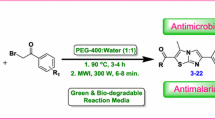
Abstract
A new series containing pyrido[1,2-a]benzimidazole derivatives of β-aryloxyquinoline has been synthesized via base catalyzed microwave-assisted multi-component cyclocondensation reaction. This methodology allowed us to achieve the desired products in good yields in very short time with the use of 10 mol% NaOH as a non-hazardous organic base. The chemical structures of compounds 6a–x were elucidated by 1H NMR, 13C NMR, FT-IR, elemental analysis, and mass spectral data. The titled derivatives were tested against a panel of pathogenic strains of bacteria and fungi for antimicrobial activity and against Mycobacterium tuberculosis H37Rv for their antitubercular activity. The structural activity relationship study revealed that antimicrobial and antitubercular potency of the title compounds depends not only on the bicyclic heteroaromatic pharmacophore appended through ether linked aryl ring but also on the nature of the peripheral substituents and may also upon their spatial relationship and positional changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberculosis is mainly caused by Mycobacterium tuberculosis bacteria (MTB). It is found to be the second leading killer disease from a single infectious agent because around billions of lives were lost during the last two centuries (WHO report, 2010). There is no universally effective vaccine against infection caused by MTB (Russell et al., 2010); therefore, the development of antitubercular drugs becomes thirsty area in the medicinal chemistry research. Moreover, the problems posed by multi-drug-resistant (MDR) microorganisms have reached an alarming level in many countries around the world. Some antimicrobial and antitubercular drugs were failed during the chemotherapy against the infections caused by MDR microorganism (Diekema et al., 2004; WHO report, 2008). These reports encompass the development of new and effective antitubercular and antimicrobial drugs. In pursuit of this goal, we have synthesized pyrido[1,2-a]benzimidazole derivatives bearing β-aryloxyquinoline nucleus with the hope that amalgamation of these two biopotent moiety into a single scaffold may produce novel heterocyclic scaffold with promising biological activity.
Quinoline derivatives are found to possess wide range of useful biological properties including antitubercular (Mital et al., 2006), antibacterial (Kalluraya et al., 2008), antifungal (Rana et al., 2008), antimalarial (Charris et al., 2005; Dave et al., 2009), anti-inflammatory (Bava and Kumar 2009), and anticancer activities (Shi et al., 2008). Moreover, the presence of aryl ring appended via ether linkage at β-position of quinoline moiety is found to be highly active against MTB (H37Rv) and plays a vital role in the development of new antituberculosis drugs (Mungra et al., 2011; Upadhaya et al., 2009).
On the other hand, pyrido[1,2-a]benzimidazole derivatives did not receive much attention until the past decades (Panda et al., 2003), but from last few years they attracted organic as well as medicinal chemist due to their diverse syntheses and various biological activities, such as antitubercular (Pieroni et al., 2011), antimicrobial (Rida et al., 2006; Takemura et al., 2004; Rida et al., 1988), anti-HIV-1 (Rida et al., 2006), anticancer (Rida et al., 2006; El-Hawash et al., 1999), antineoplastic (Badawey et al., 1995a, b; 1999), antiviral (Kotovskaya et al., 2005), and antimalarial (Ndakala et al., 2011).
Previously, pyrido[1,2-a]benzimidazoles have been prepared using different synthetic approaches (Bogdanowicz-Szwed and Czarny, 1993; Prostakov et al., 1983; Russell and Van Nievelt, 1995; Sundberg and Ellis, 1982; Yan et al., 2009; Algul et al., 2009; Dawood et al., 2010, 2011; Wu et al., 2011). However, some shortcomings were observed in these methods such as, longer reaction time, use of hazardous organic base, drastic reaction conditions, and poor yield. To overcome these drawbacks, we have adopted the microwave-assisted organic synthesis (MAOS) approach and NaOH as an eco-friendly base which facilitate to construct the title derivatives with more efficacy in shorter reaction time.
In the light of the above-mentioned reports and in continuation of our efforts toward the synthesis of quinoline-based biologically active heterocycles (Shah et al., 2012; Jardosh and Patel, 2011, 2012; Mungra et al., 2011; Makawana et al., 2012; Kathrotiya et al., 2012 ), an attempt has been through to undertake the microwave-assisted NaOH catalyzed synthesis of pyrido[1,2-a]benzimidazole derivatives bearing β-aryloxyquinoline nucleus and evaluated their antimicrobial and antitubercular activities.
Results and discussion
Chemistry
The required intermediates 2-chloroquinoline-3-carbaldehydes 1a–c were prepared by Vilsmeier-Haack reaction of various substituted acetanilide (Meth-Cohn and Bramha, 1978). The synthetic precursors β-aryloxyquinoline-3-carbaldehydes 3a–l were synthesized by nucleophilic displacement of chloro group at C-2 position of 1a–c with various phenols 2a–d by refluxing in dimethylformamide using anhydrous potassium carbonate as a base (Scheme 1) (Mungra et al., 2011). Substituted pyrido[1,2-a]benzimidazole-2,4-dicarbonitrile derivatives 6a–x have been synthesized efficiently via microwave-assisted one-pot three-component cyclocondensation of β-aryloxyquinoline-3-carbaldehydes 3a–l, malononitrile 4, and 2-cyanomethylbenzimidazole 5a,b in ethanol containing 10 mol% of NaOH at 280 W (Scheme 1).
The reaction was optimized by varying the mole ratio of catalyst (NaOH). A mixture of respective β-aryloxyquinoline-3-carbaldehyde, malononitrile, and 2-cyanomethylbenzimidazole was subjected to microwave irradiation (280 W) by varying mole ratio of NaOH 2.5, 5.0, 7.5, 10.0, and 12.5 mol%. It was observed that when the amount of NaOH was increased to 10.0 mol%, the reaction rate was increased within shorter reaction time (4 min). On the other hand, further increase in the amount of NaOH resulted into the sticky mass which required ice-water work-up and gave poor yield. The formation of products was continuously checked by thin layer chromatography (TLC) at regular time interval to optimize reaction time and it was found that reaction was completed within 4 min. The above results showed that the best results were obtained when the reaction was carried out with 10.0 mol% of NaOH under microwave irradiation at 280 W for 4 min.
The formation of compounds 6a–x may proceed via two steps. (i) Initial formation of an intermediate heterylidenenitrile by Knoevenagel condensation of β-aryloxyquinoline-3-carbaldehydes 3a–l with malononitrile 4 and (ii) intermolecular cyclization, driven through the nucleophilic attack of 2-cyanomethylbenzimidazole 5a,5b in basic reaction condition gives 7 which undergoes air oxidation to furnish pyrido[1,2-a]benzimidazole as the final product (Scheme 2).
The structures of newly synthesized compounds 6a–x were confirmed by 1H NMR, 13C NMR, FT-IR, elemental analysis, and mass spectral data. The IR spectrum of title compounds 6a–x showed absorption bands around 3,500–3,400 and 3,350–3,300 cm−1 correspond to asymmetric and symmetric stretching of primary amino group, bands around 2,250–2,190 cm−1 for –C≡N stretching of cyano group and 1,250–1,180 cm−1 for C–O–C stretching of ether linkage. In 1H NMR spectrum of the compounds 6a–x, multiplets in the range of δ 6.98–8.83 ppm appeared for aromatic protons. Moreover, a singlet around δ 2.32–2.59 and δ 3.76–3.92 ppm stands for methyl and methoxy protons, respectively. A distinctive broad singlet in the range of δ 8.75–8.97 appeared for the primary amine. In the 13C NMR spectral data of the title compounds 6a–x, the signal at around δ 77.60–87.95 ppm is assigned to carbon attached with carbonitrile while signals around δ 115.10–158.60 ppm are attributed to all the aromatic carbons. Also, distinctive signals around δ 20.88–21.85 and δ 55.85–56.16 ppm stand for methyl and methoxy of 6a–x derivatives, respectively. The obtained elemental analysis values are in good agreement with the theoretical data. Furthermore, mass spectral studies of all the title compounds showed molecular ion peak M+· corresponding to their exact mass. All physical, analytical data, as well as spectroscopic characterization data of the synthesized compounds 6a–x are given in “Experimental” section.
Antimicrobial activity
Antimicrobial activity of the title compounds 6a–x was carried out by broth microdilution method (NCCLS, 2002). Mueller–Hinton broth was used as nutrient medium to grow and dilute the compound suspension for the test bacteria and Sabouraud Dextrose broth used for fungal nutrition. The strains used for the activity were procured from [MTCC-Microbial Type Culture Collection] Institute of Microbial Technology, Chandigarh. The compounds 6a–x were screened for their antibacterial activity against Bacillus subtilis (MTCC 441), Clostridium tetani (MTCC 449), Streptococcus pneumoniae (MTCC 1936), Escherichia coli (MTCC 443), Salmonella typhi (MTCC 98), and Vibrio cholerae (MTCC 3906) as well as for antifungal activity against Aspergillus fumigatus (MTCC 3008) and Candida albicans (MTCC 227). DMSO was used as diluents to get desired concentration of compounds to test upon standard bacterial strains. Serial dilutions were prepared in primary and secondary screening. The control tube containing no antibiotic was immediately subcultured (before inoculation) by spreading a loopful evenly over a quarter of plate of medium suitable for the growth of the test organism and put for incubation at 37 °C overnight. The tubes were then incubated overnight. The MIC of the control organism was read to check the accuracy of the compound concentrations. The MIC was defined as the lowest concentration of the antibiotic or test sample allowing no visible growth. All the tubes not showing visible growth (in the same manner as control tube described above) was subcultured and incubated overnight at 37 °C. The amount of growth from the control tube before incubation (which represents the original inoculum) was compared. Subcultures might show similar number of colonies indicating bacteriostatic, a reduced number of colonies indicating a partial or slow bactericidal activity, and no growth if the whole inoculum has been killed. The test must include a second set of the same dilutions inoculated with an organism of known sensitivity. Each synthesized compound was diluted obtaining 2,000 μg/mL concentration as a stock solution. In primary screening, 500, 250, and 200 μg/mL concentrations of the synthesized compounds were taken. The active synthesized compounds found in this primary screening were further tested in a second set of dilution against all microorganisms. The compounds found active in primary screening were similarly diluted to obtain 100, 62.5, 50, and 25 μg/mL concentrations. The highest dilution showing at least 99 % inhibition is taken as MIC. In this study, ampicillin, ciprofloxacin, and norfloxacin were used as standard antibacterial drugs, whereas griseofulvin was used as standard antifungal drug.
The antibacterial screening data (Table 1) revealed that, against Gram-positive bacteria B. subtilis, compounds 6a, 6f, and 6w (MIC = 100 μg/mL) were found to be more potent than ampicillin and equipotent to norfloxacin. Compounds 6g, 6s, and 6v (MIC = 125 μg/mL) were found to have significant activity when compared with ampicillin. Compounds 6e, 6j, 6l, 6m, and 6r (MIC = 200 μg/mL) were found to possess better activity than ampicillin, while compounds 6b, 6c, 6d, 6h, 6i, 6k, 6n, 6o, 6t, 6u, and 6x (MIC = 250 μg/mL) were found equally potent to ampicillin. Against C. tetani, compound 6n (MIC = 62.5 μg/mL) showed fabulous activity when compared with ampicillin and ciprofloxacin, while compounds 6a, 6i, 6s, 6t, and 6x (MIC = 100 μg/mL) showed significant activity compare to ampicillin and equivalent to ciprofloxacin. Compounds 6j and 6r (MIC = 125 μg/mL) as well as compounds 6d, 6k, 6l, 6o, 6v, and 6w (MIC = 200 μg/mL) were found to be more potent than ampicillin, while compounds 6b, 6c, 6e, 6h, 6m, 6p, 6q, and 6u (MIC = 250 μg/mL) were found to be equipotent to ampicillin. Against S. pneumoniae, Compound 6a (MIC = 62.5 μg/mL) was found to have more efficacy, while compounds 6g, 6p, 6q, 6v, and 6x were found to have identical activity when compare with ampicillin.
Bold numbers indicates more or equally potent compounds compare to standard drugs
Against Gram-negative bacteria E coli, compounds 6e, 6n, 6p, and 6u (MIC = 62.5 μg/mL) showed remarkable activity, while compounds 6b, 6f, 6m, 6o, and 6x (MIC = 100 μg/mL) were found to be equally potent when compared with ampicillin. Against S. typhi compounds 6b, 6e, 6u, and 6v (MIC = 100 μg/mL) showed equal potency with ampicillin. Against V. cholerae compounds 6b, 6f, and 6t (MIC = 100 μg/mL) were found to be equipotent to ampicillin.
Exploration of antifungal screening data revealed that, against fungi C. albicans, compound 6o (MIC = 200 μg/mL) was found to exhibit fabulous activity; compounds 6c, 6e, 6h, and 6p (MIC = 250 μg/mL) showed remarkable activity, while compounds 6d, 6i, 6j, 6m, 6n, 6q, 6r, 6w, and 6x (MIC = 500 μg/mL) were found equally potent when compared with griseofulvin. None of the synthesized compounds was found to be active against A. fumigatus.
Antituberculosis activity
The encouraging results from the antimicrobial studies prompted us to go for the preliminary screening of the title compounds 6a–x for their in vitro antituberculosis activity against M. tuberculosis H37Rv by using Lowenstein–Jensen medium (conventional method) as described by Rattan (2000). A primary screen was conducted at primary dilution 6.25 μg/ml against M. tuberculosis H37Rv, where 6.25 μg/ml of each test compound were added to liquid Lowenstein–Jensen Medium and then media were sterilized by inspissations method. A culture of M. tuberculosis H37Rv growing on Lowenstein–Jensen Medium was harvested in 0.85 % saline in bijou bottles. DMSO was used as vehicle to get desired concentration. These tubes were then incubated at 37 °C for 24 h followed by streaking of M. tuberculosis H37Rv (5 × 104 bacilli per tube). These tubes were then incubated at 37 °C. Growth of bacilli was seen after 12, 22, and finally 28 days of incubation. Tubes having the compounds were compared with control tubes where medium alone was incubated with M. tuberculosis H37Rv. The concentration at which complete inhibition of colonies occurred was taken as active concentration of test compound. The standard strain M. tuberculosis H37Rv was tested with known drug isoniazid and rifampicin. The screening results are summarized as % inhibition relative to standard drug isoniazid and rifampicin. Compounds effecting <90 % inhibition in the primary screen were not evaluated further. Compounds demonstrating at least 90 % inhibition in the primary screen were re-tested at lower concentration (MIC) in a Lowenstein–Jensen medium and evaluated their MIC values.
The antimycobacterial activity results of the compounds 6a–x are shown in Table 2. Of the compounds screened for antituberculosis activity, compounds 6p (R1 = H, R2 = F, R3 = CH3), 6t (R1 = CH3, R2 = F, R3 = CH3), 6x (R1 = OCH3, R2 = F, R3 = CH3), and 6h (R1 = CH3, R2 = F, R3 = H) found to possess better activity against M. tuberculosis H37Rv and exhibited highest % inhibition. The MIC values of these compounds found to be 6.25, 12.5, 12.5, and 25 μg/mL respectively. In this set of heterocyclic compounds, compound 6p (R1 = H, R2 = F, R3 = CH3) is appeared as the promising antimicrobial member with significant antitubercular activity.
Structure–Activity Relationship (SAR) study
The exploration of the structure–activity relationship of antimicrobial screening revealed that the unsubstituted compound 6a shows significant activity against all the Gram-positive bacteria. Against B. subtilis, compounds having CH3 and OCH3 groups at para position of ether linked aryl ring gives better results compare to other members of the series, e.g., compounds 6f, 6w, 6g, 6s, and 6v. Compounds with unsubstituted quinoline ring and CH3 at benzimidazole ring effectively inhibits the growth of C. tetani and showed better activity to that of ampicillin and ciprofloxacin, e.g., compounds 6n, 6r, 6s, 6t, 6v, 6w, and 6x. Compounds having H/CH3 at quinoline ring and CH3 at benzimidazole ring enhance the antibacterial effectiveness against S. pneumoniae. Against E. coli, compounds with CH3 group at quinoline and benzimidazole ring found to have more potential than the other compounds, e.g., 6e, 6n, 6p, and 6u. Compounds containing CH3 group at quinoline, aryloxy, and benzimidazole rings found to have more potential to inhibit the growth of S. typhi, e.g., compounds 6b, 6e, 6u, and 6v. In case of V. cholerae, compounds having CH3 group at quinoline and aryloxy rings found to be equipotent to ampicillin, e.g., compounds 6b, 6f, and 6t. Compounds with unsubstituted quinoline ring and H/F at aryloxy ring showed more potency compare to griseofulvin, e.g., compounds 6c, 6e, 6h, 6o, and 6p.
On the other hand, the evaluation of the structure–activity relationship of antitubercular activity exposed that compounds having F group at ether linked aryl ring appended to quinoline and CH3 group at benzimidazole unit found to exhibit more potency against M. tuberculosis H37Rv, e.g., compounds 6h, 6p, 6t, and 6x except compound.
The above-mentioned results shows that electronic influence of both the OCH3 (electron-donating) group and F (electron-withdrawing) group affects the antimicrobial activity in some extent, while only F group influences the antitubercular activity of the tested compounds. On the other hand, effect of lipophilic CH3 group becomes more dominant compare to hydrophilic OCH3 group to enhance the antimicrobial and antitubercular effectiveness of the tested compounds.
Experimental
All reactions were performed with commercially available reagents and they were used without further purification. Organic solvents were purified by standard methods (Furniss, 2004) and stored over molecular sieves. The microwave-assisted reactions are conducted in a “RAGA’s Modified Electromagnetic Microwave System” whereby microwaves are generated by magnetron at a frequency of 2,450 MHz having an adjustable output power levels, i.e., 10 levels from 140 to 700 W and with an individual sensor for temperature control (fiber optic is used as a individual sensor for temperature control) with attachment of reflux condenser with constant stirring (thus avoiding the risk of high-pressure development). All melting points were taken in open capillaries and are uncorrected. Thin-layer chromatography (TLC, on aluminum plates coated with silica gel 60 F254, 0.25 mm thickness, Merck) was used for monitoring the progress of all reactions, purity, and homogeneity of the synthesized compounds. Elemental analysis (% C, H, N) was carried out by Perkin-Elmer 2400 series-II elemental analyzer and all compounds are within ±0.4 % of theory specified. The FT-IR spectra were recorded using potassium bromide disc on a Shimadzu FT-IR 8401 spectrophotometer and only the characteristic peaks are reported in cm−1. 1H NMR and 13C NMR spectra were recorded in DMSO-d 6 on a Bruker Avance 400F (MHz) spectrometer using TMS as an internal standard at 400 and 100 MHz, respectively. Chemical shifts are reported in parts per million (ppm). Mass spectra were scanned on a Shimadzu LCMS 2010 spectrometer.
General procedure for the synthesis of pyrido-[1,2-a]benzimidazole derivatives 6a–x
In a 50-mL round-bottomed flask, β-aryloxyquinoline-3-carbaldehyde 3a–l (3 mmol), malononitrile 4 (3 mmol), 2-cyanomethyl-benzimidazole 5a,b (3 mmol), and NaOH (10 mol%) in ethanol (15 mL) were thoroughly mixed and subjected to microwave irradiation at 280 W (40 % of output power) for 4 min. After the completion of reaction (evidenced by TLC—Hexane::Ethyl acetate::4:6), the mixture was allowed to stir at room temperature for 10–15 min, the solid mass separated was filtered, washed well with ethanol (10 mL), dried and purified by leaching in equal volume ratio of chloroform and methanol (40 mL) to obtain pure solid sample. Analytical, physical, and spectroscopic characterization data of synthesized compounds 6a–x are mentioned below.
1-Amino-3-(2-phenoxyquinolin-3-yl)pyrido[1,2-a]benzimidazole-2,4-dicarbonitrile (6a)
Yield 87 %; m.p. 240–241 °C; IR (KBr, cm−1): 3,455 and 3,315 (asym. and sym. str. of –NH2), 2,215 (–C≡N str.), 1,215 (C–O–C ether str.); 1H NMR (400 MHz, DMSO-d 6 ): δ 7.00 (d, 2H, J = 8.4 Hz, H-20,24), 7.12 (m, 3H, H-21,22,23), 7.41 (t, 1H, J 1 = 7.6, J 2 = 8.0, H-9), 7.52 (m, 2H, H-8,14), 7.68 (d, 1H, J = 8.0 Hz, H-13), 7.75 (t, 1H, J 1 = 7.2, J 2 = 8.0, H-15), 7.87 (d, 1H, J = 8.0 Hz, H-10), 8.08 (d, 1H, J = 8.0 Hz, H-7), 8.59 (d, 1H, J = 8.4 Hz, H-16), 8.67 (s, 1H, H-12), 8.80 (br s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6) δ: 77.68 (C–CN), 87.79 (C–CN), 115.15, 115.91, 119.11, 120.03, 123.37, 124.09, 125.19, 127.02, 127.22, 127.65, 128.54, 132.72, 134.02, 135.60, 141.13, 144.88, 145.61, 146.76, 147.01, 148.71, 152.19, 154.48, 156.91, 158.58, (Ar–C); Anal. Calcd. for C28H16N6O (452.47 g/mol): C 74.33, H 3.56, N 18.57 % Found: C 74.15, H 3.49, N 18.53 %; MS (m/z): 452.2 (M+).
1-Amino-3-[2-(4-methylphenoxy)quinolin-3-yl]pyrido[1,2-a]benzimidazole-2,4-dicarbonitrile (6b)
Yield 82 %; m.p. 233–235 °C; IR (KBr, cm−1): 3,430 and 3,330 (asym. and sym. str. of –NH2), 2,205 (–C≡N str.), 1,195 (C–O–C ether str.); 1H NMR (400 MHz, DMSO-d 6 ): δ 2.34, (s, 3H, CH3, C-22), 7.18 (d, 2H, J = 8.4 Hz, H-23,21), 7.26 (d, 2H, J = 8.0 Hz, H-20,24), 7.44 (t, 1H, J 1 = 7.6, J 2 = 8.0, H-9), 7.58 (m, 2H, H-8,14), 7.70 (d, 1H, J = 8.0 Hz, H-13), 7.77 (t, 1H, J 1 = 7.2, J 2 = 8.0, H-15), 7.90 (d, 1H, J = 8.0 Hz, H-10), 8.11 (d, 1H, J = 8.0 Hz, H-7), 8.63 (d, 1H, J = 8.4 Hz, H-16), 8.72 (s, 1H, H-12), 8.85 (br s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6 ) δ: 20.92 (CH3), 78.10 (C–CN), 87.84 (C–CN), 115.49, 115.98, 116.39, 119.53, 120.18, 122.18, 122.68, 125.20, 126.19, 127.10, 127.40, 128.85, 128.96, 130.48, 132.06, 134.87, 141.81, 145.03, 146.56, 147.40, 148.79, 151.15, 152.49, 158.24 (Ar–C); Anal. Calcd. for C29H18N6O (466.49 g/mol): C 74.67, H 3.89, N 18.02 % Found: C 74.42, H 4.04, N 17.95 %; MS (m/z): 466.1 (M+).
1-Amino-3-[2-(4-methoxyphenoxy)quinolin-3-yl]pyrido[1,2-a]benzimidazole-2,4-dicarbonitrile (6c)
Yield 79 %; m.p. 277–279 °C; IR (KBr, cm−1): 3,400 and 3,320 (asym. and sym. str. of –NH2), 2,200 (–C ≡ N str.), 1,200 (C–O–C ether str.); 1H NMR (400 MHz, DMSO-d 6 ): δ 3.77 (s, 3H, OCH3, C-22), 7.09 (d, 2H, J = 8.4 Hz, H-23,21), 7.20 (d, 2H, J = 8.0 Hz, H-20,24), 7.41 (t, 1H, J 1 = 7.6, J 2 = 8.0, H-9), 7.54 (m, 2H, H-8,14), 7.69 (d, 1H, J = 8.0 Hz, H-13), 7.75 (t, 1H, J 1 = 7.2, J 2 = 8.0, H-15), 7.88 (d, 1H, J = 8.0 Hz, H-10), 8.09 (d, 1H, J = 8.0 Hz, H-7), 8.57 (d, 1H, J = 8.4 Hz, H-16), 8.62 (s, 1H, H-12), 8.77 (br s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6 ) δ: 55.87 (OCH3), 78.03 (C–CN), 87.77 (C–CN), 115.04, 115.94, 116.55, 119.42, 120.21, 122.20, 122.71, 125.22, 126.21, 127.11, 127.25, 129.05, 129.01, 130.52, 132.11, 134.88, 141.92, 144.92, 146.55, 147.43, 148.80, 151.15, 152.51, 158.00 (Ar–C); Anal. Calcd. for C29H18N6O2 (482.49 g/mol): C 72.19, H 3.76, N 17.42 % Found: C 72.32, H 4.00, N 17.55 %; MS (m/z): 482.7 (M+).
1-Amino-3-[2-(4-fluorophenoxy)quinolin-3-yl]pyrido[1,2-a]benzimidazole-2,4-dicarbonitrile (6d)
Yield 75 %; m.p. 254–255 °C; IR (KBr, cm−1): 3,425 and 3,340 (asym. and sym. str. of –NH2), 2,210 (–C≡N str.), 1,225 (C–O–C ether str.); 1H NMR (400 MHz, DMSO-d 6 ): δ 7.22 (d, 2H, J = 8.4 Hz, H-23,21), 7.30 (d, 2H, J = 8.4 Hz, H-20,24), 7.49 (t, 1H, J 1 = 7.6, J 2 = 8.0, H-9), 7.61 (m, 2H, H-8,14), 7.74 (d, 1H, J = 8.0 Hz, H-13), 7.79 (t, 1H, J 1 = 7.2, J 2 = 8.0, H-15), 7.93 (d, 1H, J = 8.0 Hz, H-10), 8.15 (d, 1H, J = 8.0 Hz, H-7), 8.66 (d, 1H, J = 8.4 Hz, H-16), 8.75 (s, 1H, H-12), 8.88 (br s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6 ) δ: 77.95 (C–CN), 87.93 (C–CN), 115.28, 116.03, 116.41, 119.62, 120.17, 122.20, 122.94, 125.30, 126.22, 127.13, 127.44, 128.77, 129.05, 130.49, 132.06, 134.84, 141.76, 145.01, 146.60, 147.45, 148.80, 151.37, 152.50, 157.95 (Ar–C); Anal. Calcd. for C28H15FN6O (470.46 g/mol): C 71.48, H 3.21, N 17.86 % Found: C 71.44, H 3.37, N 17.94 %; MS (m/z): 470.7 (M+).
1-Amino-3-[2-(4-phenoxy)-6-methylquinolin-3-yl]pyrido[1,2-a]benzimidazole-2,4-dicarbonitrile (6e)
Yield 89 % m.p. 261–263 °C; IR (KBr, cm−1): 3,450 and 3,335 (asym. and sym. str. of –NH2), 2,205 (–C≡N str.), 1,205 (C–O–C ether str.); 1H NMR (400 MHz, DMSO-d 6 ): δ 2.37 (s, 3H, CH3, C-14), 7.15 (d, 2H, J = 8.4 Hz, H-20,24), 7.26 (m, 3H, H-21,22,23), 7.56 (t, 1H, J 1 = 7.6, J 2 = 7.6, H-9), 7.72 (m, 3H, H-13,8,15), 7.89 (d, 1H, J = 8.0 Hz, H-16), 7.98 (d, 1H, J = 8.0 Hz, H-10), 8.64 (s, 1H, H-12), 8.79 (d, 1H, J = 8.0, H-7), 8.97 (br s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6 ) δ: 21.33 (CH3), 78.25 (C–CN), 87.84 (C–CN), 115.60, 115.83, 116.32, 119.21, 119.94, 122.53, 123.40, 125.14, 126.98, 127.89, 129.12, 134.04, 135.58, 141.18, 145.02, 145.18, 146.80, 147.60, 148.89, 152.60, 154.78, 157.03, 157.84, 158.08 (Ar–C); Anal. Calcd. for C29H18N6O2 (466.49 g/mol): C 74.67, H 3.89, N 18.02 % Found: C 74.59, H 4.01, N 17.89 %; MS (m/z): 466.4 (M+).
1-Amino-3-[2-(4-methylphenoxy)-6-methylquinolin-3-yl]pyrido[1,2-a]benzimidazole-2,4-dicarbonitrile (6f)
Yield 83 %; m.p. 243–245 °C; IR (KBr, cm−1): 3,470 and 3,315 (asym. and sym. str. of –NH2), 2,195 (–C≡N str.), 1,220 (C–O–C ether str.); 1H NMR (400 MHz, DMSO-d 6 ): δ 2.32, (s, 3H, CH3, C-22), 2.53, (s, 3H, CH3, C-14), 7.02 (d, 2H, J = 8.4 Hz, H-23,21), 7.24 (d, 2H, J = 8.4 Hz, H-20,24), 7.48 (t, 1H, J 1 = 7.6, J 2 = 7.6, H-9), 7.61 (m, 3H, H-13,8,15), 7.86 (d, 1H, J = 8.0 Hz, H-16), 7.93 (d, 1H, J = 8.0 Hz, H-10), 8.61 (s, 1H, H-12), 8.72 (d, 1H, J = 8.0, H-7), 8.87 (br s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6 ) δ: 20.93 (CH3), 21.30 (CH3), 78.20 (C–CN), 87.93 (C–CN), 115.11, 115.66, 116.31, 119.99, 120.06, 122.73, 123.41, 125.14, 127.20, 127.82, 129.11, 133.84, 135.61, 141.38, 144.87, 145.17, 146.71, 147.60, 149.00, 152.61, 154.75, 157.01, 158.00, 158.60 (Ar–C); Anal. Calcd. for C30H20N6O (480.52 g/mol): C 74.99, H 4.20, N 17.49 % Found: C 75.09, H 4.12, N 17.31 %; MS (m/z): 480.3 (M+).
1-Amino-3-[2-(4-methoxyphenoxy)-6-methylquinolin-3-yl]pyrido[1,2-a]benzimidazole-2,4-dicarbonitrile (6g)
Yield 81 %; m.p. 273–275 °C; IR (KBr, cm−1): 3,475 and 3,325 (asym. and sym. str. of –NH2), 2,225 (–C≡N str.), 1,240 (C–O–C ether str.); 1H NMR (400 MHz, DMSO-d 6 ): δ 2.52 (s, 3H, CH3, C-14), 3.78 (s, 3H, OCH3, C-22), 7.00 (d, 2H, J = 8.4 Hz, H-23,21), 7.21 (d, 2H, J = 8.4 Hz, H-20,24), 7.45 (t, 1H, J 1 = 7.6, J 2 = 7.6, H-9), 7.62 (m, 3H, H-13,8,15), 7.87 (d, 1H, J = 8.0 Hz, H-16), 7.90 (d, 1H, J = 8.0 Hz, H-10), 8.58 (s, 1H, H-12), 8.64 (d, 1H, J = 8.0, H-7), 8.82 (br s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6 ) δ: 21.33 (CH3), 55.89 (OCH3), 78.15 (C–CN), 87.89 (C–CN), 115.08, 115.65, 116.01, 119.58, 120.04, 122.72, 123.32, 125.13, 127.18, 127.75, 129.05, 133.99, 135.60, 141.08, 144.94, 145.09, 146.79, 147.54, 148.99, 152.58, 154.72, 156.97, 157.95, 158.48 (Ar–C); Anal. Calcd. for C30H20N6O2 (496.52 g/mol): C 72.57, H 4.06, N 16.93 % Found: C 72.59, H 4.12, N 17.01 %; MS (m/z): 496.6 (M+).
1-Amino-3-[2-(4-fluorophenoxy)-6-methylquinolin-3-yl]pyrido[1,2-a]benzimidazole-2,4-dicarbonitrile (6h)
Yield 78 %; m.p. 265–267 °C; IR (KBr, cm−1): 3,450 and 3,320 (asym. and sym. str. of –NH2), 2,210 (–C≡N str.), 1,250 (C–O–C ether str.); 1H NMR (400 MHz, DMSO-d 6 ): δ 2.38 (s, 3H, CH3, C-22), 7.02 (d, 2H, J = 8.4 Hz, H-23,21), 7.27 (d, 2H, J = 8.4 Hz, H-20,24), 7.50 (t, 1H, J 1 = 7.6, J 2 = 7.6, H-9), 7.65 (m, 3H, H-13,8,15), 7.88 (d, 1H, J = 8.0 Hz, H-16), 7.91 (d, 1H, J = 8.0 Hz, H-10), 8.61 (s, 1H, H-12), 8.72 (d, 1H, J = 8.0, H-7), 8.90 (br s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6 ) δ: 21.34 (CH3), 78.06 (C–CN), 87.90 (C–CN), 115.24, 115.73, 116.06, 119.60, 120.01, 122.62, 123.30, 125.16, 127.19, 127.82, 128.95, 134.01, 135.59, 141.07, 144.88, 145.14, 146.80, 147.55, 148.92, 152.44, 154.80, 157.02, 157.91, 158.38 (Ar–C); Anal. Calcd. for C29H17FN6O (484.48 g/mol): C 71.89, H 3.54, N 17.35 % Found: C 72.14, H 3.70, N 17.19 %; MS (m/z): 484.2 (M+).
1-Amino-3-[2-(4-phenoxy)-6-methoxyquinolin-3-yl]pyrido[1,2-a]benzimidazole-2,4-dicarbonitrile (6i)
Yield 85 %; m.p. 237–238 °C; IR (KBr, cm−1): 3,420 and 3,345 (asym. and sym. str. of –NH2), 2,190 (–C≡N str.), 1,190 (C–O–C ether str.); 1H NMR (400 MHz, DMSO-d 6 ): δ 3.80 (s, 3H, OCH3, C-14), 7.20 (d, 2H, J = 8.4 Hz, H-20,24), 7.32 (m, 3H, H-21,22,23), 7.61 (t, 1H, J 1 = 7.6, J 2 = 7.6, H-9), 7.77 (m, 3H, H-13,8,15), 7.86 (d, 1H, J = 8.0 Hz, H-16), 7.98 (d, 1H, J = 8.0 Hz, H-10), 8.62 (s, 1H, H-12), 8.81 (d, 1H, J = 8.0, H-7), 8.92 (br s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6 ) δ: 56.03 (OCH3), 78.08 (C–CN), 87.75 (C–CN), 115.31, 115.71, 116.05, 119.42, 120.10, 122.70, 123.29, 125.32, 127.23, 127.74, 128.91, 133.98, 135.60, 141.33, 144.90, 145.13, 146.69, 147.63, 149.03, 152.43, 154.71, 156.93, 157.94, 158.25 (Ar–C); Anal. Calcd. for C29H18N6O2 (482.49 g/mol): C 72.19, H 3.76, N 17.42 % Found: C 72.23, H 3.65, N 17.58 %; MS (m/z): 482.6 (M+).
1-Amino-3-[2-(4-methylphenoxy)-6-methoxyquinolin-3-yl]pyrido[1,2-a]benzimidazole-2,4-dicarbonitrile (6j)
Yield 80 %; m.p. 269–270 °C; IR (KBr, cm−1): 3,400 and 3,310 (asym. and sym. str. of –NH2), 2,205 (–C≡N str.), 1,225 (C–O–C ether str.); 1H NMR (400 MHz, DMSO-d 6 ): δ 2.34 (s, 3H, CH3, C-22), 3.92 (s, 3H, OCH3, C-14), 7.14 (d, 2H, J = 8.4 Hz, H-23,21), 7.27 (d, 2H, J = 8.4 Hz, H-20,24), 7.51 (t, 1H, J 1 = 7.6, J 2 = 7.6, H-9), 7.67 (m, 3H, H-13,8,15), 7.85 (d, 1H, J = 8.0 Hz, H-16), 7.94 (d, 1H, J = 8.0 Hz, H-10), 8.57 (s, 1H, H-12), 8.62 (d, 1H, J = 8.0, H-7), 8.78 (br s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6 ) δ: 20.88 (CH3), 56.12, (OCH3), 77.65 (C–CN), 87.89 (C–CN), 115.20, 115.60, 115.99, 119.48, 119.95, 122.70, 123.30, 125.10, 126.98, 127.80, 129.02, 133.88, 135.61, 140.99, 144.95, 145.07, 146.80, 147.60, 149.02, 152.60, 154.69, 157.01, 157.90, 157.90 (Ar–C); Anal. Calcd. for C30H20N6O2 (496.52 g/mol): C 72.57, H 4.06, N 16.93 % Found: C 72.39, H 3.92, N 17.11 %; MS (m/z): 496.2 (M+).
1-Amino-3-[2-(4-methoxyphenoxy)-6-methoxyquinolin-3-yl]pyrido[1,2-a]benzimidazole-2,4-dicarbonitrile (6k)
Yield 77 %; m.p. 270–272 °C; IR (KBr, cm−1): 3,440 and 3,350 (asym. and sym. str. of –NH2), 2,200 (–C≡N str.), 1,200 (C–O–C ether str.); 1H NMR (400 MHz, DMSO-d 6 ): δ 3.78 (s, 3H, OCH3, C-22), 3.85 (s, 3H, OCH3, C-14), 6.98 (d, 2H, J = 8.4 Hz, H-23,21), 7.14 (d, 2H, J = 8.4 Hz, H-20,24), 7.42 (t, 1H, J 1 = 7.6, J 2 = 7.6, H-9), 7.58 (m, 3H, H-13,8,15), 7.86 (d, 1H, J = 8.0 Hz, H-16), 7.96 (d, 1H, J = 8.0 Hz, H-10), 8.59 (s, 1H, H-12), 8.65 (d, 1H, J = 8.0, H-7), 8.84 (br s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6 ) δ: 55.85 (OCH3), 55.95 (OCH3), 78.08 (C–CN), 87.88 (C–CN), 115.40, 115.70, 116.13, 119.32, 120.14, 122.80, 123.20, 125.10, 127.19, 127.80, 129.17, 133.89, 135.61, 141.03, 145.02, 145.21, 146.89, 147.55, 149.03, 152.61, 154.74, 157.02, 157.90, 157.99 (Ar–C); Anal. Calcd. for C30H20N6O3 (512.52 g/mol): C 70.30, H 3.93, N 16.40 % Found: C 70.45, H 4.17, N 16.52 %; MS (m/z): 512.5 (M+).
1-Amino-3-[2-(4-fluorophenoxy)-6-methoxyquinolin-3-yl]pyrido[1,2-a]benzimidazole-2,4-dicarbonitrile (6l)
Yield 76 %; m.p. 260–261 °C; IR (KBr, cm−1): 3,435 and 3,300 (asym. and sym. str. of –NH2), 2,180 (–C≡N str.), 1,220 (C–O–C ether str.); 1H NMR (400 MHz, DMSO-d 6 ): δ 3.82 (s, 3H, OCH3, C-14), 7.12 (d, 2H, J = 8.4 Hz, H-23,21), 7.25 (d, 2H, J = 8.4 Hz, H-20,24), 7.53 (t, 1H, J 1 = 7.6, J 2 = 7.6, H-9), 7.68 (m, 3H, H-13,8,15), 7.87 (d, 1H, J = 8.0 Hz, H-16), 7.95 (d, 1H, J = 8.0 Hz, H-10), 8.57 (s, 1H, H-12), 8.64 (d, 1H, J = 8.0, H-7), 8.80 (br s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6 ) δ: 55.00 (OCH3), 77.60 (C–CN), 87.80 (C–CN), 115.55, 115.78, 116.19, 119.56, 120.05, 122.74, 123.40, 125.17, 127.27, 127.64, 129.11, 133.88, 135.77, 141.12, 144.89, 145.19, 146.81, 147.60, 149.01, 152.61, 154.73, 156.95, 157.99, 158.07 (Ar–C); Anal. Calcd. for C29H17FN6O2 (500.48 g/mol): C 69.59, H 3.42, N 16.79 % Found: C 69.67, H 3.28, N 17.03 %; MS (m/z): 500.1 (M+·).
1-Amino-3-[2-(4-phenoxy)quinolin-3-yl]-8-methylpyrido[1,2-a]benzimidazole-2,4-dicarbonitrile (6m)
Yield 88 %; m.p. 233–235 °C; IR (KBr, cm−1): 3,420 and 3,340 (asym. and sym. str. of –NH2), 2,220 (–C≡N str.), 1,230 (C–O–C ether str.); 1H NMR (400 MHz, DMSO-d 6 ): δ 1H NMR (400 MHz, DMSO-d 6 ): δ 2.57 (s, 3H, CH3, C-8), 7.18 (d, 2H, J = 8.4 Hz, H-20,24), 7.25 (m, 3H, H-21,22,23), 7.51 (d, 1H, J = 8.0 Hz, H-9), 7.64 (m, 2H, H-14,13), 7.75 (t, 1H, J 1 = 7.2, J 2 = 8.0, H-15), 7.82 (d, 1H, J = 8.0 Hz, H-10), 7.94 (s, 1H, H-7), 8.51 (d, 1H, J = 8.4 Hz, H-16), 8.62 (s, 1H, H-12), 8.75 (br s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6 ) δ: 21.78 (CH3), 78.21 (C–CN), 87.94 (C–CN), 115.22, 115.83, 116.38, 119.54, 120.15, 122.09, 122.70, 125.22, 126.20, 127.11, 127.52, 128.90, 129.01, 130.52, 132.11, 134.90, 141.85, 145.13, 146.60, 147.42, 148.80, 151.14, 152.50, 158.50 (Ar–C); Anal. Calcd. for C29H18N6O (466.49 g/mol): C 74.67, H 3.89, N 18.02 % Found: C 74.45, H 4.06, N 17.84 %; MS (m/z): 466.5 (M+·).
1-Amino-3-[2-(4-methylphenoxy)quinolin-3-yl]-8-methylpyrido[1,2-a]benzimidazole-2,4-dicarbonitrile (6n)
Yield 84 %; m.p. 257–259 °C; IR (KBr, cm−1): 3,455 and 3,320 (asym. and sym. str. of –NH2), 2,215 (–C≡N str.), 1,180 (C–O–C ether str.); 1H NMR (400 MHz, DMSO-d 6 ): δ 1H NMR (400 MHz, DMSO-d 6 ): δ 2.34 (s, 3H, CH3, C-22), 2.58 (s, 3H, CH3, C-8), 7.00 (d, 2H, J = 8.4 Hz, H-23,21), 7.19 (d, 1H, J = 8.8 Hz, H-20), 7.26 (d, 1H, J = 8.8 Hz, H-24), 7.41 (d, 1H, J = 8.0 Hz, H-9), 7.57 (m, 2H, H-14,13), 7.74 (t, 1H, J 1 = 7.2, J 2 = 8.0, H-15), 7.82 (d, 1H, J = 8.0 Hz, H-10), 7.91 (s, 1H, H-7), 8.53 (d, 1H, J = 8.4 Hz, H-16), 8.68 (s, 1H, H-12), 8.84 (br s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6 ) δ: 20.92 (CH3), 21.81 (CH3), 77.84 (C–CN), 87.80 (C–CN), 115.00, 115.88, 116.40, 119.43, 120.34, 122.30, 122.70, 125.19, 125.99, 127.11, 127.44, 128.80, 129.01, 130.40, 132.07, 134.90, 141.80, 144.98, 146.60, 147.32, 148.80, 151.16, 152.51, 157.92 (Ar–C); Anal. Calcd. for C30H20N6O (480.52 g/mol): C 74.99, H 4.20, N 17.49 % Found: C 75.12, H 3.94, N 17.29 %; MS (m/z): 480.8 (M+·).
1-Amino-3-[2-(4-methoxyphenoxy)quinolin-3-yl]-8-methylpyrido[1,2-a]benzimidazole-2,4-dicarbonitrile (6o)
Yield 81 %; m.p. 269–271 °C; IR (KBr, cm−1): 3,400 and 3,330 (asym. and sym. str. of –NH2), 2,200 (–C≡N str.), 1,245 (C–O–C ether str.); 1H NMR (400 MHz, DMSO-d 6 ): δ 1H NMR (400 MHz, DMSO-d 6 ): δ 2.56 (s, 3H, CH3, C-8), 3.76 (s, 3H, OCH3, C-22), 7.15 (d, 2H, J = 8.4 Hz, H-23,21), 7.23 (d, 1H, J = 8.8 Hz, H-20), 7.33 (d, 1H, J = 8.8 Hz, H-24), 7.45 (d, 1H, J = 8.0 Hz, H-9), 7.56 (m, 2H, H-14,13), 7.75 (t, 1H, J 1 = 7.2, J 2 = 8.0, H-15), 7.84 (d, 1H, J = 8.0 Hz, H-10), 7.95 (s, 1H, H-7), 8.61 (d, 1H, J = 8.4 Hz, H-16), 8.75 (s, 1H, H-12), 8.94 (br s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6 ) δ: 21.75 (CH3), 55.90, (OCH3), 78.12 (C–CN), 87.77 (C–CN), 115.43, 115.94, 116.42, 119.50, 120.23, 122.17, 122.70, 125.21, 126.16, 126.91, 127.42, 128.90, 129.02, 130.59, 132.03, 134.88, 141.84, 145.00, 146.60, 147.38, 148.81, 151.19, 152.66, 158.39 (Ar–C); Anal. Calcd. for C30H20N6O2 (496.52 g/mol): C 72.57, H 4.06, N 16.93 % Found: C 72.61, H 4.19, N 17.13 %; MS (m/z): 496.7 (M+·).
1-Amino-3-[2-(4-fluorophenoxy)quinolin-3-yl]-8-methylpyrido[1,2-a]benzimidazole-2,4-dicarbonitrile (6p)
Yield 80 %; m.p. 237–239 °C; IR (KBr, cm−1): 3,445 and 3,310 (asym. and sym. str. of –NH2), 2,185 (–C≡N str.), 1,200 (C–O–C ether str.); 1H NMR (400 MHz, DMSO-d 6 ): δ 1H NMR (400 MHz, DMSO-d 6 ): δ 2.57 (s, 3H, CH3, C-8), 7.09 (d, 2H, J = 8.4 Hz, H-23,21), 7.25 (d, 1H, J = 8.8 Hz, H-20), 7.38 (d, 1H, J = 8.8 Hz, H-24), 7.47 (d, 1H, J = 8.0 Hz, H-9), 7.55 (m, 2H, H-14,13), 7.76 (t, 1H, J 1 = 7.2, J 2 = 8.0, H-15), 7.87 (d, 1H, J = 8.0 Hz, H-10), 7.96 (s, 1H, H-7), 8.51 (d, 1H, J = 8.4 Hz, H-16), 8.66 (s, 1H, H-12), 8.81 (br s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6 ) δ: 21.84 (CH3), 78.10 (C–CN), 87.95 (C–CN), 115.03, 116.00, 116.33, 119.50, 120.01, 122.22, 122.80, 125.21, 126.20, 127.11, 127.45, 128.90, 129.00, 130.50, 132.06, 134.88, 141.79, 145.04, 146.43, 147.39, 148.80, 151.16, 152.90, 158.48 (Ar–C); Anal. Calcd. for C29H17FN6O (484.48 g/mol): C 71.89, H 3.54, N 17.35 % Found: C 71.75, H 4.71, N 17.42 %; MS (m/z): 484.4 (M+·).
1-Amino-3-[2-(4-phenoxy)-6-methylquinolin-3-yl]-8-methylpyrido[1,2-a]benzimidazole-2,4-dicarbonitrile (6q)
Yield 87 %; m.p. 246–248 °C; IR (KBr, cm−1): 3,410 and 3,325 (asym. and sym. str. of –NH2), 2,195 (–C≡N str.), 1,235 (C–O–C ether str.); 1H NMR (400 MHz, DMSO-d 6 ): δ 2.53 (s, 3H, CH3, C-8), 2.56 (s, 3H, CH3, C-14), 7.16 (d, 2H, J = 8.4 Hz, H-20,24), 7.28 (m, 3H, H-21,22,23), 7.46 (d, 1H, J = 8.0 Hz, H-9), 7.63 (s, 1H, H-13), 7.73 (d, 1H, J = 8.4 Hz, H-15), 7.79 (d, 1H, J = 8.0 Hz, H-10), 7.89 (s, 1H, H-7), 8.51 (d, 1H, J = 8.0 Hz, H-16), 8.68 (s, 1H, H-12), 8.87 (br s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6 ) δ: 21.32 (CH3), 21.78 (CH3), 55.87 (OCH3), 78.13 (C–CN), 87.84 (C–CN), 115.50, 115.95, 118.89, 120.04, 123.30, 124.10, 125.09, 127.00, 127.24, 127.70, 128.50, 132.65, 134.06, 135.44, 141.33, 145.01, 145.63, 146.70, 147.08, 148.70, 152.66, 154.52, 157.35, 158.09, (Ar–C); Anal. Calcd. for C30H20N6O (480.52 g/mol): C 74.99, H 4.20, N 17.49 % Found: C 74.83, H 4.33, N 17.52 %; MS (m/z): 480.5 (M+·).
1-Amino-3-[2-(4-methylphenoxy)-6-methylquinolin-3-yl]-8-methylpyrido[1,2-a]benzimidazole-2,4-dicarbonitrile (6r)
Yield 83 %; m.p. 244–245 °C; IR (KBr, cm−1): 3,480 and 3,310 (asym. and sym. str. of –NH2), 2,190 (–C≡N str.), 1,210 (C–O–C ether str.); 1H NMR (400 MHz, DMSO-d 6 ): δ 1H NMR (400 MHz, DMSO-d 6 ): δ 2.33 (s, 3H, CH3, C-22), 2.52 (s, 3H, CH3, C-8), 2.59 (s, 3H, CH3, C-14), 7.08 (d, 2H, J = 8.4 Hz, H-23,21), 7.26 (d, 1H, J = 8.8 Hz, H-20), 7.34 (d, 1H, J = 8.8 Hz, H-24), 7.49 (d, 1H, J = 8.0 Hz, H-9), 7.65 (s, 1H, H-13), 7.70 (d, 1H, J = 8.4 Hz, H-15), 7.78 (d, 1H, J = 8.0 Hz, H-10), 7.87 (s, 1H, H-7), 8.54 (d, 1H, J = 8.0 Hz, H-16), 8.68 (s, 1H, H-12), 8.83 (br s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6 ) δ: 20.94 (CH3), 21.31 (CH3), 21.85 (CH3), 78.25 (C–CN), 87.89 (C–CN), 115.37, 116.02, 118.99, 120.08, 123.30, 124.20, 125.17, 126.90, 127.21, 127.80, 128.60, 132.70, 133.95, 135.60, 141.13, 144.77, 145.51, 146.80, 147.03, 148.65, 152.20, 154.50, 156.90, 157.96, (Ar–C); Anal. Calcd. for C31H22N6O (494.55 g/mol): C 75.29, H 4.48, N 16.99 % Found: C 75.38, H 4.30, N 17.17 %; MS (m/z): 494.6 (M+·).
1-Amino-3-[2-(4-methoxyphenoxy)-6-methylquinolin-3-yl]-8-methylpyrido[1,2-a]benzimidazole-2,4-dicarbonitrile (6s)
Yield 78 %; m.p. 251–253 °C; IR (KBr, cm−1): 3,470 and 3,320 (asym. and sym. str. of –NH2), 2,220 (–C≡N str.), 1,245 (C–O–C ether str.); 1H NMR (400 MHz, DMSO-d 6 ): δ 2.53 (s, 3H, CH3, C-8), 2.56 (s, 3H, CH3, C-14), 3.78 (s, 3H, OCH3, C-22), 7.01 (d, 2H, J = 8.4 Hz, H-23,21), 7.21 (d, 1H, J = 8.8 Hz, H-20), 7.27 (d, 1H, J = 8.8 Hz, H-24), 7.43 (d, 1H, J = 8.0 Hz, H-9), 7.61 (s, 1H, H-13), 7.70 (d, 1H, J = 8.4 Hz, H-15), 7.77 (d, 1H, J = 8.0 Hz, H-10), 7.86 (s, 1H, H-7), 8.49 (d, 1H, J = 8.0 Hz, H-16), 8.58 (s, 1H, H-12), 8.77 (br s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6 ) δ: 21.31 (CH3), 21.78 (CH3), 55.87 (OCH3), 77.68 (C–CN), 87.79 (C–CN), 115.04, 116.01, 119.09, 120.01, 123.33, 124.15, 125.13, 126.95, 127.13, 127.72, 128.53, 132.66, 133.96, 135.59, 141.10, 144.91, 145.50, 146.75, 147.00, 148.69, 152.21, 154.48, 156.95, 157.95, (Ar–C); Anal. Calcd. for C31H22N6O2 (510.55 g/mol): C 72.93, H 4.34, N 16.46 % Found: C 73.04, H 4.32, N 16.51 %; MS (m/z): 510.2 (M+).
1-Amino-3-[2-(4-fluorophenoxy)-6-methylquinolin-3-yl]-8-methylpyrido[1,2-a]benzimidazole-2,4-dicarbonitrile (6t)
Yield 75 %; m.p. 275–277 °C; IR (KBr, cm−1): 3,465 and 3,345 (asym. and sym. str. of –NH2), 2,225 (–C≡N str.), 1,230 (C–O–C ether str.); 1H NMR (400 MHz, DMSO-d 6 ): δ 1H NMR (400 MHz, DMSO-d 6 ): δ 2.50 (s, 3H, CH3, C-8), 2.58 (s, 3H, CH3, C-14), 6.99 (d, 2H, J = 8.4 Hz, H-23,21), 7.18 (d, 1H, J = 8.8 Hz, H-20), 7.25 (d, 1H, J = 8.8 Hz, H-24), 7.40 (d, 1H, J = 8.0 Hz, H-9), 7.57 (s, 1H, H-13), 7.69 (d, 1H, J = 8.4 Hz, H-15), 7.75 (d, 1H, J = 8.0 Hz, H-10), 7.85 (s, 1H, H-7), 8.47 (d, 1H, J = 8.0 Hz, H-16), 8.65 (s, 1H, H-12), 8.79 (br s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6 ) δ: 21.33 (CH3), 21.80 (CH3), 77.64 (C–CN), 87.89 (C–CN), 115.08, 116.04, 119.06, 120.02, 123.23, 124.12, 124.93, 127.05, 127.09, 127.92, 128.83, 132.46, 134.07, 135.79, 141.17, 144.81, 145.56, 146.77, 147.07, 148.59, 152.29, 154.38, 157.11, 158.60, (Ar–C); Anal. Calcd. for C30H19FN6O (498.51 g/mol): C 72.28, H 3.84, N 16.86 % Found: C 72.44, H 3.72, N 17.01 %; MS (m/z): 498.1 (M+·).
1-Amino-3-[2-(4-phenoxy)-6-methoxyquinolin-3-yl]-8-methylpyrido[1,2-a]benzimidazole-2,4-dicarbonitrile (6u)
Yield 86 %; m.p. 275–276 °C, IR (KBr, cm−1): 3,410 and 3,340 (asym. and sym. str. of –NH2), 2,210 (–C≡N str.), 1,185 (C–O–C ether str.); 1H NMR (400 MHz, DMSO-d 6 ): δ 2.56 (s, 3H, CH3, C-8), 3.90 (s, 3H, OCH3, C-14), 7.12 (d, 2H, J = 8.4 Hz, H-20,24), 7.21 (m, 3H, H-21,22,23), 7.40 (d, 1H, J = 8.0 Hz, H-9), 7.58 (s, 1H, H-13), 7.68 (d, 1H, J = 8.4 Hz, H-15), 7.75 (d, 1H, J = 8.0 Hz, H-10), 7.84 (s, 1H, H-7), 8.53 (d, 1H, J = 8.0 Hz, H-16), 8.67 (s, 1H, H-12), 8.82 (br s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6 ) δ: 21.80 (CH3), 56.10 (OCH3), 77.73 (C–CN), 87.80 (C–CN), 115.07, 116.05, 119.19, 120.21, 123.53, 124.25, 125.17, 127.05, 127.34, 127.82, 128.63, 132.72, 134.11, 135.75, 141.33, 144.97, 145.60, 146.75, 147.09, 148.59, 152.11, 154.58, 156.87, 157.18, (Ar–C); Anal. Calcd. for C30H20N6O2 (496.52 g/mol): C 72.57, H 4.06, N 16.93 % Found: C 72.51, H 4.26, N 16.84 %; MS (m/z): 496.3 (M+·).
1-Amino-3-[2-(4-methylphenoxy)-6-methoxyquinolin-3-yl]-8-methylpyrido[1,2-a]benzimidazole-2,4-dicarbonitrile (6v)
Yield 82 %; m.p. 250–252 °C; IR (KBr, cm−1): 3,440 and 3,325 (asym. and sym. str. of –NH2), 2,200 (–C≡N str.), 1,200 (C–O–C ether str.); 1H NMR (400 MHz, DMSO-d 6 ): δ 2.34 (s, 3H, CH3, C-22), 2.56 (s, 3H, CH3, C-8), 3.92 (s, 3H, OCH3, C-14), 7.11 (d, 2H, J = 8.4 Hz, H-23,21), 7.28 (d, 1H, J = 8.8 Hz, H-20), 7.32 (d, 1H, J = 8.8 Hz, H-24), 7.48 (d, 1H, J = 8.0 Hz, H-9), 7.62 (s, 1H, H-13), 7.72 (d, 1H, J = 8.4 Hz, H-15), 7.79 (d, 1H, J = 8.0 Hz, H-10), 7.88 (s, 1H, H-7), 8.54 (d, 1H, J = 8.0 Hz, H-16), 8.73 (s, 1H, H-12), 8.92 (br s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6 ) δ: 20.91 (CH3), 21.85 (CH3), 56.16 (OCH3), 78.15 (C–CN), 87.84 (C–CN), 115.49, 116.03, 118.99, 120.18, 123.16, 124.20, 125.16, 126.90, 127.05, 127.68, 128.50, 132.70, 133.94, 135.60, 141.11, 144.77, 145.54, 146.80, 147.01, 148.70, 152.20, 154.50, 156.90, 158.24, (Ar–C); Anal. Calcd. for C31H22N6O2 (510.55 g/mol): C 72.93, H 4.34, N 16.46 % Found: C 73.08, H 4.29, N 16.54 %; MS (m/z): 510.2 (M+·).
1-Amino-3-[2-(4-methoxyphenoxy)-6-methoxyquinolin-3-yl]-8-methylpyrido[1,2-a]benzimidazole-2,4-dicarbonitrile (6w)
Yield 80 %; m.p. 279–280 °C; IR (KBr, cm−1): 3,405 and 3,320 (asym. and sym. str. of –NH2), 2,200 (–C≡N str.), 1,235 (C–O–C ether str.); 1H NMR (400 MHz, DMSO-d 6 ): δ 2.57 (s, 3H, CH3, C-8), 3.78 (s, 3H, OCH3, C-22), 3.83 (s, 3H, OCH3, C-14), 7.01 (d, 2H, J = 8.4 Hz, H-23,21), 7.22 (d, 1H, J = 8.8 Hz, H-20), 7.28 (d, 1H, J = 8.8 Hz, H-24), 7.42 (d, 1H, J = 8.0 Hz, H-9), 7.60 (s, 1H, H-13), 7.69 (d, 1H, J = 8.4 Hz, H-15), 7.78 (d, 1H, J = 8.0 Hz, H-10), 7.92 (s, 1H, H-7), 8.64 (d, 1H, J = 8.0 Hz, H-16), 8.71 (s, 1H, H-12), 8.85 (br s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6 ) δ: 21.83 (CH3), 55.89 (OCH3), 55.98 (OCH3), 78.00 (C–CN), 87.92 (C–CN), 115.08, 115.90, 119.16, 120.05, 123.24, 124.17, 125.18, 127.12, 127.22, 127.68, 128.86, 132.73, 133.78, 135.60, 141.17, 144.99, 145.54, 146.70, 147.06, 148.70, 152.20, 154.50, 157.24, 158.55, (Ar–C); Anal. Calcd. for C31H22N6O3 (526.54 g/mol): C 70.71, H 4.21, N 15.96 % Found: C 70.55, H 4.33, N 16.03 %; MS (m/z): 526.6 (M+·).
1-Amino-3-[2-(4-fluorophenoxy)-6-methoxyquinolin-3-yl]-8-methylpyrido[1,2-a]benzimidazole-2,4-dicarbonitrile (6x)
Yield 77 %; m.p. 255–256 °C; IR (KBr, cm−1): 3,460 and 3,330 (asym. and sym. str. of –NH2), 2,210 (–C≡N str.), 1,240 (C–O–C ether str.); 1H NMR (400 MHz, DMSO-d 6 ): δ 2.55 (s, 3H, CH3, C-8), 3.82 (s, 3H, OCH3, C-14), 7.04 (d, 2H, J = 8.4 Hz, H-23,21), 7.23 (d, 1H, J = 8.8 Hz, H-20), 7.29 (d, 1H, J = 8.8 Hz, H-24), 7.42 (d, 1H, J = 8.0 Hz, H-9), 7.60 (s, 1H, H-13), 7.71 (d, 1H, J = 8.4 Hz, H-15), 7.79 (d, 1H, J = 8.0 Hz, H-10), 7.87 (s, 1H, H-7), 8.51 (d, 1H, J = 8.0 Hz, H-16), 8.65 (s, 1H, H-12), 8.95 (br s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6 ) δ: 21.77 (CH3), 56.05 (OCH3), 78.15 (C–CN), 87.83 (C–CN), 115.23, 116.09, 119.11, 119.98, 123.24, 124.26, 125.32, 126.98, 127.34, 127.68, 128.60, 132.59, 133.90, 135.61, 141.23, 144.77, 144.99, 146.81, 147.05, 148.72, 152.20, 155.97, 157.44, 158.04, (Ar–C); Anal. Calcd. for C30H19FN6O2 (514.51 g/mol): C 70.03, H 3.72, N 16.33 % Found: C 69.95, H 3.63, N 16.50 %; MS (m/z): 514.3 (M+·).
Conclusion
A series of some new pyrido[1,2-a]benzimidazole derivatives 6a–x containing β-aryloxyquinoline moiety has been synthesized via microwave-assisted one-pot multicomponent reaction in the presence of non-hazardous base (NaOH). This synthetic strategy allows the construction of relatively complicated pyridine frameworks equipped with a benzimidazole unit and introduction of substituted β-aryloxyquinolines at the fourth positions of pyridine with fascinating yield in short time. From the appraisal of the antimicrobial activity data, it can be concluded that compounds 6a, 6e, 6n, 6p, and 6u having excellent antibacterial property, while compounds 6o and 6p showed better antifungal property. Evaluation of antitubercular activity shows that compounds 6t and 6x found to have better antitubercular activity and compound 6p is appeared as the promising antimicrobial member with significant antitubercular activity. From the SAR study, it is commendable to mention that the antimicrobial and antitubercular activities of the title compounds depends not only on the bicyclic heteroaromatic pharmacophore appended through ether linked aryl ring but also on the nature of the peripheral substituents and may also upon their spatial relationship and positional changes. This study highlights the identification of new molecules as good antimicrobial and antitubercular agents which can be of interest for further detailed pre-clinical investigations.
References
Algul O, Meric A, Polat S, Yuksek ND, Serin MS (2009) Comparative studies on conventional and microwave-assisted synthesis of a series of 2,4-di and 2,3,4-trisubstituted benzimidazo[1,2-a]pyrimidines and their antimicrobial activities. Cent Eur J Chem 7:337–342
Badawey ESAM, Kappe C (1995a) Benzimidazole condensed ring system. IX. Potential antineoplastics. New synthesis of some pyrido[1,2-a]benzimidazoles and related derivatives. Eur J Med Chem 30:327–332
Badawey ESAM, Kappe C (1995b) Synthesis of some new imidazo-[1,2-a]pyrimidine-5(1H)-ones as potential antineoplastic agents. J Heterocycl Chem 32:1003–1006
Badawey ESAM, Kappe T (1999) Benzimidazole condensed ring systems. XI. Synthesis of some substituted cycloalkyl pyrido[1,2-a]benzimidazoles with anticipated antineoplastic activity. Eur J Med Chem 34:663–667
Bava S, Kumar S (2009) Synthesis of schiff’s bases of 8-methyltetrazolo[1,5-a]quinoline as potential anti-inflammatory and antimicrobial agents. Indian J Chem 48B:142–145
Bogdanowicz-Szwed K, Czarny A (1993) Synthesis of polyazaheterocycles by Michael addition of CH acids to α, β-unsaturated nitriles. Synthesis of pyrido[1,2-a]benzimidazole and pyrimido[5′,4′: 5,6]pyrido[1,2-a]benzimidazole derivatives. J Prakt Chem 335:279–282
Charris JE, Dominguez JN, Gamboa N, Rodrigues JR, Angel JE (2005) Synthesis and antimalarial activity of E-2-quinolinylben-zocycloalcanones. Eur J Med Chem 40:875–881
Dave SS, Ghatole AM, Rahatgaonkar AM, Chorghade MS, Chuhan PMS, Srivastava K (2009) Experimental and computational evaluation of new quinolyl chalcones as potent antiplasmodium agents. Indian J Chem 48B:1780–1793
Dawood KM, Elwan NM, Farahat AA, Abdel-Wahab BF (2010) 1H-Benzimidazole-2-acetonitriles as synthon in fused benzimidazole synthesis. J Heterocyclic Chem 47:243–267
Diekema DJ, Bootsmiller BJ, Vaughn TE, Woolson RF, Yankey JW, Ernst EJ (2004) Antimicrobial resistance trends and outbreak frequency in united states hospitals. Clin Infect Dis 38:78–85
El-Hawash SAM, Badawey ESAM, Kappe T (1999) Benzimidazole condensed ring systems. XII. Synthesis and anticancer evaluation of certain pyrido[1,2-a]benzimidazole derivatives. Pharmazie 54:341–346
Furniss BS, Hannaford AJ, Smith PWG, Tatchell AR (2004) Vogel’s Textbook of Practical Organic Chemistry, 5th edn. Longman, Harlow
Jardosh HH, Patel MP (2011) Lanthanum triflate triggered synthesis of tetrahydroquinazolinone derivatives of N-allyl quinolone and their biological assessment. J Serb Chem Soc. doi: 10.2298/JSC120121039J
Jardosh HH, Patel MP (2012) Microwave-assisted CAN-catalyzed solvent-free synthesis of N-allyl quinolone-based pyrano[4,3-b]chromene and benzopyrano[3,2-c]chromene derivatives and their antimicrobial activity. Med Chem Res doi:10.1007/s00044-012-0085-z
Dawood KM, Elwan NM, Abdel-Wahab BF (2011) Recent advances on the synthesis of azoles, azines and azepines fused to benzimidazole. Arkivoc i:111–195
Kalluraya B, Nayak J, Adhikari A, Sujith KV, Sucheta N, Shetty MW (2008) Synthesis and characterization of some novel quinolino-thiazines of biological interest. Phosphorus, Sulfur Silicon Relat Elem 183:1870–1883
Kathrotiya HG, Patel NA, Patel RG, Patel MP (2012) An efficient synthesis of 3′-quinolinyl substituted imidazole-5-one derivatives catalyzed by zeolite and their antimicrobial activity. Chin Chem Lett 23:273–276
Kotovskaya SK, Baskakova ZM, Charushin VN, Chupakhin ON, Belanov EF, Bormotov NI, Balakhnin SM, Serova OA (2005) Synthesis and antiviral activity of fluorinated pyrido[1,2-a]benzimidazoles. Pharm Chem J 39:12–16
Makawana JA, Patel MP, Patel RG (2012) Synthesis and in vitro antimicrobial evaluation of penta-substituted pyridine derivatives bearing the quinoline nucleus. Med Chem Res 21:616–623
Meth-Cohn O, Bramha NA (1978) A versatile new synthesis of quinolines, thienopyridine and related fused pyridines. Tetrahedron Lett 23:2045–2048
Mital A, Negi V, Ramachandran U (2006) Synthesis and antimycobacterial activities of certain trifluoromethyl-aminoquinoline derivatives. Arkivoc X:220–227
Mungra DC, Patel MP, Rajani DP, Patel RG (2011) Synthesis and identification of β-aryloxyquinolines and their pyrano[3,2-c]chromene derivatives as a new class of antimicrobial and antituberculosis agents. Eur J Med Chem 46:4192–4200
National Committee for Clinical Laboratory Standards (NCCLS) (2002) Performance standards for antimicrobial susceptibility testing: twelfth informational supplement. ISBN 1-56238-454-6, M100-S12 (M7)
Ndakala AJ, Gessner RK, Gitari PW, October N, White KL, Hudson A, Fakorede F, Shackleford DM, Kaiser M, Yeates C, Charman SA, Chibale K (2011) Antimalarial pyrido[1,2-a]benzimidazoles. J Med Chem 54:4581–4589
Panda K, Suresh JR, Ila H, Junjappa H (2003) Heteroaromatic annulation of 2-methyl/2-cyanomethylbenzimidazole dianions with-oxoketene dithioacetals: a highly regioselective synthetic protocol for 1,2-and 2,3-substituted/annulated pyrido[1,2-a]benzimidazoles. J Org Chem 68:3498–3506
Pieroni M, Tipparaju SK, Lun S, Song Y, Sturm AW, Bishai WR, Kozikowski AP (2011) Pyrido[1,2-a]benzimidazole-Based agents active against tuberculosis (TB), multidrug-resistant (MDR) TB and extensively drug-resistant (XDR) TB. Chem Med Chem 6:334–342
Prostakov NS, Varlamov AV, Shendrik IV, Anisimov BN, Krapivko AP, Lavani-Edogiaverie S, Fomichev AA (1983) New method for the synthesis of pyrido[1,2-a]benzimidazole. Chem Heterocycl Compd 19:1102–1104
Rana PB, Mistry BD, Desai KR (2008) Green chemistry: conventional and microwave induced synthesis of various thiazolidinone derivatives from 3-{[(1E)-(2′-chloro-7′-methoxyquinoline-3′-yl)methylene]amino}-4 (substitutedphenyldiazenyl)phenol and their antimicrobial screening. Arkivoc xv:262-279
Rattan A (2000) Antimicrobials in Laboratory Medicine. Churchill B I. Livingstone, New Delhi, p 85
Rida SM, Soliman FSG, Badawey ESAM, El-Ghazzawi E, Kader O, Kappe T (1988) Benzimidazole condensed ring systems. 1. Syntheses and biological investigations of some substituted pyrido[1,2-a]benzimidazoles. J Heterocycl Chem 25:1087–1093
Rida SM, EI-Hawash SAM, Fahmy HTY, Hazzaa AA, EI-Meligy MMM (2006) Synthesis of novel benzofuran and related benzimidazole derivatives for evaluation of in vitro anti-HIV-1, anticancer and antimicrobial activities. Arch Pharm Res 29:826–833
Russell RK, Van Nievelt CE (1995) The synthesis of cycloalkylpyrido[1,2-a]benzimidazole carbonitrile analogs. J Heterocyclic Chem 32:299–306
Russell DG, Barry CE, Flynn JL (2010) Tuberculosis: what we don’t know can, and does, hurt us. Science 328:852–856
Shah NM, Patel MP, Patel RG (2012) New N-arylamino biquinoline derivatives: Synthesis, antimicrobial, antituberculosis and antimalarial evaluation. Eur J Med Chem, doi:10.1016/j.ejmech.2012.05.004
Shi A, Nguyen TA, Battina SK, Rana S, Takemoto DJ, Chiang PK, Hua DH (2008) Synthesis and anti-breast cancer activities of substituted quinolines. Bioorg Med Chem Lett 18:3364–3368
Sundberg RJ, Ellis JE (1982) Synthesis of pyrido[1,2-a]benzimidazoles by cyclizative condensation of 2-halopyridines and 1,2-benzenediamines. Scope and mechanism of the reaction. J Heterocyclic Chem 19:585–588
Takemura M, Takashi H, Kawakami K, Takeshita H, Kimura Y, Watanabe J, Sugimoto Y, Kitamura A, Nakajima R, Kanai K, Fujisawa T. (2004) Imidazo[1,2-a]pyridine derivative. Eur. Pat. Appl.1479681
Upadhaya RS, Kulkarni GM, Vasireddy NR, Vandavasi JK, Dixit SS, Sharma V, Chattopadhyaya J (2009) Design, synthesis and biological evaluation of novel triazole, urea and thiourea derivatives of quinoline against Mycobacterium tuberculosis. Bioorg Med Chem 17:4681–4692
World Health Organization. (2008) Global TB control: surveillance, planning, financing: WHO report. Geneva, Switzerland: WHO Press
World Health Organization. (2010) Global tuberculosis control: WHO report 2010; WHO Press: Geneva, Switzerland
Wu Z, Huang Q, Zhou X, Yu L, Li Z, Wu D (2011) Synthesis of Pyrido[1,2-a]benzimidazoles through a copper-catalyzed cascade C-N coupling process. Eur J Org Chem 2011:5242–5245
Yan CG, Wang QF, Song XK, Sun J (2009) One-step synthesis of pyrido[1,2-a]benzimidazole derivatives by a novel multicomponent reaction of chloroacetonitrile, malononitrile, aromatic aldehyde, and pyridine. J Org Chem 74:710–718
Acknowledgments
The authors are thankful to Department of Chemistry, Sardar Patel University for providing research facilities and NMR facility. We are also thankful to Vaibhav Analytical Laboratory, Ahmedabad for the FT-IR and Sophisticated Instrumentation Centre for Applied Research and Training (SICART), Vallabh Vidyanagar for elemental analysis. As well as Oxygen Healthcare Research Pvt. Ltd., Ahmedabad for providing mass spectrometry facilities and Microcare Laboratory, Surat for antimicrobial screening of the compounds reported herein. One of the authors is grateful to UGC, New Delhi for a Research Fellowship in Sciences for Meritorious Students.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sangani, C.B., Jardosh, H.H., Patel, M.P. et al. Microwave-assisted synthesis of pyrido[1,2-a]benzimidazole derivatives of β-aryloxyquinoline and their antimicrobial and antituberculosis activities. Med Chem Res 22, 3035–3047 (2013). https://doi.org/10.1007/s00044-012-0322-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-012-0322-5