Abstract
A convenient, regioselective, novel, and elegant one-pot, four-component reaction was designed for synthesis of a series of new of imidazo[1,2-a]pyridines using aryl glyoxal monohydrates, ethyl acetoacetate, hydrazine hydrate, and 2-aminopyridine in presence of tetrapropylammonium bromide under reflux in EtOH as solvent. The main advantages of this protocol include the availability and low cost of the starting materials, short reaction time, convenient operation, easy workup process, highly facile operation, nonhazardous byproducts, and high yield (82–94%).
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One-pot, multicomponent reactions (MCRs) are effective, time-saving, and elaborate processes in which three or more simple and readily available components are combined together to form novel complex molecules containing features of each reagent. Therefore, these reactions have been developed as powerful, highly efficient, and useful tools for synthesis of fused heterocycle compounds in various branches of organic and medicinal chemistry [1,2,3,4,5], due to their minimization of cost and waste.
Imidazo[1,2-a]pyridines are fused [5,6]-bicyclic heterocycles with one imidazole moiety embedded with the pyridine ring, being an important class of privileged bicyclic scaffolds with widespread applications in pharmaceuticals, as organic functional materials, and for their diverse biological activities [6].
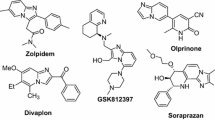
The imidazo[1,2-a]pyridine moiety can be found in many pharmaceuticals, natural products, bioactive compounds, biomolecules, and agrochemicals [7]. Imidazo[1,2-a]pyridines exhibit biological activities such as anticancer [8], antibacterial [9], antifungal [10], antiviral [11], antiinflammatory [12], antimalarial [13], antiparkinsonian [14], and antituberculosis effects [15]. In addition, a few derivatives exhibit enzyme inhibition [16] and are used as drugs for Alzheimer’s disease [17]. Compounds containing the imidazo[1,2-a]pyridine moiety are present in many natural products and marketed drugs, such as alpidem (an anxiolytic agent) [18], saripidem and necopidem (both anxiolytic agents) [19], zolpidem (an hypnotic agent used in treatment of insomnia) [20], zolimidine (an antiulcer drug) [21], miroprofen (an analgesic drug) [22], olprinone (a cardiotonic agent for treatment of acute heart failure) [23], GSK812397 [with anti-human immunodeficiency virus (HIV) properties] [24], and minodronic acid (for treatment of anxiety, heart failure, and osteoporosis) [25] (Fig. 1).
All these bioactivities make the imidazo[1,2-a]pyridine scaffold remarkable for chemists due to its excellent pharmaceutical and medicinal applications and use in advanced organic chemistry.
Over recent years, several methods have been described for synthesis of fused bicyclic imidazo[1,2-a]pyridines, as summarized in a review [26].
A popular approach involves condensation reaction of 2-aminopyridines with various precursors such as α-halocarbonyl compounds [27], one-pot reaction of 2-aminopyridines, aldehydes, and nitroalkane [28], isonitrile [29], using copper catalysis, one-pot multicomponent reaction of aryl glyoxals, 2-aminopyridine, and cyclic 1,3-dicarbonyls [30], oxidative coupling between substituted 2-aminopyridine and propargylic alcohols [31], nitroolefins [32], α,β-unsaturated ketones [33], alkynes [34], copper-catalyzed oxidative coupling trough C–H functionalization between pyridine derivatives and N-(alkylidene)-4H-1,2,4-triazole-4-amines [35], etc.
Nevertheless, these strategies have drawbacks in terms of harsh reaction conditions, use of excess amounts of expensive catalyst, complex workup procedures, extended reaction time, extensive by-product formation, and low yield. Hence, development of economic, practicable methods offering operational simplicity and high yield using basic chemicals as starting materials for multicomponent synthesis of this series of compounds in a single step is still desired.
Tetrapropylammonium bromide (TPAB) was first synthesized in 1990 [36]. This quaternary ammonium salt is commonly used as a phase-transfer catalyst [37]. Recently, we used this compound as catalyst for synthesis of heterocycle scaffolds [38]. TPAB is a cheap, readily available, ecocompatible catalyst with noncorrosive nature.
Results and discussion
In continuation of our work on aryl-glyoxal-based synthesis of heterocyclic compounds using one-pot, multicomponent strategies [39,40,41,42], we report herein a convenient one-pot, four-component process for synthesis of 2,3-disubstituted imidazo[1,2-a]pyridine derivatives using aryl glyoxals, 2-aminopyridine, ethyl acetoacetate, and hydrazine hydrate in presence of TPAB in EtOH. This synthetic strategy is promising for synthesis of novel alkaloid structures which may exhibit pharmaceutical and biological activities (Scheme 1).
The aryl glyoxal monohydrates applied as starting material in this study were prepared by oxidation of corresponding aryl methyl ketones with SeO2 in H2O:dioxane under reflux condition according to standard literature process [43].
We started our study on the synthesis of a new series of imidazo[1,2-a]pyridine derivatives by investigating the model reaction of phenyl glyoxal monohydrate (1a), 2-aminopyridine (2), and 5-methyl-2,4-dihydro-3H-pyrazol-3-one (6) using various solvents, catalysts, and temperatures to establish the optimum reaction conditions (Table 1).

To maintain green chemistry features, nonhazardous and safe solvents such as water, ethanol, water:ethanol, and ethylene glycol were favored as solvents in all the optimization tests. The one-pot reaction of the mixture of substrates in absence of catalyst in various solvents did not afford the desired product 5a either at room temperature or under stirring at reflux even after 24 h (Table 1, entries 1–5).
Next, the same model reaction was tested in presence of a series of Brønsted acids including p-toluene sulfonic acid (p-TSA), l-proline, l-alanine, AcOH, and sulfamic acid in refluxing H2O or EtOH, forming the desired product in 17–35% yield (Table 1, entries 6–11).
Common basic catalysts such as Et3N, K2CO3 and 1,4-diazabicyclo[2.2.2]octane (DABCO) were also investigated but unfortunately did not lead to a noticeable amount of product or increase in the yield (Table 1, entries 12–16).
When we shifted our attention to TPAB as a catalyst in water, water:acetone, or water:ethanol under reflux condition, the reaction proceeded well, and the desired product was obtained within 3 h in 65–70% yield (Table 1, entries 17–19). Finally, the optimized condition was obtained as TPAB (20 mol%) in ethanol as solvent under stirring at reflux within 3 h, which produced the desired product 5a in 87% yield (Table 1, entry 20).
This method was successfully applied for synthesis of 3-methyl-4-(2-phenylimidazo[1,2-a]pyridin-3-yl)-1H-pyrazol-5-ol (5a) using phenylglyoxal monohydrate (1a), 2-aminopyridine (2), ethyl acetoacetate (3), and hydrazine hydrate (4) (Table 2). The feasibility of this four-component strategy appears to rely on the structure of 5-methyl-2,4-dihydro-3H-pyrazol-3-one (6) with the optimum reaction conditions previously set up for the three-component synthesis.

As indicated in Table 2, the reaction in absence of catalyst in various solvents under high-temperature condition failed to afford the product (Table 2, entries 1–3). In presence of TPAB (15 mol%), the reaction gave the product in 60% yield in water (Table 2, entry 4). Interestingly using the same amount of TPAB (15 mol%) in ethanol with stirring under reflux for 3 h gave the desired four-component product in 91% yield (Table 2, entry 5). Elevation or reduction of the molar ratio of catalyst or changing the temperature to room temperature with duration of 3–10 h could not improve the yield (Table 2, entries 6–9). The reusability of TPAB in synthesis of 5a was also examined. For this purpose, the recovered catalyst was recycled up to four times; the catalytic performance results are shown in Fig. 2.
Using the optimized condition, different aryl glyoxal derivatives 1a–l (monohydrate form), 2-aminopyridine (2), ethyl acetoacetate (3), and hydrazine hydrate (4) also afforded the desired products 5a–l in high yield. The results obtained with various aryl glyoxals, including the product, melting point (m.p.), and yield, are summarized in Table 3.
The structure of all the imidazo[1,2-a]pyridine derivatives 5a–l was elucidated using Fourier-transform infrared (FT-IR) spectroscopy, 1H and 13C nuclear magnetic resonance (NMR) spectroscopy, and mass analysis. The FT-IR (KBr) spectra of 5a–l showed absorption bonds due to vibrations of NH and OH group at 3433–3243 cm−1. In the 1H NMR spectra, N–H and OH group of the products showed broad doublets at δ = 11.20–10.92 ppm, which disappeared on addition of D2O. The protons of aromatic rings were located at around δ = 8.22–6.73 ppm. The methyl hydrogens of the pyrazole ring of the products appeared as a singlet at δ = 1.83–1.58 ppm, and in the 13C NMR spectra as a carbon signal located at around δ = 11.7–10.2 ppm. In the 13C NMR spectra of 5a–l, signals located at around δ = 172.5–160.1 ppm and δ = 160.3–159.0 ppm were ascribed to C–OH and N–C=N group, respectively. The signals located at around δ = 148.9–90.7 ppm were assigned to other carbons of aromatic rings. The analytical and spectral data of the synthesized scaffolds are all in agreement with their proposed structure.
A reasonable reaction mechanism for the synthesis of imidazo[1,2-a]pyridine derivatives 5a–l by a one-pot four-component strategy is depicted in Scheme 2. In the first step, 5-methyl-2,4-dihydro-3H-pyrazol-3-one (6) forms by condensation of hydrazine hydrate (4) and ethyl acetoacetate (3), which would be stabilized by keto–enol tautomerization. In the next step, intermediate 6 in enolate form reacts with aryl glyoxal monohydrate 1a–l in presence of TPAB to form intermediate A. TPAB plays an important role in the formation of intermediate A via Knoevenagel condensation followed by loss of one molecule of water. Subsequently, 2-aminopyridine (2) undergoes intermolecular condensation with the carbonyl group of intermediate A to form intermediate B, which then undergoes Baldwin 5-exo–trig cyclization [44] to give intermediate C. Finally, loss of a water molecule from intermediate C gives the desired imidazo[1,2-a]pyridine derivative 5a–l in presence of TPAB, as shown in Scheme 2.
Conclusions
We developed a simple and efficient protocol for regioselective synthesis of a series of [5,6]-bicyclic fused imidazo[1,2-a]pyridines by one-pot, four-component reaction between aryl glyoxal monohydrates, 2-aminopyridine, ethyl acetoacetate, and hydrazine hydrate in EtOH under reflux conditions, using TPAB as an inexpensive, readily available, and ecocompatible catalyst, showing advantages in terms of reaction time, easy workup, and facile operation. Considering the presence of pyrazole linked to the 3-position of the imidazo[1,2-a]pyridine moiety, it is expected that these fused structures will show promising pharmaceutical activities. The use of an inexpensive catalyst, metal-free conditions, short reaction time, high yield, and lack of hazardous byproducts are the main advantages of this procedure.
Experimental
Materials and methods
The chemicals used in this work were obtained from Arcos and Merck companies and used without purification. Melting points were measured on a Philip Harris C4954718 apparatus and are uncorrected. Reaction progress was monitored by thin-layer chromatography (TLC) on Merck silica plates. Infrared spectra were recorded on a Thermo Nicolet Nexus 670 FT-IR instrument using KBr discs. 1H and 13C NMR spectra were recorded on a Bruker Advance AQS 300 MHz spectrometer at 300 and 75.5 MHz, respectively. Chemical shifts were measured in dimethyl sulfoxide (DMSO)-d6 as solvent relative to tetramethylsilane (TMS) as internal standard. Mass spectra and high-resolution mass spectra were recorded on a Kratos MS 25RF spectrometer; relative abundance of fragments is quoted in parentheses after m/z values.
General procedure for synthesis of 3-methyl-4-(2-arylimidazo[1,2-a]pyridin-3-yl)-1H-pyrazol-5-ol derivatives (5a–k)
Hydrazine hydrate (4, 1 mmol) was added dropwise to a solution of ethyl acetoacetate (3, 1 mmol) in ethanol (5 mL). The reaction mixture was stirred at room temperature and monitored by TLC (using chloroform:methanol 20:1 as eluent). After reaction completion (5–10 min), white precipitate of 5-methyl-2,4-dihydro-3H-pyrazol-3-one (6) formed. To the reaction mixture, aryl glyoxal monohydrate 1a–k (1 mmol), 2-aminopyridine (2, 1 mmol), and TPAB (15 mol%) were added, and the mixture was refluxed for appropriate time as mentioned in Table 3. After reaction completion as monitored by TLC (using chloroform:methanol 3:1 as eluent), the reaction mixture was cooled to room temperature and the precipitate filtered, washed with water, and cold ethanol to give the desired products 5a–k in high yield (82–94%).
Synthesis of 4,4′-(1,4-phenylenebis(imidazo[1,2-a]pyridine-2,3-diyl))bis(3-methyl-1H-pyrazol-5-ol) (5l)
Hydrazine hydrate (4, 2 mmol) was added dropwise to a solution of ethyl acetoacetate (3, 2 mmol) in ethanol (10 mL). The reaction mixture was stirred at room temperature and monitored by TLC (using chloroform:methanol 20:1 as eluent). After reaction completion (5–10 min), 5-methyl-2,4-dihydro-3H-pyrazol-3-one (6) formed as white precipitate. To the reaction mixture, bis-phenylene glyoxal monohydrate (1l, 1 mmol), 2-aminopyridine (2, 2 mmol), and TPAB (15 mol%) were added, and the mixture was refluxed for 6 h. After reaction completion as monitored by TLC (using chloroform:methanol 3:1 as eluent), the reaction mixture was cooled to room temperature, and the orange precipitate filtered, washed with water, and cold ethanol to give the desired product 5l in 84% yield.
Physical and spectral data for compounds 5a–l
3-Methyl-4-(2-phenylimidazo[1,2-a]pyridin-3-yl)-1H-pyrazol-5-ol (5a)
White solid; yield 91% (264 mg); m.p. 290 °C (dec.). FT-IR (KBr) υ: 3243, 2976, 2923, 1633, 1606, 1571, 1505, 1440, 1337, 1283, 1236, 1149, 1106, 1031, 983, 738, 694 cm−1. 1H NMR (300 MHz, DMSO-d6) δ (ppm): 11.78 (bs, 1H, OH, D2O exch.), 10.36 (bs, 1H, NH, D2O exch.), 7.85 (d, J = 6.9 Hz, 1H, Ar), 7.78 (d, J = 7.2 Hz, 2H, Ar), 7.61 (d, J = 9 Hz, 1H, Ar), 7.37–7.26 (m, 4H, Ar), 6.88 (t, J = 6.9 Hz, 1H, Ar), 1.76 (s, 3H, Me). 13C NMR (75.5 MHz, DMSO-d6) δ (ppm): 160.2, 160.1, 144.7, 142.3, 135.4, 132.0, 129.7, 128.6, 127.9, 126.7, 125.9, 124.3, 112.9, 91.3, 10.2. LRMS (EI, 70 eV) m/z (%): 290 (M+, 100), 275 (5), 262 (13), 244 (5), 233 (29), 218 (19), 205 (22), 194 (6), 179 (3), 167 (2), 154 (4), 140 (2), 128 (8), 115 (5), 105 (7), 91 (3), 78 (36), 65 (4), 51 (3). HRMS (ESI): m/z (M)+ calcd. for C17H14N4O+: 290.1168; found: 290.1163.
4-(2-(4-Methoxyphenyl)imidazo[1,2-a]pyridin-3-yl)-3-methyl-1H-pyrazol-5-ol (5b)
White solid; yield 89% (285 mg); m.p. 294 °C (dec.). FT-IR (KBr) υ: 3433, 3110, 3036, 3005, 2907, 2837, 2782, 2613, 1614, 1512, 1459, 1385, 1299, 1255, 1174, 1111, 1032, 973, 837, 788, 754 cm−1. 1H NMR (300 MHz, DMSO-d6) δ (ppm): 11.05 (bs, 2H, OH, NH, D2O exch.), 7.83 (d, J = 6.6 Hz, 1H, Ar), 7.72 (d, J = 6.9 Hz, 2H, Ar), 7.58 (d, J = 8.7 Hz, 1H, Ar), 7.24 (t, J = 6.9 Hz, 1H, Ar), 6.93–6.82 (m, 3H, Ar), 3.76 (s, 3H, OMe), 1.78 (s, 3H, Me). 13C NMR (75.5 MHz, DMSO-d6) δ (ppm): 160.2, 159.0, 144.7, 142.4, 140.3, 129.4, 127.9, 127.2, 125.8, 124.0, 114.7, 113.7, 111.9, 91.4, 56.2, 10.3. LRMS (EI, 70 eV) m/z (%): 320 (M+, 100), 305 (31), 290 (5), 277 (5), 262 (17), 248 (6), 234 (4), 219 (10), 205 (6), 192 (5), 181 (2), 170 (2), 153 (5), 142 (2), 131 (3), 115 (3), 103 (3), 91 (2), 78 (10), 65 (2), 51 (3). HRMS (ESI): m/z (M)+ calcd. for C18H16N4O2+: 320.1273; found: 320.1275.
4-(2-(3-Methoxyphenyl)imidazo[1,2-a]pyridin-3-yl)-3-methyl-1H-pyrazol-5-ol (5c)
White solid; yield 85% (272 mg); m.p. 168–169 °C. FT-IR (KBr) υ: 3407, 3206, 2943, 2840, 1606, 1503, 1459, 1435, 1390, 1343, 1288, 1246, 1219, 1042, 787, 754 cm−1. 1H NMR (300 MHz, DMSO-d6) δ (ppm): 11.00 (bs, 2H, OH, NH, D2O exch.), 7.85 (d, J = 6.3 Hz, 1H, Ar), 7.62 (d, J = 9 Hz, 1H, Ar), 7.36 (d, J = 8.1 Hz, 2H, Ar), 7.30–7.22 (m, 2H, Ar), 6.90–6.82 (m, 2H, Ar), 3.72 (s, 3H, OMe), 1.79 (s, 3H, Me). 13C NMR (75.5 MHz, DMSO-d6) δ (ppm): 160.2, 159.6, 144.6, 142.1, 140.4, 136.6, 130.8, 128.8, 126.3, 124.3, 123.8, 120.3, 118.4, 113.1, 111.2, 91.2, 55.9, 10.2. LRMS (EI, 70 eV) m/z (%): 320 (M+, 100), 305 (17), 290 (10), 275 (3), 263 (20), 247 (8), 231 (5), 219 (19), 205 (14), 192 (5), 159 (4), 145 (2), 127 (2), 109 (3), 98 (5), 78 (19), 67 (4), 57 (8). HRMS (ESI): m/z (M)+ calcd. for C18H16N4O2+: 320.1273; found: 320.1278.
4-(2-(3,4-Dimethoxyphenyl)imidazo[1,2-a]pyridin-3-yl)-3-methyl-1H-pyrazol-5-ol (5d)
White solid; yield 94% (329 mg); m.p. 168–169 °C; FT-IR (KBr) υ: 3367, 3097, 2995, 2940, 2836, 1589, 1523, 1464, 1434, 1392, 1257, 1237, 1178, 1142, 1024, 757 cm−1. 1H NMR (300 MHz, DMSO-d6) δ (ppm): 11.00 (bs, 2H, OH, NH, D2O exch.), 7.84 (d, J = 6.6 Hz, 1H, Ar), 7.60 (d, J = 9 Hz, 1H, Ar), 7.45 (s, 1H, Ar), 7.32–7.23 (m, 2H, Ar), 6.93 (d, J =8.4 Hz, 1H, Ar), 6.86 (t, J = 6.6 Hz, 1H, Ar), 3.75 (s, 3H, OMe), 3.68 (s, 3H, OMe), 1.70 (s, 3H, Me). 13C NMR (75.5 MHz, DMSO-d6) δ (ppm): 172.5, 160.3, 148.9, 144.5, 142.4, 140.4, 128.0, 126.1, 124.0, 120.4, 118.5, 115.7, 113.4, 112.6, 112.0, 91.4, 56.6, 55.2, 10.2. LRMS (EI, 70 eV) m/z (%): 350 (M+, 44), 335 (8), 320 (100), 305 (23), 291 (6), 277 (10), 262 (31), 247 (8), 234 (7), 219 (19), 205 (17), 192 (7), 179 (3), 160 (3),145 (2), 130 (3), 115 (3), 105 (4), 93 (3), 78 (19), 67 (2), 57 (5). HRMS (ESI): m/z (M)+ calcd. for C19H18N4O3+: 350.1379; found: 350.1378.
3-Methyl-4-(2-(p-tolyl)imidazo[1,2-a]pyridin-3-yl)-1H-pyrazol-5-ol (5e)
White solid; yield 88% (268 mg); m.p. 294 °C (dec.). FT-IR (KBr) υ: 3405, 3182, 3133, 3028, 2923, 2862, 2738, 1609, 1520, 1476, 1443, 1419, 1387, 1332, 1237, 1100, 823, 747 cm−1. 1H NMR (300 MHz, DMSO-d6) δ (ppm): 10.92 (bs, 2H, OH, NH, D2O exch.), 7.82 (d, J = 7.2 Hz, 1H, Ar), 7.66 (d, J = 7.5 Hz, 2H, Ar), 7.58 (d, J = 9.6 Hz, 1H, Ar), 7.26 (t, J = 7.5 Hz, 1H, Ar), 7.15 (d, J = 7.8 Hz, 2H, Ar), 6.87 (t, J = 6.6 Hz, 1H, Ar), 2.29 (s, 3H, Me), 1.58 (s, 3H, Me). 13C NMR (75.5 MHz, DMSO-d6) δ (ppm): 160.2, 160.1, 144.7, 142.4, 140.2, 136.9, 132.5, 130.4, 128.6, 128.1, 126.1, 124.1, 112.5, 91.4, 22.4, 10.3. LRMS (EI, 70 eV) m/z (%): 304 (M+, 100), 289 (18), 273 (2), 261 (3), 247 (14), 232 (7), 219 (5), 205 (2), 195 (2), 183 (2), 169 (2),154 (2), 144 (4), 130 (3), 119 (5), 103 (2), 91 (5), 78 (14), 65 (2), 51 (3). HRMS (ESI): m/z (M)+ calcd. for C18H16N4O+: 304.1324; found: 304.1319.
4-(2-(4-Hydroxy-3-methoxyphenyl)imidazo[1,2-a]pyridin-3-yl)-3-methyl-1H-pyrazol-5-ol (5f)
White solid; yield 91% (306 mg); m.p. 266–267 °C. FT-IR (KBr) υ: 3351, 3094, 3025, 2925, 2726, 2572, 1600, 1576, 1531, 1487, 1465, 1436, 1395, 1285, 1254, 1217, 1172, 1138, 1112, 1035, 819, 786, 754, 683, 640 cm−1. 1H NMR (300 MHz, DMSO-d6) δ (ppm): 10.95 (bs, 2H, OH, NH, D2O exch.), 9.05 (s, 1H, OH, D2O exch.), 7.81 (d, J = 6.9 Hz, 1H, Ar), 7.57 (d, J = 8.7 Hz, 1H, Ar), 7.42 (s, 1H, Ar), 7.26–7.17 (m, 2H, Ar), 6.85 (t, J = 6.6 Hz, 1H, Ar), 6.73 (d, J = 8.1 Hz, 1H, Ar), 3.70 (s, 3H, OMe), 1.80 (s, 3H, Me). 13C NMR (75.5 MHz, DMSO-d6) δ (ppm): 160.6, 160.2, 147.8, 146.5, 144.5, 142.8, 140.4, 126.7, 123.8, 120.7, 118.8, 117.0, 115.8, 144.7, 111.6, 91.5, 56.7, 11.7. LRMS (EI, 70 eV) m/z (%): 336 (M+, 100), 320 (16), 306 (12), 293 (3), 277 (6), 264 (5), 247 (3), 235 (4), 221 (3), 207 (4), 193 (2), 181 (1), 168 (4),161 (2), 153 (1), 139 (1), 131 (1), 118 (1), 110 (1). HRMS (ESI): m/z (M)+ calcd. for C18H16N4O3+: 336.1222; found: 336.1227.
4-(2-(4-Chlorophenyl)imidazo[1,2-a]pyridin-3-yl)-3-methyl-1H-pyrazol-5-ol (5g)
White solid; yield 92% (298 mg); m.p. 317 °C (dec.). FT-IR (KBr) υ: 3427, 3174, 3130, 3027, 2927, 2860, 2740, 1610, 1542, 1509, 1464, 1443, 1418, 1387, 1329, 1283, 1238, 1098, 974, 831, 743 cm−1. 1H NMR (300 MHz, DMSO-d6) δ (ppm): 11.10 (bs, 2H, OH, NH, D2O exch.), 7.85 (d, J = 6.6 Hz, 1H, Ar), 7.79 (d, J = 8.4 Hz, 2H, Ar), 7.60 (d, J = 9 Hz, 1H, Ar), 7.41 (d, J = 8.4 Hz, 2H, Ar), 7.29 (t, J = 7.5 Hz, 1H, Ar), 6.89 (t, J = 6.9 Hz, 1H, Ar), 1.80 (s, 3H, Me). 13C NMR (75.5 MHz, DMSO-d6) δ (ppm): 160.1, 160.0, 144.9, 141.1, 140.3, 134.3, 132.3, 129.7, 127.5, 126.5, 124.6, 118.3, 113.2, 91.0, 10.6. LRMS (EI, 70 eV) m/z (%): 326 [(M + 2)+, 45], 324 (M+, 100), 309 (18), 295 (3), 281 (3), 267 (14), 254 (4), 243 (2), 231 (9), 218 (3), 205 (5), 186 (1), 174 (1), 162 (3), 144 (2), 128 (2), 116 (1), 103 (2), 89 (1), 78 (10), 65 (1), 52 (1). HRMS (ESI): m/z (M)+ calcd. for C17H13ClN4O+: 324.0778; found: 324.0783.
4-(2-(4-Bromophenyl)imidazo[1,2-a]pyridin-3-yl)-3-methyl-1H-pyrazol-5-ol (5h)
White solid; yield 90% (331 mg); m.p. 300 °C (dec.). FT-IR (KBr) υ: 3430, 3178, 3130, 3028, 2926, 2858, 2738, 1610, 1505, 1540, 1465, 1441, 1418, 1385, 1327, 1238, 1100, 1077, 1007, 826, 744 cm−1. 1H NMR (300 MHz, DMSO-d6) δ (ppm): 11.00 (bs, 2H, OH, NH, D2O exch.), 7.85 (d, J = 6.6 Hz, 1H, Ar), 7.72 (d, J = 8.4 Hz, 2H, Ar), 7.62–7.53 (m, 1H, Ar), 7.28 (t, J = 7.5 Hz, 2H, Ar), 6.89 (t, J = 6.6 Hz, 1H, Ar), 1.80 (s, 3H, Me). 13C NMR (75.5 MHz, DMSO-d6) δ (ppm): 160.5, 160.1, 144.6, 141.1, 134.6, 132.9, 130.7, 130.1, 127.8, 126.5, 124.5, 120.9, 113.3, 91.0, 11.5. LRMS (EI, 70 eV) m/z (%): 370 [(M + 2)+, 100], 368 (M+, 90), 353 (16), 341 (1), 324 (9), 311 (10), 300 (2), 288 (2), 274 (9), 262 (12), 243 (3), 231 (17), 218 (5), 205 (8), 184 (2), 168 (1), 154 (1), 137 (7), 126 (1), 115 (1), 103 (2), 89 (1), 78 (17), 63 (1), 51 (2). HRMS (ESI): m/z (M)+ calcd. for C17H13BrN4O+: 368.0273; found: 368.0279.
4-(2-(4-Fluorophenyl)imidazo[1,2-a]pyridin-3-yl)-3-methyl-1H-pyrazol-5-ol (5i)
White solid; yield 86% (265 mg); m.p. 290 °C (dec.). FT-IR (KBr) υ: 3174, 3127, 3029, 2927, 2862, 2745, 1608, 1519, 1477, 1420, 1388, 1331, 1298, 1233, 1155, 1099, 840, 803, 748 cm−1. 1H NMR (300 MHz, DMSO-d6) δ (ppm): 11.02 (bs, 2H, OH, NH, D2O exch.), 7.86–7.78 (m, 3H, Ar), 7.60 (d, J = 9 Hz, 1H, Ar), 7.27 (t, J = 7.8 Hz, 1H, Ar), 7.19 (t, J = 8.7 Hz, 2H, Ar), 6.88 (t, J = 6.6 Hz, 1H, Ar), 1.78 (s, 3H, Me). 13C NMR (75.5 MHz, DMSO-d6) δ (ppm): 163.5, 160.1, 144.8, 141.5, 140.3, 131.9, 130.1, 127.8, 126.3, 123.9, 116.7, 114.9, 112.7, 91.1, 10.3. LRMS (EI, 70 eV): m/z (%) = 308 (M+, 100), 293 (14), 279 (3), 264 (2), 251 (17), 236 (8), 223 (11), 212 (2), 199 (1), 187 (1), 172 (3), 158 (2), 145 (4), 133 (2), 118 (1), 103 (1), 90 (1), 78 (17), 63 (2), 51 (4). HRMS (ESI): m/z (M)+ calcd. for C17H13FN4O+: 308.1073; found: 308.1075.
3-Methyl-4-(2-(4-nitrophenyl)imidazo[1,2-a]pyridin-3-yl)-1H-pyrazol-5-ol (5j)
Yellow solid; yield 82% (275 mg); m.p. 300 °C (dec.). FT-IR (KBr) υ: 3418, 3110, 3028, 2888, 2783, 2614, 1602, 1513, 1453, 1346, 1280, 1108, 858, 753 cm−1. 1H NMR (300 MHz, DMSO-d6) δ (ppm): 11.20 (bs, 2H, OH, NH, D2O exch.), 8.22 (d, J = 8.7 Hz, 2H, Ar), 8.03 (d, J = 8.4 Hz, 2H, Ar), 7.90 (d, J = 6.6 Hz, 1H, Ar), 7.65 (d, J = 9 Hz, 1H, Ar), 7.34 (t, J = 7.8 Hz, 1H, Ar), 6.94 (t, J = 6.6 Hz, 1H, Ar), 1.83 (s, 3H, Me). 13C NMR (75.5 MHz, DMSO-d6) δ (ppm): 160.8, 160.0, 146.5, 145.1, 142.1, 139.9, 128.9, 128.6, 127.2, 126.7, 125.2, 123.3, 115.3, 90.7, 10.4. LRMS (EI, 70 eV) m/z (%): 335 (M+, 100), 324 (12), 314 (1), 304 (8), 288 (10), 274 (18), 262 (6), 244 (2), 231 (7), 218 (4), 205 (4), 192 (1), 177 (1), 168 (2), 150 (1), 140 (1), 123 (1), 113 (1), 103 (1) 92 (1), 78 (1), 67 (1), 56 (1). HRMS (ESI): m/z (M)+ calcd. for C17H13N5O3+: 335.1018; found: 335.1015.
4-(2-([1,1′-Biphenyl]-4-yl)imidazo[1,2-a]pyridin-3-yl)-3-methyl-1H-pyrazol-5-ol (5k)
White solid; yield 87% (319 mg); m.p. 290 °C (dec.). FT-IR (KBr) υ: 3415, 3030, 2875, 2778, 2607, 1613, 1502, 1448, 1386, 1344, 1281, 1111, 847, 761, 740, 694 cm−1. 1H NMR (300 MHz, DMSO-d6) δ (ppm): 11.15 (bs, 2H, OH, NH, D2O exch.), 7.90 (t, J = 8.1 Hz, 3H, Ar), 7.68–7.58 (m, 5H, Ar), 7.45 (t, J = 6.9 Hz, 2H, Ar), 7.36–7.25 (m, 2H, Ar), 6.89 (t, J = 6.9 Hz, 1H, Ar), 1.83 (s, 3H, Me). 13C NMR (75.5 MHz, DMSO-d6) δ (ppm): 160.4, 160.2, 144.9, 141.9, 140.1, 139.1, 134.5, 130.5, 128.8, 128.6, 126.4, 124.3, 123.9, 118.2, 116.0, 113.1, 112.1, 91.3, 11.7. LRMS (EI, 70 eV) m/z (%): 366 (M+, 100), 351 (15), 337 (2), 320 (8), 309 (14), 294 (7), 281 (8), 262 (5), 243 (3), 231 (10), 218 (4), 205 (7), 194 (2), 183 (4), 165 (2), 154 (3), 141 (1), 130 (3), 115 (1), 103 (1), 91 (1), 78 (15), 67 (1), 52 (1). HRMS (ESI): m/z (M)+ calcd. for C23H18N4O+: 366.1481; found: 366.1489.
4,4′-(1,4-Phenylenebis(imidazo[1,2-a]pyridine-2,3-diyl))bis(3-methyl-1H-pyrazol-5-ol) (5l)
Pale-yellow solid; yield 84% (422 mg); m.p. 324 °C (dec.). FT-IR (KBr) υ: 3388, 3215, 3043, 2926, 1609, 1508, 1439, 1388, 1343, 1239, 1104, 1046, 978, 843, 743 cm−1. 1H NMR (300 MHz, DMSO-d6) δ (ppm): 11.01 (bs, 4H, 2 × OH, 2 × NH, D2O exch.), 7.85 (d, J = 6.6 Hz, 2H, Ar), 7.76 (s, 4H, Ar), 7.60 (d, J = 8.7 Hz, 2H, Ar), 7.28 (t, J = 8.1 Hz, 2H, Ar), 6.89 (t, J = 6.6 Hz, 2H, Ar), 1.79 (s, 6H, 2 × Me). 13C NMR (75.5 MHz, DMSO-d6) δ (ppm): 160.3, 160.1, 144.7, 142.0, 140.4, 134.1, 128.0, 125.8, 123.9, 118.1, 115.9, 113.1, 91.3, 11.6. LRMS (EI, 70 eV) m/z (%): 502 (M+, 15), 369 (32), 354 (10), 313 (11), 299 (10), 285 (10), 260 (10), 236 (23), 222(10), 211 (11), 152 (11), 135 (10), 123 (13), 109 (19), 97 (37), 83 (52), 71 (53), 69 (58), 57 (86), 55 (67), 43 (100), 41 (57). HRMS (ESI): m/z (M)+ calcd. for C28H22N8O2+: 502.1866; found: 502.1861.
References
S. Jiang, J. Gao, L. Han, Res. Chem. Intermed. 42, 1017 (2016)
M. Rimaz, H. Mousavi, L. Nikpey, B. Khalili, Res. Chem. Intermed. 43, 3925 (2017)
M.H. Sayahi, S.J. Saghanezhad, M. Mahdavi, Res. Chem. Intermed. 44, 739 (2018)
S. Golchin, M.H. Mosslemin, A. Yazdani-Elah-Abadi, N. Shams, Res. Chem. Intermed. 43, 1735 (2017)
A. Dömling, Chem. Rev. 106, 17 (2006)
A. Linton, P. Kang, M. Ornelas, S. Kephart, Q. Hu, M. Pairish, Y. Jiang, C. Guo, J. Med. Chem. 54, 7705 (2011)
A.K. Bagdi, S. Santra, K. Monir, A. Hajra, Chem. Commun. 51, 1555 (2015)
N. Perin, R. Nhili, K. Ester, W. Laine, G. Karminski-Zamola, M. Kralj, M.-H. David-Cordonnier, M. Hranjec, Eur. J. Med. Chem. 80, 218 (2014)
S. Ramachandran, M. Panda, K. Mukherjee, N.R. Choudhury, S.J. Tantry, C.K. Kedari, V. Ramachandran, S. Sharma, V.K. Ramya, S. Guptha, V.K. Sambandamurthy, Bioorg. Med. Chem. Lett. 23, 4996 (2013)
H. Takeshita, J. Watanabe, Y. Kimura, K. Kawakami, H. Takahashi, M. Takemura, A. Kitamura, K. Someya, R. Nakajima, Bioorg. Med. Chem. Lett. 20, 3893 (2010)
M.L. Bode, D. Gravestock, S.S. Moleele, C.W. van der Westhuyzen, S.C. Pelly, P.A. Steenkamp, H.C. Hoppe, T. Khan, L.A. Nkabinde, Bioorg. Med. Chem. 19, 4227 (2011)
R.B. Lacerda, C.K. de Lima, L.L. da Silva, N.C. Romeiro, A.L.P. Miranda, E.J. Barreiro, C.A.M. Fraga, Bioorg. Med. Chem. 17, 74 (2009)
A.J. Ndakala, R.K. Gessner, P.W. Gitari, N. October, K.L. White, A. Hudson, F. Fakorede, D.M. Shackleford, M. Kaiser, C. Yeates, S.A. Charman, K.J. Chibale, Med. Chem. 54, 4581 (2011)
B.F. McGuiness, A.W. Cole, G. Dong, M.R. Brescia, Y. Shao, I. Henderson, L.L. Rokosz, T.M. Stauffer, N. Mannava, E.F. Kimble, C. Hicks, N. White, P.G. Wines, E. Quadros, Bioorg. Med. Chem. Lett. 20, 6845 (2010)
C.B. Sangani, H.H. Jardosh, M.P. Patel, R.G. Patel, Med. Chem. Res. 22, 3035 (2013)
M. Hieke, C.B. Rödl, J.M. Wisniewska, E. la Buscató, H. Stark, M. Schubert-Zsilavecz, D. Steinhilber, B. Hofmann, E. Proschak, Bioorg. Med. Chem. Lett. 22, 1969 (2012)
Z. Fanxing, A.S. Jeanine, J.V. Ronald, R.V. John, W. Larry, J.C. Brian, I.L. Allan, M.G. Mark, Bioorg. Med. Chem. Lett. 16, 3015 (2006)
T. Okubo, R. Yoshikawa, S. Chaki, S. Okuyamac, A. Nakazato, Bioorg. Med. Chem. 12, 423 (2004)
A.N. Jain, J. Med. Chem. 47, 947 (2004)
S.M. Hanson, E.V. Morlock, K.A. Satyshur, C. Czajkowski, J. Med. Chem. 51, 7243 (2008)
C.M. Hendriks, P. Nürnberg, C. Bolm, Synthesis 47, 1190 (2015)
Y. Maruyama, K. Anami, M. Terasawa, K. Goto, T. Imayoshi, Y. Kadobe, Y. Mizushima, Arzneim. Forsch. 31, 1111 (1981)
C. Enguehard-Gueiffier, A. Gueiffier, Mini Rev. Med. Chem. 7, 888 (2007)
S. Boggs, V.I. Elitzin, K. Gudmundsson, M.T. Martin, M. Sharp, J. Org. Process Res. Dev. 13, 781 (2009)
M. Ito, T. Sone, M. Fukunaga, J. Bone Miner. Metab. 28, 334 (2010)
S. Mohana Roopan, S.M. Patil, J. Palaniraja, Res. Chem. Intermed. 42, 2749 (2015)
A.J. Stasyuk, M. Banasiewicz, M.K. Cyrański, D.T. Gryko, J. Org. Chem. 77, 5552 (2012)
H. Yan, Y. Wang, C. Pan, H. Zhang, S. Yang, X. Ren, J. Li, G. Huang, Eur. J. Org. Chem. 13, 2754 (2014)
T. Shao, Z. Gong, T. Su, W. Hao, C. Che, Beilstein J. Org. Chem. 13, 817 (2017)
S. Karamthulla, M.N. Khan, L.H. Choudhury, RSC Adv. 5, 19724 (2015)
C. Cheng, L. Ge, X. Lu, J. Huang, H. Huang, J. Chen, W. Cao, X. Wu, Tetrahedron 72, 6866 (2016)
R.-L. Yan, H. Yan, C. Ma, Z.-Y. Ren, X.-A. Gao, G.-S. Huang, Y.-M. Liang, J. Org. Chem. 77, 2024 (2012)
K. Monir, A.K. Bagdi, S. Mishra, A. Majee, A. Hajra, Adv. Synth. Catal. 356, 1105 (2014)
N. Chernyak, V. Gevorgyan, Angew. Chem. Int. Ed. 49, 2743 (2010)
J. Yu, Y. Jin, H. Zhang, X. Yang, H. Fu, Chem. Eur. J. 19, 16804 (2013)
M. Siray, P. Kleinschmit, U.S. Patent 4, 931, 593 (1990)
P.P. Mpungose, N.I. Sehloko, G.E.M. Maguire, H.B. Friedrich, New J. Chem. 41, 13560 (2017)
M. Ezzati, J. Khalafy, A.P. Marjani, R.H. Prager, Aust. J. Chem. 71, 435 (2018)
J. Khalafy, N. Etivand, S. Dilmaghani, M. Ezzati, A. Poursattar Marjani, Tetrahedron Lett. 55, 3781 (2014)
J. Khalafy, M. Mohammadlou, M. Mahmoody, F. Salami, A. Poursattar Marjani, Tetrahedron Lett. 56, 1528 (2015)
A. Poursattar, J. Khalafy, S. Mahmoodi, ARKIVOC 3, 262 (2016)
J. Khalafy, S. Ilkhanizadeh, M. Ranjbar, J. Heterocycl. Chem. 55, 951 (2018)
H.A. Riley, A.R. Gray Organic syntheses, in Collect, vol. 2 (Wiley, New York, 1943), pp. 509–510
J.E. Baldwin, J. Chem. Soc., Chem. Commun. 18, 734 (1976)
Acknowledgements
The authors gratefully acknowledge financial assistance from Urmia University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Etivand, N., Khalafy, J. & Poursattar Marjani, A. Facile, one-pot, four-component synthesis of a new series of imidazo[1,2-a]pyridines in presence of TPAB in EtOH under reflux conditions. Res Chem Intermed 45, 3379–3394 (2019). https://doi.org/10.1007/s11164-019-03797-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-03797-1