), 1580 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.08, 1.04 (2s, 6H, 2CH3), 1.12 (t, 3H, J = 6.32 Hz, CH3), 2.23, 2.38 (2s, 4H, 2CH2), 4.21 (q, 2H, J = 6.32 Hz, CH2), 6.53 (s, 1H, CH- pyridine), 7.22–7.54 (m, 9H, C6H5, C6H4), 8.30 (s, 1H, NH). 13C NMR (DMSO-d6, 75 MHz): δ 16.2 (OCH2CH3), 36.4, 56.8 (2CH2), 24.8 (2CH3), 52.6 (OCH2CH3), 94.2 (pyridine C-4), 120.3, 120.6, 121.3, 121.9, 122.2, 124.6, 125.1, 126.8 (C6H5, C6H4), 139.5, 140.5, 142.7, 143.2 (pyridine C-2, C3, C-5, C-6), 165.3, 168.7 (2C=O). Analysis Calculated for: C26H26ClNO3 (435.94): C, 71.63; H, 6.01; N, 3.21%. Found: C, 71.42; H, 5.86; N, 3.33%. EIMS: m/z 435 [M]+ (30%).
General procedure for the synthesis of the thieno[3,2-f]chromene-8-carboxylate 16a–f
To a solution of either compounds 14a (4.02 g, 0.01 mol), 14b (4.32 g, 0.01 mol) or 14c (4.36 g, 0.01 mol) in 1,4-dioxane (50 mL) containing triethylamine (0.50 mL) each of either malononitrile (0.66 g, 0.01 mol) or ethyl cyanoacetate (1.13 g, 0.01 mol) were added. The reaction mixture was heated under reflux for 2 h then poured onto ice/water containing a few drops of hydrochloric acid and the precipitated solid product was collected by filtration.
Ethyl 2-amino-1-cyano-4,4-dimethyl-7,9-diphenyl-5,9-dihydro-4H-thieno[3,2-f]chromene-8-carboxylate (16a)
Pale orange crystals from acetic acid, yield (3.27 g, 68%), m.p. 162–165 °C. IR (KBr) ν max (cm−1): 3474–3328 (NH2), 3050 (CH-aromatic), 2953 (CH-aliphatic), 2220 (CN), 1689 (CO), 1580 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.08, 1.04 (2s, 6H, 2CH3), 1.12 (t, 3H, J = 5.86 Hz, CH3), 2.43 (s, 2H, CH2), 4.22 (q, 2H, J = 5.86 Hz, CH2), 5.18 (s, 2H, D2O exchangeable, NH2), 6.50 (s, 1H, CH- pyran), 7.25–7.40 (m, 10H, 2C6H5). 13C NMR (DMSO-d6, 75 MHz): δ 16.2 (OCH2CH3), 24.5 (2CH3), 56.0 (CH2), 52.1 (OCH2CH3), 90.6 (pyridine C-4), 116.8 (CN), 120.1, 122.6, 123.3, 123.8, 124.7, 125.1, 125.4, 126.4 (2C6H5), 136.3, 138.8, 139.4, 141.4, 142.5, 143.0, 143.2, 144.5 (thiophene C, pyran C-2, C-3, C-5, C-6), 165.8 (CO). Analysis Calculated for: C29H26N2O3S (482.59): C, 72.17; H, 5.43; N, 5.80; S, 6.64%. Found: C, 72.29; H, 5.50; N, 5.71; S, 6.93%. EIMS: m/z 482 [M]+ (44%).
Diethyl 2-amino-4,4-dimethyl-7,9-diphenyl-5,9-dihydro-4H-thieno[3,2-f]chromene-1,8-dicarboxylate (16b)
Pale orange crystals from acetic acid, yield 78%, m.p. 129–131 °C. IR (KBr) ν max (cm−1): 3458–3341 (NH2), 3050 (CH-aromatic), 2953 (CH-aliphatic), 1689, 1687 (2CO), 1580 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.07, 1.04 (2s, 6H, 2CH3), 1.11, 1.12 (2t, 6H, J = 5.86, 6.83 Hz, 2CH3), 2.43 (s, 2H, CH2), 4.19, 4.22 (2q, 4H, J = 5.86, 6.83 Hz, 2CH2), 5.17 (s, 2H, D2O exchangeable, NH2), 6.53 (s, 1H, CH- pyran), 7.23–7.46 (m, 10H, 2C6H5). 13C NMR (DMSO-d6, 75 MHz): δ 16.1, 16.2 (two OCH2CH3), 24.7 (2CH3), 56.2 (CH2), 52.1, 52.3 (two OCH2CH3), 90.5 (pyran C-4), 120.3, 122.7, 123.2, 123.5, 124.9, 125.3, 125.6, 126.8 (2C6H5), 136.5, 138.7, 139.9, 140.3, 142.8, 143.2, 143.7, 143.3 (thiophene C, pyran C-2, C-3, C-5, C-6), 165.6, 166.2 (2CO). Analysis Calculated for: C31H31NO5S (529.65): C, 70.30; H, 5.90; N, 2.64; S, 6.05. Found: C, 70.52; H, 5.74; N, 2.83; S, 6.25%. EIMS: m/z 529 [M]+ (26%).
Ethyl 2-amino-1-cyano-9-(4-methoxyphenyl)-4,4-dimethyl-7-phenyl-5,9-dihydro-4H-thieno[3,2-f]chromene-8-carboxylate (16c)
Pale orange crystals from acetic acid, yield (3.07 g, 60%), m.p. 143–145 °C. IR (KBr) ν max (cm−1): 3483–3342 (NH2), 3050 (CH-aromatic), 2953 (CH-aliphatic), 2220 (CN), 1688 (CO), 1580 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.07, 1.05 (2s, 6H, 2CH3), 1.13 (t, 3H, J = 7.22 Hz, CH3), 2.45 (s, 2H, CH2), 3.70 (s, 3H, OCH3), 4.23 (q, 2H, J = 7.22 Hz, CH2), 5.21 (s, 2H, D2O exchangeable, NH2), 6.50 (s, 1H, CH- pyran), 7.22–7.48 (m, 9H, C6H5, C6H4). 13C NMR (DMSO-d6, 75 MHz): δ 16.1 (OCH2CH3), 24.8 (2CH3), 50.2 (OCH3), 56.2 (CH2), 52.2 (OCH2CH3), 90.5 (pyran C-5), 116.8 (CN), 120.3, 121.4, 121.8, 122.5, 123.67, 125.8, 126.8, 127.4 (C6H5, C6H4), 136.1, 138.6, 139.2, 141.7, 142.6, 142.8, 143.5, 144.8 (thiophene C, pyran C-2, C-3, C-5, C-6), 165.6, 166.3 (2CO). Analysis Calculated for: C30H28N2O4S (512.62): C, 70.29; H, 5.51; N, 5.46; S, 6.26. Found: C, 70.42; H, 5.46; N, 5.61; S, 6.38%. EIMS: m/z 512 [M]+ (46%).
Diethyl 2-amino-9-(4-methoxyphenyl)-4,4-dimethyl-7-phenyl-5,9-dihydro-4H-thieno[3,2-f]chromene-1,8-dicarboxylate (16d)
Yellow crystals from acetic acid, yield (3.91 g, 70%, m.p. 231–233 °C. IR (KBr) ν max (cm−1): 3472–3330 (NH2), 3050 (CH-aromatic), 2955 (CH-aliphatic), 1688, 1686 (2CO), 1580 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.09, 1.05 (2s, 6H, 2CH3), 1.12, 1.13 (2t, 6H, J = 7.22, 7.31 Hz, 2CH3), 2.43 (s, 2H, CH2), 4.19, 3.67 (s, 3H, OCH3), 4.22 (2q, 4H, J = 7.22, 7.31 Hz, 2CH2), 5.18 (s, 2H, D2O exchangeable, NH2), 6.51 (s, 1H, CH- pyran), 7.22–7.52 (m, 9H, C6H5, C6H4). 13C NMR (DMSO-d6, 75 MHz): δ 16.1, 16.3 (two OCH2CH3), 24.8 (2CH3), 56.3 (CH2), 50.8 (OCH3), 52.2, 52.3 (two OCH2CH3), 90.6 (pyran C-4), 120.1, 121.3, 123.7, 123.9, 124.3, 125.1, 125.4, 126.5 (C6H5, C6H4), 136.3, 138.6, 139.5, 140.7, 143.2, 143.6, 143.3, 143.4 (thiophene C, pyran C-2, C-3, C-54, C-6), 165.3, 166.6 (2CO). Analysis Calculated for: C32H33NO6S (559.67): C, 68.67; H, 5.94; N, 2.50; S, 5.73%. Found: C, 68.52; H, 5.88; N, 2.71; S, 5.62%. EIMS: m/z 559 [M]+ (33%).
Ethyl 2-amino-9-(4-chlorophenyl)-1-cyano-4,4-dimethyl-7-phenyl-5,9-dihydro-4H-thieno[3,2-f]chromene-8-carboxylate (16e)
Pale orange crystals from acetic acid, yield (3.72 g, 72%), m.p. 111–113 °C. IR (KBr) ν max (cm−1): 3493–3340 (NH2), 3050 (CH-aromatic), 2951 (CH-aliphatic), 2220 (CN), 1688 (CO), 1580 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.08, 1.03 (2s, 6H, 2CH3), 1.13 (t, 3H, J = 7.02 Hz, CH3), 2.46 (s, 2H, CH2), 4.22 (q, 2H, J = 7.02 Hz, CH2), 5.21 (s, 2H, D2O exchangeable, NH2), 6.54 (s, 1H, CH- pyran), 7.25–7.40 (m, 9H, C6H5, C6H4). 13C NMR (DMSO-d6, 75 MHz): δ 16.1 (OCH2CH3), 24.7 (2CH3), 56.3 (CH2), 52.2 (OCH2CH3), 90.7 (pyran C-4), 116.8 (CN), 120.0, 121.8, 123.6, 124.5, 125.2, 125.7, 126.1, 127.0 (C6H5, C6H4), 136.4, 138.7, 139.2, 141.1, 142.8, 143.2, 143.6, 144.7 (thiophene C, pyran C-2, C-3, C-5, C-6), 165.5 (CO). Analysis Calculated for: C29H25ClN2O3S (517.04): C, 67.37; H, 4.87; N, 5.42; S, 6.20%. Found: C, 67.55; H, 5.04; N, 5.60; S, 6.42%. EIMS: m/z 517 [M]+ (32%).
Diethyl 2-amino-9-(4-chlorophenyl)-4,4-dimethyl-7-phenyl-5,9-dihydro-4H-thieno-[3,2-f]chromene-1,8-dicarboxylate (16f)
Yellow crystals from acetic acid, yield (33.77 g, 67%), m.p. 85 °C. IR (KBr) ν max (cm−1): 3483–3327 (NH2), 3050 (CH-aromatic), 2955 (CH-aliphatic), 1688, 1686 (2CO), 1584 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.08, 1.04 (2s, 6H, 2CH3), 1.12, 1.14 (2t, 6H, J = 6.25, 7.51 Hz, 2CH3), 2.43 (s, 2H, CH2), 4.19, 4.22 (2q, 4H, J = 6.25, 7.51 Hz, 2CH2), 5.18 (s, 2H, D2O exchangeable, NH2), 6.51 (s, 1H, CH- pyran), 7.22–7.52 (m, 9H, C6H5, C6H4). 13C NMR (DMSO-d6, 75 MHz): δ 16.1, 16.3 (two OCH2CH3), 24.8 (2CH3), 56.3 (CH2), 52.1, 52.4 (two OCH2CH3), 90.6 (pyran C-4), 120.5, 120.9, 122.3, 123.5, 124.1, 125.4, 125.8, 127.9 (C6H5, C6H4), 136.5, 137.8, 139.2, 140.5, 143.9, 143.3, 143.6, 144.1 (thiophene C, pyran C-2, C-3, C-5, C-6), 165.6, 166.9 (2CO). Analysis Calculated for: C31H30ClNO5S (564.09): C, 66.01; H, 5.36; N, 2.48; S, 5.68%. Found: C, 66.26; H, 5.46; N, 2.62; S, 5.80%. EIMS: m/z 564 [M]+ (26%).
Synthesis of the chromeno[5,6-d]thiazole derivatives 18a–c
Equimolar amounts of either 14a (4.02 g, 0.01 mol), 14b (4.32 g, 0.01 mol) or 14c (4.36 g, 0.01 mol) in absolute ethanol (50 mL) containing triethylamine (1.0 mL), elemental sulfur (0.32 g, 0.01 mol) and phenylisothiocyanate (1.30 g, 0.01 mol) were mixed together. The reaction mixture, in each case was heated under reflux for 2 h then poured onto ice/water containing a few drops of hydrochloric acid and the formed solid product was collected by filtration.
Ethyl 4,4-dimethyl-1,7,9-triphenyl-2-thioxo-2,4,5,9-tetrahydro-1H-chromeno[5,6-d]thiazole-8-carboxylate (18a)
Orange crystals from ethanol, yield (3.30 g, 60%), m.p. 221–223 °C. IR (KBr) ν max (cm−1): 3050 (CH-aromatic), 2956 (CH-aliphatic), 1688 (CO), 1580 (C=C), 1210 (C=S). 1H NMR (DMSO-d6, 300 MHz): δ = 1.08, 1.03 (2s, 6H, 2CH3), 1.16 (t, 3H, J = 7.05 Hz, OCH2CH3), 2.40 (s, 2H, CH2), 4.22 (q, 2H, J = 7.05 Hz, OCH2CH3), 6.56 (s, 1H, CH- pyran), 7.25–7.42 (m, 15H, 3C6H5). 13C NMR (DMSO-d6, 75 MHz): δ 16.2 (OCH2CH3), 24.3 (2CH3), 50.2 (OCH2CH3), 56.0 (CH2), 90.8 (pyran C-4),119.0, 119.6, 120.4, 121.5, 122.2, 122.6, 123.1, 123.6, 124.6, 124.9, 125.1, 125.8 (3C6H5), 134.1, 136.7, 139.2, 140.1, 142.8, 143.2, 143.8, 145.8 (thiazole C, pyran C-2, C-3, C-5, C-6), 178.7 (C=S). Analysis Calculated for C33H29NO3S2 (551.72): C, 71.84; H, 5.30; N, 2.54; S, 11.62%. Found: C, 71.96; H, 5.48; N, 2.80; S, 11.80%. EIMS: m/z 551 [M]+ (80%).
Ethyl 9-(4-methoxyphenyl)-4,4-dimethyl-1,7-diphenyl-2-thioxo-2,4,5,9-tetrahydro-1H-chromeno[5,6-d]thiazole-8-carboxylate (18b)
Orange crystals from 1,4-dioxane, yield (3.83 g, 66%), m.p. 201–204 °C. IR (KBr) ν max (cm−1): 3050 (CH-aromatic), 2955 (CH-aliphatic), 1688 (CO), 1620 (C=C), 1218 (C=S). 1H NMR (DMSO-d6, 300 MHz): δ = 1.07, 1.05 (2s, 6H, 2CH3), 1.13 (t, 3H, J = 6.95 Hz, OCH2CH3), 2.43 (s, 2H, CH2), 3.70 (s, 3H, CH3), 4.22 (q, 2H, J = 6.95 Hz, OCH2CH3), 6.53 (s, 1H, CH- pyran), 7.24–7.56 (m, 14H, 2C6H5, C6H4). 13C NMR (DMSO-d6, 75 MHz): δ 16.5 (OCH2CH3), 24.6 (2CH3), 50.1 (OCH2CH3), 50.7 (OCH3), 56.0 (CH2), 90.7 (pyran C-4), 119.2, 119.5, 120.3, 120.7, 122.1, 122.8, 123.3, 123.8, 124.2, 124.7, 125.5, 126.4 (2C6H5, C6H4), 133.8, 135.2, 139.1, 140.3, 142.2, 143.5, 143.4, 145.9 (thiazole C, pyran C-2, C-3, C-5, C-6), 178.9 (C=S). Analysis Calculated for C34H31NO4S2 (581.74): C, 70.20; H, 5.37; N, 2.41; S, 11.02%. Found: C, 70.39; H, 5.43; N, 2.67; S, 11.19%. EIMS: m/z 581 [M]+ (76%).
Ethyl 9-(4-chlorophenyl)-4,4-dimethyl-1,7-diphenyl-2-thioxo-2,4,5,9-tetrahydro-1H-chromeno[5,6-d]thiazole-8-carboxylate (18c)
Orange crystals from ethanol, yield (2.90 g, 50%), m.p. 156–158 °C. IR (KBr) ν max (cm−1): 3050 (CH-aromatic), 2954 (CH-aliphatic), 1689 (CO), 1580 (C=C), 1212 (C=S). 1H NMR (DMSO-d6, 300 MHz): δ = 1.07, 1.03 (2s, 6H, 2CH3), 1.16 (t, 3H, J = 6.30 Hz, OCH2CH3), 2.43 (s, 2H, CH2), 4.22 (q, 2H, J = 6.30 Hz, OCH2CH3), 6.58 (s, 1H, CH- pyran), 7.25–7.56 (m, 14H, 2C6H5, C6H4). 13C NMR (DMSO-d6, 75 MHz): δ 16.5 (OCH2CH3), 24.6 (2CH3), 50.5 (OCH2CH3), 56.3 (CH2), 90.8 (pyran C-4), 119.3, 120.0, 120.4, 121.8, 122.1, 122.7, 123.1, 123.9, 124.2, 124.5, 125.3, 125.5 (2C6H5, C6H4), 133.6, 136.9, 139.1, 140.1, 142.5, 143.2, 143.7, 145.3 (thiazole C, pyran C-2, C-3, C-5, C-6), 178.9 (C=S). Analysis Calculated for C33H28ClNO3S2 (586.16): C, 67.62; H, 4.81; N, 2.39; S, 10.94%. Found: C, 67.82; H, 5.02; N, 2.42; S, 11.19%. EIMS: m/z 586 [M]+ (58%).
General procedure for the synthesis of the hydrazide derivatives 20a–f
To a solution of either 18a (5.51 g, 0.01 mol), 18b (5.81 g, 0.01 mol) or 18c (5.85 g, 0.01 mol) in 1,4-dioxane (40 mL) either hydrazine hydrate (1.0 mL, 0.20 mol) or phenylhydrazine (2.16 g, 0.02 mol) was added. The reaction mixture, in each case, was heated under reflux for 3 h then left to cool and the produced solid product was collected by filtration.
2-Hydrazono-4,4-dimethyl-1,7,9-triphenyl-2,4,5,9-tetrahydro-1H-chromeno[5,6-d]thiazole-8-carbohydrazide (20a)
Orange crystals from ethanol/DMF, yield (3.21 g, 60%), m.p. > 300 °C. IR (KBr) ν max (cm−1): 3520–3342 (NH2, NH), 3050 (CH-aromatic), 2955 (CH-aliphatic), 1687 (CO), 1623 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.08, 1.03 (2s, 6H, 2CH3), 2.40 (s, 2H, CH2), 4.58 (s, 4H, D2O exchangeable, 2NH2), 6.53 (s, 1H, CH- pyran), 7.25–7.48 (m, 15H, 3C6H5), 8.20 (s, 1H, D2O exchangeable, NH). 13C NMR (DMSO-d6, 75 MHz): δ 24.3 (2CH3), 56.2 (CH2), 90.6 (pyran C-4), 119.5, 119.9, 120.1, 121.4, 121.8, 122.2, 123.5, 123.9, 124.6, 124.8, 125.4, 125.5 (3C6H5), 134.0, 136.4, 138.6, 140.4, 142.3, 143.6, 143.9, 145.3 (thiazole C, pyran C-2, C-3, C-5, C-6, 164.8 (CO), 174.9 (C=N). Analysis Calculated for C31H29N5O2S (535.66): C, 69.51; H, 5.46; N, 13.07; S, 5.99%. Found: C, 69.24; H, 5.49; N, 13.26; S, 6.16%. EIMS: m/z 535 [M]+ (85%).
4,4-Dimethyl-N',1,7,9-tetraphenyl-2-(2-phenylhydrazono)-2,4,5,9-tetrahydro-1H-chromeno[5,6-d]thiazole-8-carbohydrazide (20b)
Orange crystals from ethanol/DMF, yield (4.25 g, 62%), m.p. 268–271 °C. IR (KBr) ν max (cm−1): 3520–3342 (NH2, NH), 3050 (CH-aromatic), 2955 (CH-aliphatic), 1689 (CO), 1623 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.06, 1.04 (2s, 6H, 2CH3), 2.38 (s, 2H, CH2), 6.56 (s, 1H, CH- pyran), 7.25–7.48 (m, 25H, 5C6H5), 8.20, 8.24, 8.29 (3 s, 3H, D2O exchangeable, 3NH). 13C NMR (DMSO-d6, 75 MHz): δ 24.3 (2CH3), 56.2 (CH2), 90.4 (pyran C-4), 118.7, 119.5, 119.9, 120.1, 120.4, 120.6, 121.4, 121.8, 122.2, 122.4, 122.3, 123.5, 123.9, 124.6, 124.8, 125.1, 125.4, 125.5 (5C6H5), 133.8, 134.1, 137.2, 139.3, 142.3, 143.4, 143.9, 144.1 (thiazole C, pyran C-2, C-3, C-5, C-6), 164.4 (CO), 174.8 (C=N). Analysis Calculated for C43H37N5O2S (687.95): C, 75.08; H, 5.42; N, 10.18; S, 4.66%. Found: C, 74.23; H, 5.60; N, 10.31; S, 4.82%. EIMS: m/z 687 [M]+ (80%).
2-Hydrazono-9-(4-methoxyphenyl)-4,4-dimethyl-1,7-diphenyl-2,4,5,9-tetrahydro-1H-chromeno[5,6-d]thiazole-8-carbohydrazide (20c)
Orange crystals from ethanol/DMF, yield (3.39 g, 60%), m.p. 245–248 °C. IR (KBr) ν max (cm−1): 3492–3335 (NH2, NH), 3050 (CH-aromatic), 2955 (CH-aliphatic), 1689 (CO), 1631 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.08, 1.05 (2s, 6H, 2CH3), 2.43 (s, 2H, CH2), 3.69 (s, 3H, OCH3), 4.58 (s, 4H, D2O exchangeable, 2NH2), 6.53 (s, 1H, CH- pyran), 7.22–7.58 (m, 14H, 2C6H5, C6H4), 8.23 (s, 1H, D2O exchangeable, NH). 13C NMR (DMSO-d6, 75 MHz): δ 24.5 (2CH3), 56.6 (CH2), 50.6 (OCH3), 90.6 (pyran C-4), 119.2, 119.7, 120.3, 121.6, 121.9, 122.2, 123.5, 123.7, 124.4, 124.8, 125.1, 125.8 (2C6H5, C6H4), 133.8, 135.7, 138.3, 140.2, 142.6, 143.8, 143.9, 144.6 (thiazole C, pyran C-2, C-3, C-5, C-6), 164.8 (CO), 174.6 (C=N). Analysis Calculated for C32H31N5O3S (565.69): C, 67.94; H, 5.52; N, 12.38; S, 5.67%. Found: C, 68.25; H, 5.46; N, 12.51; S, 6.43%. EIMS: m/z 565 [M]+ (65%).
9-(4-Methoxyphenyl)-4,4-dimethyl-N',1,7-triphenyl-2-(2-phenylhydrazono)-2,4,5,9-tetrahydro-1H-chromeno[5,6-d]thiazole-8-carbohydrazide (20d)
Orange crystals from ethanol/DMF, yield (4.44 g, 62%), m.p. 221–225 °C. IR (KBr) ν max (cm−1): 3484–3327 (NH2, NH), 3050 (CH-aromatic), 2955 (CH-aliphatic), 1687 (CO), 1635 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.07, 1.04 (2s, 6H, 2CH3), 2.35 (s, 2H, CH2), 3.66 (s, 3H, OCH3), 6.52 (s, 1H, CH- pyran), 7.26–7.52 (m, 24H, 4C6H5, C6H4), 8.22, 8.26, 8.28 (3 s, 3H, D2O exchangeable, 3NH). 13C NMR (DMSO-d6, 75 MHz): δ 24.6 (2CH3), 56.3 (CH2), 90.7 (pyran C-4), 119.5, 119.8, 119.9, 120.4, 120.7, 120.8, 121.2, 121.5, 122.5, 122.6, 122.8, 123.3, 123.5, 124.8, 124.8, 125.3, 125.6, 125.8 (4C6H5, C6H4), 133.5, 134.3, 137.5, 138.2, 141.1, 143.8, 143.5, 144.3 (thiazole C, pyran C-2, C-3, C-5, C-6), 164.5 (CO), 174.7 (C=N). Analysis Calculated for C44H39N5O3S (717.88): C, 73.62; H, 5.48; N, 9.59; S, 4.47%. Found: C, 73.80; H, 5.32; N, 9.79; S, 4.51%. EIMS: m/z 717 [M]+ (50%).
9-(4-Chlorophenyl)-2-hydrazono-4,4-dimethyl-1,7-diphenyl-2,4,5,9-tetrahydro-1H-chromeno[5,6-d]thiazole-8-carbohydrazide (20e)
Pale brown crystals from ethanol/DMF, yield (3.30 g, 58%), m.p. 196–197 °C. IR (KBr) ν max (cm−1): 3478–3342 (NH2, NH), 3050 (CH-aromatic), 2956 (CH-aliphatic), 1687 (CO), 1634 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.07, 1.06 (2s, 6H, 2CH3), 2.45 (s, 2H, CH2), 4.52 (s, 4H, D2O exchangeable, 2NH2), 6.56 (s, 1H, CH- pyran), 7.24–7.55 (m, 14H, 2C6H5, C6H4), 8.24 (s, 1H, D2O exchangeable, NH). 13C NMR (DMSO-d6, 75 MHz): δ 24.7 (2CH3), 56.6 (CH2), 50.8 (OCH3), 90.8 (pyan C-4), 119.0, 119.4, 120.7, 121.1, 121.9, 122.0, 123.5, 123.9, 124.4, 124.2, 125.3, 125.6 (2C6H5, C6H4), 133.4, 135.4, 138.6, 140.1, 142.3, 143.8, 143.7, 144.2 (thiazole C, pyran C-2, C-3, C-5, C-6), 164.8 (CO), 174.6 (C=N). Analysis Calculated for C31H28ClN5O2S (570.10): C, 65.31; H, 4.95; N, 12.28; S, 5.62%. Found: C, 65.52; H, 5.13; N, 12.40; S, 6.39%. EIMS: m/z 570 [M]+ (42%).
9-(4-Chlorophenyl)-4,4-dimethyl-N',1,7-triphenyl-2-(2-phenylhydrazono)-2,4,5,9-tetrahydro-1H-chromeno[5,6-d]thiazole-8-carbohydrazide (20f)
Orange crystals from ethanol/DMF, yield (3.46 g, 48%), m.p. 195–198 °C. IR (KBr) ν max (cm−1): 3498–3332 (NH), 3050 (CH-aromatic), 2955 (CH-aliphatic), 1687 (CO), 1632 (C=C). 1H NMR (DMSO-d6, 300 MHz): δ = 1.07, 1.04 (2s, 6H, 2CH3), 2.38 (s, 2H, CH2), 6.56 (s, 1H, CH- pyran), 7.25–7.48 (m, 24H, 4C6H5, C6H4), 8.20, 8.24, 8.29 (3 s, 3H, D2O exchangeable, 3NH). 13C NMR (DMSO-d6, 75 MHz): δ 24.3 (2CH3), 56.2 (CH2), 90.6 (pyran C-4), 118.7, 119.5, 119.9, 120.1, 120.4, 120.6, 121.4, 121.8, 122.2, 122.4, 122.3, 123.5, 123.9, 124.6, 124.8, 125.1, 125.4, 125.5 (4C6H5, C6H4), 133.8, 134.1, 137.2, 139.3, 142.3, 143.4, 143.9, 144.1 (thiazole C, pyran C-2, C-3, C-5, C-6), 164.4 (CO), 174.8 (C=N). Analysis Calculated for C43H36ClN5O2S (722.30): C, 71.50; H, 5.02; N, 9.70; S, 4.44%. Found: C, 71.23; H, 5.37; N, 10.01; S, 4.62%. EIMS: m/z 722 [M]+ (80%).
Conclusion
The target molecules were synthesized using dimedone through multi-component reactions reactions to produce fused thiophene, thiazole, coumarin, pyran and pyridine derivatives. Some multi-component reactions were carried out using the effective magnetically separable nanocatalyst Fe3O4@MCM-41-SO3H@[HMIm][HSO4] could efficiently catalyze the one-pot three-component reaction. The anti-proliferative activity of the newly synthesized compounds toward the six cancer cell lines namely A549, H460, HT-29, MKN-45, U87MG, and S + MMC-7721 was studied. In addition, inhibitions of the most active compounds the thieno[3,2-f]chromene derivatives 16a–f toward cancer cell lines classified according to the disease were also studied. Moreover, the newly synthesized compounds were screened for their anticancer potentials against hepatocellular carcinoma HepG2 and cervical carcinoma HeLa cell lines. The results obtained in this work encourage further work in the future since many compounds were considered as promising anticancer agents.
References
K. Li, Y. Yingl, Z. Lu, Y. Xinghan, S. Yan, Green Synth. Catal. 2022(3), 59 (2022)
N. Zumbrägel, H. Gröger, J. Biotechnol. 291, 35 (2019)
K. Nikoofar, F.M.A. Yielzoleh, J. Saudi Chem. Soc. 22, 715 (2018)
A. Kumar, S. Sharma, R.A. Maurya, Tetrahedron Lett. 50, 5973 (2009)
F. Shi, X.N. Zeng, G. Zhang, N. Ma, B. Jiang, S. Tu, Bioorg. Med. Chem. Lett. 21, 7119 (2011)
V. Cecchetti, F. Schiaffella, O. Tabarrini, W. Zhou, A. Fravolini, A. Goi, G. Bruni, G. Segre, Eur. J. Med. Chem. 26, 381 (1991)
H.A. Abuelizz, R.A. El-Dib, M. Marzouk, R. Al-Salahi, Microbial. Pathogen. 117, 60 (2018)
J.A. Kumar, G. Saidachary, G. Mallesham, B. Sridhar, N. Jain, S.V. Kalivendi, V. Jayathirtha, B. Raod, B.C. Raju, Eur. J. Med. Chem. 65, 389 (2013)
I. Fatima, R. Saxena, G. Kharkwal, M.K. Hussain, N. Yadav, K. Hajela, P.L. Sankhwar, A. Dwived, J. Steroid Biochem. Mol. Biol. 138, 123 (2013)
M. Beyrati, A. Hasaninejad, Tetrahedron Lett. 58, 1947 (2017)
B. Ganem, Acc. Chem. Res. 42, 463 (2009)
S. Nemouchi, R. Boulcina, B. Carboni, A. Debachea, C. R. Chim. 15, 394 (2012)
S.K. Mansoor, K. Logaiya, K. Aswin, P.N. Sudhan, J. Taibah Univ. Sci. 9, 213 (2015)
C. Yao, Y. Wang, T. Li, C. Yu, L. Li, C. Wang, Tetrahedron 69, 10593 (2013)
G. Brahmachari, K. Nurjamal, Tetrahedron Lett. 60, 1904 (2019)
A. Ivanets, N. Kitikova, I. Shashkova, Y. Matrunchik, L. Kulbitskaya, M. Sillanpää, Environ. Technol. Innov. 6, 152 (2016)
M. Rahman, A. Sarkar, M. Ghosh, A. Majee, A. Hajra, Tetrahedron Lett. 55, 235 (2014)
M.M. Heravi, E. Hashemi, Y.S. Beheshtiha, K. Kamjou, M. Toolabi, N. Hosseintash, J. Mol. Catal. A Chem. 392, 173 (2014)
M. Khalil, A. Amanda, R.T. Yunarti, B.M. Jan, S. Irawan, J. Pet. Sci. Eng. 195, 107660 (2020)
M.G. Dekamin, M.E. Maleki, Tetrahedron 69, 1074–1085 (2013)
E.S. Dragan, D.F. Loghin, A.I. Cocarta, M. Doroftei, React. Funct. Polym. 105, 66 (2016)
N.Y. Abdo, R.M. Mohareb, P.A. Halim, Bioorg. Chem. 97, 103667 (2020)
R. Mohan, N. Kalla, A. Varyambath, M.R. Kim, I. Kim, Appl. Catal. A Gen. 538, 9 (2017)
P. Cottu, O. Tredan, A. Livartowski, D. Pero, M. Gilberg, R. Ghorbal, J. Dupin, C. Maillard, A. Kador, Value Health 23, S456 (2020)
M. Burgera, M.N.M. van der Aa, J.M.M. van Oers, A. Brinkmann, T.H. van der Kwast, E.C. Steyerberg, R. Stoehr, W.J. Kirkels, S. Denzinger, P.J. Wild, W.F. Wieland, F. Hofstaedter, A. Hartmann, E.C. Zwarthoff, Eur. Urol. 54, 835 (2008)
H. Sung, J.M.E. Ferlay, Rebecca, L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal, F. Bray, Cancer J. Clin. 71, 209 (2021)
R.M. Mohareb, T.M. Klapotke, E. Reinhardt, Med. Chem. Res. 27, 249 (2018)
R.M. Mohareb, N.Y. Abdo, M.S. Gamaan, J. Heterocycl. Chem. 57, 2512 (2020)
R.M. Mohareb, E.S. Elwan, Anticancer Agent Med. Chem. 20, 1209 (2020)
I.M. Paczkowski, E.P. Guedes, E.B. Mass, E.W. de Meneses, L.A. Marques, M.S. Mantovani, D. Russowsky, J. Heterocycl. Chem. 57, 2597 (2020)
Y. Dong, Q. Shi, K.N. Goto, P.C. Wu, S.L. Natschke, A. Brossi, K.F. Bastow, J.Y. Lang, M.C. Hung, K.H. Lee, Bioorg. Med. Chem. 18, 803 (2010)
W.H. El-Shwiniy, W.S. Shehab, W.A. Zordok, J. Mol. Struct. 1199, 126993 (2020)
T. Uengwetwanit, N. Chutiwitoonchai, K. Wichapong, N. Karoonuthaisiri, Comput. Struct. Biotechnol. J. 20, 882 (2022)
E.P. Gere, A.S. Tóth, P. Szabó, T. Steinmetzer, E.F. Nyúl, M. Poór, Biomed. Pharmacother. 151, 113124 (2022)
G. Routholla, S. Pulya, T. Patel, N. Adhikari, S.A. Amin, M. Paul, S. Bhagavatula, S. Biswas, T. Jha, B. Ghosh, Bioorg. Chem. 117, 105446 (2021)
S. Zare, M. Fereidoonnezhad, D. Afshar, Z. Ramezani, Comput. Biol. Chem. 67, 22 (2017)
F. Javadi, R. Tayebee, Microporous Mesoporous Mater. 231, 100 (2016)
Y.H.W. Quan, T.C.G. Yan, Tetrahedron Lett. 55, 1441 (2014)
Z. Puterová, A. Krutošíkov, D. Végh, ARKIVOC i, 209 (2010)
J. Safari, Z. Zarnegar, C. R. Chim. 16, 920 (2013)
N. Azgomi, M. Mokhtary, J. Mol. Catal. A Chem. 398, 58 (2015)
A. Monks, D. Scudiero, P. Skehan, R. Shoemaker, K. Paull, D. Vistica, C. Hose, J. Langley, P. Cronise, M.A. Vaigro-Wolff, Gray-Goodrich, H. Cambell, J. Mayo, M. Boyd, J. Nat. Cancer Inst. 83, 757 (1991)
S. Elmore, Apoptosis: a review of programmed cell death. Toxicol. Pathol. 35, 495 (2007)
S. Fink, B. Cookson, Infect. Immun. 73, 1907 (2005)
M.R. Boyd, K.D. Paull, Drug Dev. Res. 34, 91 (1995)
M.R. Boyd, in Cancer Drug Discovery and Development, 2nd edn., ed. by B.A. Teicher (Humana Press, 1997), pp.23–43
S. Garofalo, R. Rosa, R. Bianco, G. Tortora, Expert Opin. Therap. Patents 18, 889 (2008)
A.I. Al-Suwaidan, I.N. Abdel-Aziz, A.S. El-Azab, M.A. ElSayed, A.M. Alanazi, M.B. El-Ashmawy, A.A. Abdel-Aziz, J. Enzyme Inhib. Med. Chem. 30, 679 (2015)
V. Pogacic, A.N. Bullock, O. Fedorov, P. Filippakopoulos, C. Gasser, A. Biondi, S. Meyer-Monard, S. Knapp, J. Schwaller, Cancer Res. 2007(67), 6916 (2007)
I.W. Cheney, S. Yan, T. Appleby, H. Walker, T. Vo, N. Yao, R. Hamatake, Z. Hong, Bioorg. Med. Chem. Lett. 17, 1679 (2007)
M.A.O. Abdelf-Fattah, M.A.M. El-Naggar, R.M.H. Rashied, B.D. Gary, G.A. Piazza, A.H. Abadi, Med. Chem. 8, 392 (2012)
A.M. Clark, C. Magawa, A.P. Zamora, P. Low, M. Reynolds, S.J. Ralph, Biomed. Pharmacoth. 2021(140), 111790 (2021)
Z. Ding, W. Pan, Y. Xiao, B. Cheng, G. Huang, J. Chen, Eur. J. Med. Chem. 2022(237), 114401 (2022)
N.N. Kumar, M.E. Pizzo, G. Nehra, B.W. Resman, S. Boroumand, R.G. Thorne, Bioconj. Chem. 2018(29), 3937 (2018)
R. Wang, B. Chen, H. Wei, W. Yan, Y. Wu, C. Wang, B. Zhang, F. Liu, H. Tian, X. Chen, W. Tian, Collecting and deactivating TGF-β1 hydrogel for anti-scarring therapy in post-glaucoma filtration surgery. Mater. Today Biol. 14, 100260 (2022)
E.A. Fayed, Y.A. Ammar, M.A. Saleh, A.H. Bayoumi, A. Belal, A.B.M. Mehany, A. Ragab, J. Mol. Struct. 2021(1236), 130317 (2021)
M.A.I. Elbastawesy, A.A. Aly, M. Ramadan, Y.A.M.M. Elshaier, B.G.M. Youssif, A.B. Brown, Bioorg. Chem. 90, 103045 (2019)
K. Khaldoun, A. Safer, N. Boukabcha, N. Dege, S. Ruchaud, M. Souab, S. Bach, A. Chouaih, S.S. Besbes, J. Mol. Struct. 1192, 82 (2019)
S. Madhusudan, T.S. Ganesan, Clin. Biochem. 37, 618 (2004)
N. Hesabi, A. Ebrahimi, J. Mol. Liq. 307, 112874 (2020)
Acknowledgements
R. M. Mohareb would like to express his great thanks to the Alexander von Humboldt Foundation in Bonn, Germany for affording him regular fellowships in Germany that help to finance this work.
Funding
This work was not financed by any source.
Author information
Authors and Affiliations
Contributions
First author RMM had the idea of writing this article, and he performed the literature survey and data research. The second author and the third author were responsible about revising the manuscript and writing the text and the references of this work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest, financial, or otherwise.
Ethical approval
No related ethical issues.
Informed consent
Informed consent was obtained from all participants included in the study.
Consent for publication
This work is consent for publication through the Journal formats.
Consent to participate
The authors promise that the work described has not been published previously, that it is not under consideration for publication elsewhere, that its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out.
Consent to publish
The authors promise that if the manuscript is accepted, it will not be published elsewhere in the same form, in English or in any other language, without the written consent of the Publisher. There are no conflicts of interest to declar.
Human and animal rights
No Animals/Humans were used for studies that are the basis of this research.
Additional information
Communicated by Richard G.F. Visser.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohareb, R.M., Ibrahim, R.A. & Samir, E.M. Synthesis and docking studies of pyran, pyridine and thiophene derivatives and their antitumor evaluations against cancer, hepatocellular carcinoma and cervical carcinoma cell lines. J IRAN CHEM SOC 20, 2163–2187 (2023). https://doi.org/10.1007/s13738-023-02793-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-023-02793-y