Abstract
Aims
Negative interactions in the rhizosphere between entomopathogenic nematodes (EPNs) and plant-parasitic nematodes, such as root-knot nematodes (RKNs), have been documented over the past two decades but the mechanisms and dynamics of such interactions remain largely elusive.
Methods
Here, we evaluated the effect of the inoculation position of two EPN species, Steinernema feltiae and Heterorhabditis bacteriophora, as well as different facets of the EPN-bacterial symbiont complex on the migration of RKNs toward tomato roots, both in sand and in Pluronic gel conditions.
Results
When EPNs were placed between the position of the RKNs and the roots, the movement of RKNs toward the roots was inhibited. We observed this same pattern both in sand and in Pluronic F-127 (PF-127) gel for two species of EPNs. We also observed that different components of the EPNs/bacterial symbiont complex (bacteria separate from the nematodes vs. the nematode-bacterium complex), and particularly the cell-free supernatant produced by the bacterial culture, displayed inhibitory effects on RKNs.
Conclusion
Therefore, the EPNs/bacterial complex, by slowing down the movement of RKNs toward the host plant roots, could partially contribute to RKN control. By screening for the most repulsive strains of EPNs that are also effective against insect pests, the combined target approach should alleviate EPNs application costs in integrated pest management practices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Root-knot nematodes (RKNs) are economically important polyphagous pests (Agrios 2005; Jones et al. 2013), causing losses to global crop production up to US $157 billion annually (Chitwood 2003; Elling 2013). RKNs damage plants through direct consumption of the root system, but also indirectly by forming complexes with soil-borne plant pathogens, such as Fusarium or Pythium, which also inhibit plant growth and yield (Morris et al., 2016). Meloidogyne incognita is one of the most harmful species of RKN, leading to dramatical losses in crops’ yield worldwide (Barbary et al. 2015; Jones et al. 2013; Ralmi et al. 2016; Trudgill and Blok 2001). Another recently-emerging RKN species is Meloidogyne enterolobii, which has become an economically important plant-parasitic nematode worldwide because of its high level of aggressiveness, its increasingly wide geographic distribution (Khanal and Harshman 2022), and its ability to weaken crop resistance to other RKN species (Philbrick et al. 2020). The life cycle of most RKNs can be described as follows (Moens et al. 2009; Shukla et al. 2018):
Pre-parasitic second-stage juveniles (J2s) invade plant roots, induce the formation of multiple giant cells, and develop into adult female after multiple molting’s. Later (swollen) life stages burst out of the root and adult females produce an eggs sac – a gelatinous matrix with hundreds of eggs. Mobile pre-parasitic J2s travel through the soil and start searching for a suitable host root system.
The host-seeking behavior of RKNs is generally mediated by chemotaxis in relation to a chemical gradient of root exudates (Dutta et al. 2011; Leitao et al. 2021; Rasmann et al. 2012; Tsai et al. 2021). Once the host roots are found, the J2s enter the root tip and continue their development to produce a new generation of J2 ready to colonize other nearby root systems. To date, the commonly used means of controlling RKNs have been the application of chemical nematicides and resistant cultivars (Verdejo-Lucas et al. 2019, Liu and Grabau 2022). For example, the Mi-1.2 gene confers resistance in tomatoes to M. incognita (Milligan et al. 1998). In general, effective management of RKNs has relied upon the application of chemical nematicides (Chen et al. 2020), which have been shown to cause undesirable adverse side effects on non-target organisms, humans, and the environment (Oka 2020). However, growing concerns about environmental safety and public health led to the withdrawal or restricted usage of a wide range of commonly used chemical nematicides. Accordingly, more ecologically-sound means of RKN control are needed (Ahmad et al. 2021).
Entomopathogenic nematodes (EPNs) are also soil-dwelling nematodes that can be attracted by the roots of crop plants, particularly, when a host-insect larva is feeding on the roots (Rasmann et al. 2005; Tonelli et al. 2016). The two families of EPNs, Steinernematidae and Heterorhabditidae have been extensively studied for the development of biological control products to control root arthropod pests (Divya and Sankar 2009; Shapiro-Ilan et al. 2020; Zhang et al. 2019). The life cycle of EPNs includes an egg stage, four juvenile stages and an adult stage. A specialized third juvenile stage EPN is referred to as the “infective juvenile” (IJ) or a parallel of the “dauer” stage. This is the only free-living stage; the IJs persist in soil for several days or months without food (Mitani et al. 2004; Poinar 1990). Interestingly, EPNs have been shown to have antagonistic effects on RKNs (Grewal et al. 1997; Sayedain et al. 2021), but these effects vary depending on the EPN species (Damascena et al. 2019; Lewis and Grewal, 2005). For instance, when Steinernema feltiae EPNs were present in the rhizosphere of tomato plants, the galling and egg hatching of M. incognita RKNs was reduced by 34.12% and 62.42% respectively, and the number of eggs per egg mass in treatment plants was not different from that in control plants (Lewis et al. 2001). Four Philippine EPN isolates significantly reduced the extent of root penetration and gall development of M. incognita in tomato roots with the lowest numbers of M. incognita in plants treated with S. abbasi (1.90 ± 2.20) and H. indica (2.05 ± 2.61) (Felicitas et al. 2021). Similarly, S. brazilense, S. rarum, S. feltiae, Heterorhabditis amazonensis and H. bacteriophora were all observed to reduce galls, egg mass, egg hatching and reproduction of M. enterolobii (Damascena et al. 2019). Moreover, it has been shown that EPN-infected insect cadavers themselves can reduce root colonization by RKNs (Caccia et al. 2018; Kepenekci et al. 2016; Molina et al. 2007). In a greenhouse-based study, an aqueous suspension of IJs, the nematode-infected cadaver, or the Xenorhabdus bovienii (bacterial symbiont of S. feltiae), significantly reduced damage caused by M. incognita and M. arenaria on tomato plants (Kepenekci et al. 2016). Hence, EPNs could partially contribute to RKN control considering their multi-faceted potential against both insect pests and plant-parasitic nematodes.
To date, the mechanisms driving the inhibitory effects of EPNs on RKNs still remain largely untested, as these antagonistic effects can be direct and indirect. In an indirect manner, an allelopathic mechanism was indicated for EPNs suppression of PPNs (Grewal et al. 1999). Additionally, EPNs have been shown to enhance the activation of plant defense pathways against RKNs (Helms et al. 2019, Kamali et al. 2022). For instance, the EPN Steinernema carpocapsae and its symbiotic bacterium Xenorhabdus nematophila have also been shown to induce the expression of PATHOGENESIS-RELATED PROTEIN-1 (PR-1) in the roots, and increase the activity of peroxidase and catalase in leaves of Arabidopsis thaliana (Jagdale et al. 2009). On the other hand, it is also plausible that EPNs can inhibit the virulence of RKNs directly through interference competition. Indeed, both EPNs and RKNs can be attracted toward the plant root system, likely leading to potential encounters, followed by avoidance.
In this study, we aimed to address the potential direct antagonisms between EPNs and RKNs near the root system. We hypothesized that the presence of EPNs between the location of the RKNs in soil and the root system would inhibit the movement of RKNs to the roots of the host plant. Secondly, as it was previously observed that EPN-infested insect cadavers were also repulsive to RKNs, we hypothesized that the EPN-mediated interference is regulated by chemical compounds produced by the EPN-symbiont complex. Therefore, the objectives of the present study were (1) to evaluate the effect of the inoculation position of EPNs on RKNs migration toward host plant roots, and (2) to address the effect of the EPN-bacterial symbiont complex on RKNs migration toward host plant roots.
Materials and methods
To address the effect of two EPN species on the attraction of RKNs toward tomato roots, we performed three separate experiments, a first one in sand, a second one in Pluronic gel, and a third one, also in Pluronic gel, but that included EPN-symbiotic bacterial treatments.
Organisms
Tomato plants
The seeds of tomato (Solanum lycopersicum) cultivar “Hezuo 903” susceptible to M. incognita without the resistant gene Mi-1 (Guan et al. 2017), were soaked in 2% sodium hypochlorite for 15 min, and then rinsed with sterilized water for five times. Seeds were transferred on a shallow dish lined with gauze soaked in sterile water in the dark at 25 °C for approximately five days. The emerged seedlings were next transplanted into 32-hole germinating trays filled with a vermiculite/nutrient peat mixture (2:1, v/v) (KLASMANN, Germany), and placed in a greenhouse at 25℃, with 16/8 hrs light/dark photoperiod. Seedlings with 1-1.5 cm root length were used for the bioassays in Pluronic gel, while four-week-old seedlings, with two sets of leaves, were used for the bioassay in sand.
Root-knot nematodes (RKNs)
Meloidogyne incognita RKNs were reared in Ipomoea aquatica seedlings in the greenhouse of Nankai University. Briefly, egg masses were hand-picked from infested roots, readily sterilized using 1% NaOCl for 1 min, rinsed thoroughly with distilled water, and incubated at 25 °C in the dark. Freshly-hatched second-stage juveniles (J2) were collected using a 25 μm sieve in distilled water, counted using an inverted microscope (Olympus CKX41), and used in the experiments within a maximum of 3 days.
Entomopathogenic nematodes (EPNs)
Steinernema feltiae (SN strain) (Sf) and Heterorhabditis bacteriophora (HB1 strain) (Hb) were kindly provided by David Shapiro-Ilan from USDA-ARS. EPNs were reared in last-instar Galleria mellonella in the laboratory at 22 °C following Zhen et al. (2018). Infective juveniles (IJs) were collected via White traps after the third day of emergence from G. mellonella cadaver (White, 1927) and stored at 14 °C until use, within a maximum of two weeks storage.
EPN symbiotic bacteria
Xenorhabdus bovienii and Photorhabdus luminescens were isolated from S. feltiae and H. bacteriophora, respectively (their identification was previously confirmed by David Shapiro-Ilan’s laboratory). Briefly, each last-instar G. mellonella was inoculated with 40 µL of nematode suspension (approximately 100 IJs per larva) in a 24-well cell culture plate lined with filter paper. Around 30 h after infection, one drop of hemolymph was obtained from the infected insect by snipping the very end of the second proleg and adding it to nutrient bromothymol agar (NBTA). After 48 h, pure colonies of the primary variant bacteria were inoculated into Trypticase Soy Yeast (TSY) broth. Flasks were placed on a shaker at 25 °C, 200 rpm for 24 h and stored at 4 °C until use (Ansari et al. 2003). The suspension was centrifuged at 10,000 rpm for 10 min and cells were removed using a 0.22 μm membrane filter, resulting in a cell-free preparation of bacterial metabolites for testing.
Bioassay to test for the effect of EPNs on the movement of RKNs in sand conditions
The experimental set-up was built using a 90° elbow PVC pipe, which was connected to three 5 cm straight PVC pipes (Diameter 5 cm) and sealed with Parafilm. Each section was named as A, B, C and D for distinguishing the locations used for the inoculation and recollection of nematodes (Fig. 1A). A 2-mm diameter hole was made in the centre of the C and D sections for nematode inoculation. The set-up was filled with 850 g of sterilized sand (~ 1 mm) and kept at 10% relative humidity. Tomato seedlings with two sets of leaves were transplanted in the first curved connector (A zone) of each set-up, and the opening around the stem was covered with aluminium foil. The set-up was placed in a climate and light-controlled room (16 h/8 hrs light/dark photoperiod, 22 °C/18°C day/night temperature, and photosynthetic photon flux density of 100 µmol m− 2 s− 1). Similar to the study by Rasmann and Turlings (2007) three days after, the EPN treatment was initiated by adding 5000 IJs of Sf or Hb in 2 mL water to the A, C, or D zones, or no EPNs were added. Meanwhile, 2000 RKNs (J2) in 2 mL water were added to the C zone. Thus, in summary, all experimental units received RKNs, and prior to RKNs inoculation, each unit received either Sf or Hb (inoculated in one of 3 locations), or the no-EPN control. Each treatment was replicated 5 times to obtain a total of 35 experimental units (2 EPN strains ×3 EPN inoculation position × 5 replications + 1 control(without inoculation) × 5 replications). The experiment was conducted twice (two complete trials).
Experimental devices to test the effect of entomopathogenic nematodes (EPNs) on root-knot nematodes (RKNs) movement toward tomato roots. (A) Photograph of the sand-based bioassay arena. The arenas consisted of four sequentially-connected PVC pipes (A, B, C and D zones). The A zone was made from an elbow-bent pipe, which allowed the placement of a four-week-old tomato seedling. RKNs were only added to the 2-mm diameter hole in the C zone, whereas EPNs were added to A, C, or D zone. The RKN number in A, B, C, and D- zones, the rhizosphere, and the roots were counted after seven days. (B) Photograph of a Petri dish filled with Pluronic F-127 gel. For the second experiment, each Petri dish was divided into three parts; an inner zone (I), a transition zone (T), and an outer zone (O). For the third experiment, the T zone was removed (not shown here). In the middle of the Petri dish, tomato seedlings with 1-1.5 cm roots were used. RKNs were only added to O-zone whereas EPN infective juveniles (dead/ live) and symbiotic bacterial culture solution (crude/cell-free) was added to I-zone. The seedling was added to I-zone. The RKN in I-, T-, O-zone and root were sampled at 4 and 24 h post-inoculation
Seven days after nematode inoculation, the experiment was terminated. The seedlings were cut near the base of the stem. The sand attached to the plant root was collected by carefully rinsing the roots with tap water, and the nematodes in the sand solution were regarded as the nematode in the rhizosphere. The rinsed roots were then transferred in a 100 mL Ziplock bag and RKNs were quantified by the frozen-thaw method (Ruan et al. 2012). Briefly, roots were frozen in a -20 °C refrigerator for 24 h and subsequently thawed, and then this frozen-thawed process was repeated once. After that, the root samples were placed in a blender filled, immersed in tap water and blended for 30 s. The mixture was next sieved using a 200-mesh sieve nested on a 600-mesh sieve, the root tissue was thoroughly washed, and the residue on the 600-mesh sieve was collected into a 50-ml centrifuge tube and the nematodes were finally counted under an inverted microscope (Olympus CKX41).
The sand in the A, B, C and D zones of each experimental set-up was placed in ziplock bags. For RKNs recollection, the sand from each zone was washed five times with tap water. Each time 30 s after intensely stirring, and the supernatant was immediately collected in a 2 L glass beaker in order to reduce the amount of sand in the supernatant to the greatest extent. After 10 h decantation, the supernatant was removed by gentle aspiration until the volume of the remaining liquid was approximately 800 mL. The remaining part was filtered on a 10 µM nitrocellulose membrane via vacuum filtration, and the nematodes on the membrane were collected and counted under an inverted microscope (Olympus CKX41).
The effect of EPN presence in the different zones of the arena (or EPNs absence) on the presence of RKNs for each zone of the PVC pipe, separately, was assessed with generalized linear models (GLM) following a quasipoisson distribution. Trial was included in the model as blocking factor. Type-II analysis-of-variance tables were estimated using the ANOVA function in the package car (Fox and Weisberg 2019), and marginal means and contrasts among treatments were estimated using the package emmeans (Searle et al. 1980).
Bioassay to test for the effect of EPNs on the movement of RKNs in Pluronic gel
To test for the effect of EPN presence on the distribution of RKNs, we developed a second bioassay using 6 cm diameter Petri dishes, which were divided into three zones; an inner zone (I), a transition zone (T), and an outer zone (O). The boundaries of each zone to the centre of the Petri dish were 0.5, 1 and 3 cm, respectively (Fig. 1B). The EPNs treatment consisted of applying the two EPN species (Sf or Hb separately) to one of the three zones of the Petri dishes. One tomato seedling per Petri dish was placed in the I zone. Next, RKNs were added in T-zone, and two separate treatments that did not include EPNs were also included; one with the tomato seedlings only, and empty Petri dishes. Each treatment was replicated 6 times, so to obtain a total of 48 Petri dishes (2 EPN species × 3 zones ×6 replications + 2 control×6 replications.). This experiment was conducted twice (two complete trials).
For the bioassay, the different zones of the Petri dishes were filled with a PF-127 gel solution that contained the nematodes and bacteria in different mixtures. The PF-127 gel was prepared following Li et al. (2015). Approximately 25% (wt/vol) Pluronic F-127 gel (NF Prill Poloxamer 407, BASF, Mt Olive, NJ, USA) in 10 mM Tris–MES (morpholino-ethane sulfonic acid) buffer (Sigma–Aldrich) was made and stirred continuously at 4 °C overnight. The dissolved gel was stored at 4 °C until for use. Nematode suspensions (EPNs, RKNs or EPNs + RKNs) were added to the 25% PF-127 gel, and the gel was finally adjusted to 23% with Tris-MES buffer to reach the concentration (200 individuals/mL) for each nematode species. Symbiotic bacteria suspension was added to the 25% gel to reach the final concentration 107 CFU/mL, and the gel was adjusted to 23% with Tris-MES buffer. The final volume of the PF-127 gel added to the I, T and O zones was 0.5, 2 and 2mL, respectively. At the onset of the experiment, 400 RKNs were added to the T zone, and 400 EPNs of each species were added to the respective Petri dish according to the treatment position (I, T, or O). All the Petri dishes were then transferred to a dark chamber containing wetted gauze to maintain moisture. Four and 24 h after the onset of the experiment, RKNs in each zone were directly counted on an inverted microscope (Olympus CKX41). Finally, at 24 h, tomato roots were thoroughly rinsed with tap water, crushed on a microscope glass slide, and RKNs in roots were counted.
The interactive effect of EPN presence in the different zones of the Petri dishes (or EPNs absence), and the time of collection (4 and 24 h) on the presence of RKNs for each zone of the Petri dish, separately, was assessed with generalized linear models (GLM) following a quasipoisson distribution. Trial was included in the model as blocking factor. Type-II analysis-of-variance tables were estimated using the ANOVA function in the package car (Fox and Weisberg 2019), and marginal means and contrasts among treatments were estimated using the package emmeans (Searle et al. 1980). Finally, we performed the same analysis, but with the EPN position treatment and EPN species as fixed factors, for the two time points separately, and without the “no EPN” treatment; this approach allowed us to measure the effect of EPN species on the RKNs distribution within the Petri dishes (see Fig. 3, letters above boxplots of the EPNs for these results).
Effect of entomopathogenic nematodes (EPNs) on root-knot nematodes (RKNs) movement in the sand. Shown are the effects of EPN species and location within the tube arena (Zones A, C, and D) on the abundance of the RKNs in the different zones of the arena (tomato roots, rhizosphere circle, Zones A, B, C (where RKNs were originally placed), and D). Blue boxplots represent the presence of RKNs when in the tube arena there were no EPNs, but only the tomato plants (Plant). Two EPN species were tested; Steinernema feltiae (SN strain) (green boxes), and Heterorhabditis bacteriophora (HB1 strain) (orange boxes). Different capital letters above boxes show pairwise differences across EPN location treatments (p < 0.05 after sidak correction)
Bioassay for testing the effect of the EPN-bacterial symbiont complex on the movement of RKNs in Pluronic gel
We performed a third experiment to address the effect of two EPN-bacterial symbiont complexes (S. feltiae- X. bovienii and H. bacteriophora - P. luminescens) on the movement of RKNs toward tomato roots. Similarly, as described above, we performed the experiment in Petri dishes (3.5 cm diameter), which were only divided into an inner zone(I) and an outer zone(O) (Fig. 4A). The boundaries of the I and O zones were 0.5, 1.75-cm away from the centre of the Petri dish, respectively, and the volume of PF-127 gel added to the I- and O-zone was 0.5 and 2 mL, respectively. At the centre of I-zone of each Petri dish, we planted 5 days-old tomato seedlings. Next, we imposed the EPNs treatment, which consisted of 5 levels by adding in the I zone: (1) 400 alive EPNs (S. feltiae or H. bacteriophora), separately, (2) 400 dead EPNs that were previously killed with using a microwave oven (300 watts for 1 min), (3) 2 ml of the symbiotic bacteria solution only, (4) 2 ml of the cell-free supernatant of the symbiotic bacteria solution (filter of 0.22 µM pore size), and (5) no EPNs and no bacteria as control. Finally, a sixth treatment consisted of Pluronic gel only Petri dishes without plants (empty treatment). Each treatment was replicated six times, so to obtain a total of 60 Petri dishes (2 EPN strains × 4 test factors ×6 replications + 2 control×6 replications). Immediately after treatment inoculation, a total of 400 RKNs were added to the O-zone. Finally, the RKNs in the I- and O- zone were counted directly in the Pluronic gel at 4 and 24 h after the onset of the experiment under an inverted microscope (Olympus CKX41). The RKNs in root tissues were counted after 24 h as described above. The whole experiment was conducted twice in time.
Effect of entomopathogenic nematodes (EPNs) on root-knot nematodes (RKNs) movement in Pluronic gel. Shown are the effects of EPN species and their initial location within the Petri dish on the abundance of the RKNs in different zones of the Petri dish; tomato roots, inner circle, transition circle (where RKNs were originally placed, marked by a dashed line), and outer circle. Blue boxplots represent the presence of RKNs when in the Petri dish there were no EPNs, but only the tomato plants (Plant), or nothing (Empty). Two EPN species were tested; Steinernema feltiae (SN strain) (green boxes), and Heterorhabditis bacteriophora (HB1 strain) (orange boxes). Different capital letters above boxes show pairwise differences across EPN location treatments, and different lower-case letters represent differences among EPN species and location in the Petri dishes (p < 0.05 after sidak correction). NA means that no RKNs were counted
The interactive effect of the EPN/bacteria treatments plus the two controls in the different zones of the Petri dishes, and the time of collection (4 and 24 h) on the presence of RKNs in the inner and outer zones, separately, was assessed with generalized linear models (GLM) following a quasipoisson distribution. Trial was included in the model as blocking factor. Type-II analysis-of-variance tables were estimated using the ANOVA function in the package car (Fox and Weisberg 2019), and marginal means and contrasts among treatments were estimated using the package emmeans (Searle et al. 1980). Next, we performed the same analysis, but with the EPNs position treatment and EPN species as fixed factors, for the two time points separately, and without the “no EPN” treatment; this allowed us to measure the effect of EPN species on RKNs distribution within the Petri dishes (see Fig. 4, letters above boxplots of the EPNs for these results). For the RKNs in the root zone, we performed the same analysis, but we did not include time as factor, as in the roots, RKNs were only measured at 24 h.
Results
Effect of EPNs inoculation position on RKNs movement in sand
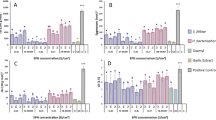
We found that most RKNs remained in zone C of the experimental set-up, while the lowest number of RKNs was found in the roots (an average of 807.8 RKNs in C, compared to an average of 148.3 in D, an average of 108.3 in A, an average of 88.3 in B, an average of 72.8 in rhizosphere and an average of 39.5 in the roots). Nonetheless, we found an effect of the EPNs treatment on RKNs movement (Table 1), particularly for the RKNs found in the roots (Fig. 2A). Specifically, we found 2.4 times more RKNs in the roots if there were no EPNs in the arena compared to when EPNs were in zone A. We observed a similar, but only marginally significant, trend for RKNs in zone A (Fig. 2C). However, we found no effect of the EPNs treatment when RKNs were counted in the rhizosphere, nor in zones B, C and D (Fig. 2B, D, E, F).
Effect of entomopathogenic nematode (EPNs)-bacteria complex on root-knot nematodes (RKNs) movement in Pluronic gel. Shown are A) the experimental set-up with the three zones of activity delimited for each Petri dish in the experiment, and the effect of EPN species and different EPN-symbiotic bacteria treatments (live EPNs, dead EPNs, live bacteria only, or the supernatant of the bacterial damaged cells on the inner zone of the Petri dish) on the abundance of the RKNs in the different zones of the Petri dish; in (A) tomato roots, (B) and D) inner circle, and E) and F) outer circle (where RKNs were originally placed, marked by a dashed line). Blue boxplots represent the presence of RKNs when in the Petri dish there were no EPNs, but only the tomato plants (Plant), or nothing (Empty). Two EPN species were tested; Steinernema feltiae (SN strain) (green boxes), and Heterorhabditis bacteriophora (HB1 strain) (orange boxes), and RKNs were measured at two time points (4 and 24 h post-inoculation). NA means that no RKNs were counted
Effect of EPNs application position on RKNs movement in Pluronic gel
Across all trials and timepoints, we found that most nematodes remained in the transition zone (average of 119.4 RKNs) and in the outer zone (average of 45.8 RKNs), rather than in the inner zone (average of 5.7 RKNs) or in tomato roots (average of 4.1 RKNs). That said, we also observed a clear effect of the EPNs treatment on the position of RKNs (Table 2; Fig. 3), but this depended on the zone and time of sampling. First, we found 4.5 times more RKNs in the roots when there were no EPNs, or when EPNs were in the outer zone than when EPNs were in the transition, or in the inner zone (Fig. 3A). In the inner zone, we found a similar effect, in which, we found 5.3 times more RKNs in the inner zone when there were no EPNs added, 2.9 times more when there was the plant only, and 3.7 times more when EPNs were in the outer zone than when EPNs were in the inner or the transition zone (Fig. 3B). When RKNs were measured in the transition or the outer zones, the effect of EPNs position (or no EPNs) was negligible (Fig. 3C, D). Therefore, to summarize, the presence of RKNs in roots or in the inner zones was affected by EPNs presence or absence in the Petri dishes, but the presence of RKNs in the transition or the outer zones was not affected the EPNs treatment in the Petri dishes.
Effect of EPNs-symbiotic bacteria complex on RKNs movement in Pluronic gel
Across all trials and timepoints, we found that most nematodes remained in the outer zone (average of 82.6 RKNs), rather than in the inner zone (average of 16.8 RKNs) or in tomato roots (average of 29.7 RKNs). Nonetheless, we also observed a clear effect of the EPN/bacteria treatments on the position of RKNs (Table 3; Fig. 4), but this depended on the zone and time of sampling. For the RKNs in the roots of tomato seedlings (Fig. 4B), and the inner zone (Fig. 4C, D), we observed a strong repulsive effect of the bacterial supernatant (Table 3). In other words, when the supernatant was added to the inner zone, it blocked the RKNs, initially placed in the outer zone, to move close to tomato roots (0.5 and 0.04 RKNs in the inner zone and in roots on average, respectively). The other EPNs treatments were less repulsive compared to the plant only, with the live EPNs being the least repulsive. In the outer zone, we found a weak but significant effect for live EPNs in the inner zone to keep the RKNs in the outer zone (Fig. 4E, F).
Discussion
We conducted three independent behavioral experiments to assess the movement of RKNs toward tomato roots in the presence or absence of EPNs. We observed that the movement of RKNs toward tomato roots or rhizosphere was generally inhibited when EPNs were present (Fig. 5). We observed this same pattern both in sand and in Pluronic gel, and for both species of EPNs. Interestingly, by testing different components of the EPNs/bacterial symbiont complex, we also observed the breakdown products produced by the bacteria displayed the highest inhibitory effect on RKNs. Therefore, the EPN/bacterial complex, by slowing down the movement of RKNs toward the host plant roots, can function as an effective biocontrol strategy. Below, we expand on each of these points.
Plant nematodes interaction in the rhizosphere. The diagram depicts the main findings of this work, particularly showing that when entomopathogenic nematodes (EPNs), or their symbiotic bacterial cells, are near the rhizosphere of host plant roots, root-knot nematodes (RKNs) tend to be inhibited (blunt grey arrow), and only relatively few RKNs will actually reach the roots. On the other hand, plant roots alone are highly attractive to RKNs (thin grey arrow). The size of the arrow indicate relative strength of inhibition, with thicker arrows indicating stronger inhibition
The present work was based on previous studies indicating that EPNs show antagonistic and/or repulsive effects on plant-parasitic nematodes, such as Meloidogyne spp. (Grewal et al. 1999; Sayedain et al. 2021), Nacobbus aberrans (Caccia et al. 2013), or even the foliar nematode Aphelenchoides fragariae (Jagdale and Grewal 2008). However, the mechanisms mediating EPNs antagonistic effects against RKNs remain to date largely unexplored. As mentioned above, potential ways in which EPNs might impact RKNs behavior including interference competition, in which both species are in competition for space in the rhizosphere, or on the root surface, or even in the root tissue. Another way in which EPNs might inhibit RKNs virulence includes the production of repulsive chemicals by the EPN-bacterial symbiont complex (Kepenekci et al. 2016).
Here, we confirmed that EPN inoculation negatively affects RKNs penetration of host plant roots, which was dependent on the EPNs inoculation position relative to the RKNs inoculation position, and therefore partially providing support to the first hypothesis. Moreover, both the 24-h PF-gel bioassay and 7-day sand bioassay, across two EPN species demonstrated consistent results. Similarly, the zones near host plants, I- and T-zone in PF-gel bioassay and rhizosphere and A-zone in sand bioassay also showed similar effects driven by EPNs inoculation position, altogether indicating the robustness of such effects. Host plants can attract both EPNs (Li et al. 2015; Rasmann et al. 2005; Tonelli et al. 2016) and plant-parasitic nematodes (Dutta et al. 2011; Wang et al. 2009). Moreover, once emerged from the insect host cadaver, EPN infective juveniles have been shown to gregariously move in a sand medium (Ruan et al. 2018). Therefore, if the EPNs can actively aggregate in the rhizosphere or around the root tip, into which RKNs preferentially penetrate, EPNs might effectively interrupt the RKNs’ host-finding and root penetration process. To date, however, we are not aware of a clear proof showing that EPNs directly interact with the RKNs, and the physical presence of EPNs can prevent RKNs movement and penetration into the host plant roots. Therefore, further studies, such as staining the nematodes with specific dyes, could be used to in situ distinguish EPNs versus RKNs in the rhizosphere, in the rhizoplane (root surface), or even in the endosphere (root interior) of root tips. From a more applied perspective, Kamali et al. (2022) showed that plants cannot clearly distinguish an EPN from an RKN, both similarly activating the plant immune system. Therefore, placing EPNs near the root system might serve the double role of physically antagonizing RKNs movement, as well as activating the plant immune system, but this hypothesis needs to be confirmed with future experiments.
We have observed different results when testing RKN inhibition by EPN in Pluronic gel or in sand substrates. Two possible reasons might explain better EPN inhibition of RKN in Pluronic gel compared to sand assays. First, we might hypothesize that there is a difference in diffusion coefficient (D) between Pluronic acid and moistened sand, in which D of Pluronic acid most likely exceeds de D of moist sand, so that water-soluble exudates might more easily disperse in Pluronic gel than moistened sand. The other reason can be related to the age of tomato seedlings. In the bioassay conducted in gel, only approximately 4-day-old seedlings with 1-1.5 cm root were used, whereas about 4-week-old seedlings were used in the sand bioassay. The potential difference in the structure and component of root exudates of seedlings of different plant ages might partially contribute to the differences observed (Zhalnina et al. 2018).
We further observed a clear negative effect of the EPN-bacterial symbiont complex on the distribution of RKNs in root tissue, I- and O-zone of the Petri dishes. Particularly, the bacterial supernatant induced an 80–90% reduction of RKNs in the I-zone compared to the treatment without RKNs. These results are consistent with previous findings showing that cell-free filtrates from symbiotic bacteria were toxic or repellent to RKNs (Grewal et al. 1999). For example, cell-free bacterial extracts were antagonistic to M. incognita, causing 98–100% mortality at 15% concentration, or the most significant negative effect on RKNs population growth (62–90% reduction), was produced by the bacterial metabolites in greenhouse tomato systems (Caccia et al. 2018). In another study, the cell-free supernatant of X. bovienii from Steinernema feltiae applied to the soil of a tomato production area also caused significant antagonistic effects against RKNs, resulting in reduced galling and higher yield (4.975 kg increase in average tomato production per plant) (Kepenekci et al. 2018). Our work confirmed the previous study of antagonism and repellence of EPNs/bacterial symbionts to RKN and further demonstrates a direct effect of EPN on RKN migratory behavior. Therefore, the cell-free supernatant of selected symbiotic bacteria added close to crop roots might serve as an ecologically-sound alternative method for repelling RKNs, thereby mitigating RKNs damage.
At this stage, we can only speculate that the toxicity of the cell-free supernatant might have been generated by the natural death and subsequent decomposition of the EPN infective juveniles in the soil matrix. Recently, it was shown that natural products from the bacterial genus Xenorhabdus, including fabclavines, rhabdopeptides, and xenocoumacins displayed strong nematicidal activity, inducing 82%, 90% and 85% mortality of M.javanica, respectively (Abebew et al. 2022). Similarly, rhabdopeptide from X. budapestensis inhibited the performance of M. incognita (Bi et al. 2018). Therefore, while some indication suggests that bacterial-specific specialized molecules can deter RKNs movement in the soil, further research is needed to evaluate this hypothesis, as well as to potentially identify broader molecular activity of such effects. Finally, concerning living EPNs, we observed that while the presence of living infective juveniles also tended to inhibit RKNs movement, their effect was generally weak. This might be because the number of inoculated EPNs was not high enough to exert significant negative effects, since it was previously shown that the suppressive effect of EPN IJs against population growth of RKNs is density dependent (Kepenekci et al. 2018).
In conclusion, we found that the position of EPNs in the experimental arenas impacted RKNs movement behavior toward host plants. EPNs added to the area close to the host plant root system exerted the highest suppressive effect against RKNs. These findings, therefore, indicate that EPNs could be used alone or as part of an Integrated Pest Management strategy as a novel management tool to control plant-parasitic nematodes attacking crop plants. From the perspective of field application of EPNs, it is further necessary to screen antagonistic characteristics of EPN strains based on the chemical signatures that are emitted by the insect cadaver or the bacterial symbiont so as to find promising EPN strains that can simultaneously control insect pests and RKNs in agricultural systems.
References
Abebew D, Sayedain FS, Bode E, Bode HB (2022) Uncovering nematicidal natural products from Xenorhabdus bacteria. J Agric Food Chem 70(2):498–506. https://doi.org/10.1021/acs.jafc.1c05454
Agrios GN (2005) Plant diseases caused by nematodes. In: Agrios GN (ed) Plant pathology, 5th edn. Academic, San Diego, pp 825–874
Ahmad G, Nishat Y, Ansari M, Khan A, Haris M, Khan AA (2021) Eco-friendly approaches for the alleviation of root-knot nematodes. In: Mohamed HI, El-Beltagi HEDS, Abd-Elsalam KA (eds) Plant growth-promoting microbes for sustainable biotic and abiotic stress management. Springer, Cham, pp 557–575
Ansari MA, Tirry L, Moens M (2003) Entomopathogenic nematodes and their symbiotic bacteria for the biological control of Hoplia philanthus (Coleoptera: Scarabaeidae). Biol Control 28(1):111–117. https://doi.org/10.1016/S1049-9644(03)00032-X
Barbary A, Djian-Caporalino C, Palloix A, Castagnone-Sereno P (2015) Host genetic resistance to root-knot nematodes, Meloidogyne spp., in Solanaceae: from genes to the field. Pest Manag Sci 71 (12):1591–1598. https://doi.org/10.1002/ps.4091
Bi YH, Gao CZ, Yu ZG (2018) Rhabdopeptides from Xenorhabdus budapestensis SN84 and their nematicidal activities against Meloidogyne incognita J Agric Food Chem 66:3833–3839. https://doi.org/10.1021/acs.jafc.8b00253
Caccia M, Lax P, Doucet ME (2013) Effect of entomopathogenic nematodes on the plant-parasitic nematode Nacobbus aberrans Biol Fertil Soils 49(1):105–109. https://doi.org/10.1007/s00374-012-0724-z
Caccia M, Marro N, Duenas JR, Doucet ME, Lax P (2018) Effect of the entomopathogenic nematode-bacterial symbiont complex on Meloidogyne hapla and Nacobbus aberrans in short-term greenhouse trials. Crop Prot 114:162–166. https://doi.org/10.1016/j.cropro.2018.07.016
Chen JX, Li QX, Song BA (2020) Chemical nematicides: recent research progress and outlook. J Agric Food Chem 68(44):12175–12188. https://doi.org/10.1021/acs.jafc.0c02871
Chitwood DJ, United States Department of Agriculture-Agricultural Research Service (2003) Research on plant-parasitic nematode biology conducted by the. Pest Manag Sci 59(6–7):748–753. https://doi.org/10.1002/ps.684
Damascena AP, Ferreira JCA, Costa MGS, de Araujo Junior LM, Wilcken SRS (2019) Hatching and mortality of Meloidogyne enterolobii under the interference of entomopathogenic nematodes in vitro. J Nematol 51:1–8. https://doi.org/10.21307/jofnem-2019-058
Divya K, Sankar M (2009) Entomopathogenic nematodes in pest management. Indian J Sci Technol 2(7):53–60. https://doi.org/10.17485/ijst/2009/v2i7/29499
Dutta TK, Powers SJ, Kerry BR, Gaur HS, Curtis RHC (2011) Comparison of host recognition, invasion, development and reproduction of Meloidogyne graminicola and M. incognita on rice and tomato. Nematology 13(5):509–520. https://doi.org/10.1163/138855410X528262
Elling AA (2013) Major emerging problems with minor Meloidogyne species. Phytopathology 103(11):1092–1102. https://doi.org/10.1094/PHYTO-01-13-0019-RVW
Felicitas EFA, Caoili BL, Latina RA (2021) Antagonistic effect of Steinernema abbasi and Heterorhabditis indica philippine isolates on root penetration and development of Meloidogyne incognita in tomato. Biocontrol Sci Technol 31(8):865–876. https://doi.org/10.1080/09583157.2021.1898541
Fox J, Weisberg S (2019) An R companion to applied regression, 3rd edn. Sage, Thousand Oaks
Grewal PS, Martin WR, Miller RW, Lewis EE (1997) Suppression of plant-parasitic nematode populations in turfgrass by application of entomopathogenic nematodes. Biocontrol Sci Technol 7(3):393–399. https://doi.org/10.1080/09583159730802
Grewal PS, Lewis EE, Venkatachari S (1999) Allelopathy a possible mechanism of suppression of plant-parasitic nematodes by entomopathogenic nematodes. Nematology 1(7–8):735–743. https://doi.org/10.1163/156854199508766
Guan TL, Shen JH, Fa Y, Su YS, Wang X, Li HM (2017) Resistance-breaking population of Meloidogyne incognita utilizes plant peroxidase to scavenge reactive oxygen species, thereby promoting parasitism on tomato carrying Mi-1 gene. Biochem Bioph Res Co 482(1):1–7. https://doi.org/10.1016/j.bbrc.2016.11.040
Helms AM, Ray S, Matulis NL, Kuzemchak MC, Grisales W, Tooker JF, Ali JG (2019) Chemical cues linked to risk: cues from below-ground natural enemies enhance plant defences and influence herbivore behavior and performance. Funct Ecol 33(5):798–808. https://doi.org/10.1111/1365-2435.13297
Jagdale GB, Grewal PS (2008) Influence of the entomopathogenic nematode Steinernema carpocapsae infected host cadavers or their extracts on the foliar nematode Aphelenchoides fragariae on Hosta in the greenhouse and laboratory. Biol Control 44(1):13–23. https://doi.org/10.1016/j.biocontrol.2007.07.001
Jagdale GB, Kamoun S, Grewal PS (2009) Entomopathogenic nematodes induce components of systemic resistance in plants biochemical and molecular evidence. Biol Control 51(1):102–109. https://doi.org/10.1016/j.biocontrol.2009.06.009
Jones JT, Haegeman A, Danchin EG, Gaur HS, Helder J, Jones MG, Kikuchi T, Manzanilla-Lopez R, Palomares-Rius JE, Wesemael WM, Perry RN (2013) Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol 14(9):946–961. https://doi.org/10.1111/mpp.12057
Kamali S, Javadmanesh A, Stelinski LL, Kyndt T, Seifi A, Cheniany M, Zaki-Aghl M, Hosseini M, Heydarpour M, Asili J, Karimi J (2022) Beneficial worm allies warn plants of parasite attack below-ground and reduce above-ground herbivore preference and performance. Mol Ecol 31(2):691–712. https://doi.org/10.1111/mec.16254
Kepenekci I, Hazir S, Lewis EE (2016) Evaluation of entomopathogenic nematodes and the supernatants of the in vitro culture medium of their mutualistic bacteria for the control of the root-knot nematodes Meloidogyne incognita and M. arenaria. Pest Manag Sci 72 (2):327–334. https://doi.org/10.1002/ps.3998
Kepenekci I, Hazir S, Oksal E, Lewis EE (2018) Application methods of Steinernema feltiae, Xenorhabdus bovienii and purpureocillium lilacinum to control root-knot nematodes in greenhouse tomato systems. Crop Prot 108:31–38. https://doi.org/10.1016/j.cropro.2018.02.009
Khanal C, Harshman D (2022) Evaluation of summer cover crops for host suitability of Meloidogyne enterolobii. Crop Prot 151. https://doi.org/10.1016/j.cropro.2021.105821
Leitao D, Pedrosa EMR, Dickson DW, Brito JA, Oliveira AKD, Rolim MM (2021) Upward migration of second-stage juveniles of Meloidogyne floridensis and M. incognita under different plant stimuli. Eur J Plant Pathol 161(2):301–311. https://doi.org/10.1007/s10658-021-02322-8
Lewis EE, Grewal PS, Sardanelli S (2001) Interactions between the Steinernema feltiae-Xenorhabdus bovienii insect pathogen complex and the root-knot nematode Meloidogyne incognita. Biol Control 21(1):55–62. https://doi.org/10.1006/bcon.2001.0918
Lewis EE, Grewal PS (2005) Interactions with plant parasitic nematodes. In: Grewal PS, Ehlers RU, Shapiro-Ilan D (eds) Nematodes as biocontrol agents. CABI, Wallingford, pp 349–362
Li CJ, Wang Y, Hua YF, Hua C, Wang CL (2015) Three dimensional study of wounded plant roots recruiting entomopathogenic nematodes with Pluronic gel as a medium. Biol Control 89:68–74. https://doi.org/10.1016/j.biocontrol.2015.05.007
Liu C, Grabau Z (2022) Meloidogyne incognita management using fumigant and non-fumigant nematicides on sweet potato. J Nematol 54(1):1–15. https://doi.org/10.2478/jofnem-2022-0026
Milligan SB, Bodeau J, Yaghoobi J, Kaloshian I, Zabel P, Williamson VM (1998) The root knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell 10(1):1307–1319. https://doi.org/10.1105/tpc.10.8.1307
Mitani DK, Kaya HK, Goodrich-Blair H (2004) Comparative study of the entomopathogenic nematode, Steinernema carpocapsae, reared on mutant and wild-type Xenorhabdus nematophila Biol Control 29(3):382–391. https://doi.org/10.1016/j.biocontrol.2003.07.005
Moens M, Perry RN, Starr JL (2009) Meloidogyne species-a diverse group of novel and important plant parasites. In: Perry RN, Moens M, Starr JL (eds) Root-knot nematodes. CAB International, Wallingford, pp 1–17
Molina JP, Dolinski C, Souza RM, Lewis EE (2007) Effect of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) on Meloidogyne mayaguensis Rammah and Hirschmann (Tylenchida: Meloidoginidae) infection in tomato plants. J Nematol 39(4):338–342. https://doi.org/10.1007/s00374-012-0724-z
Morris KA, Langston DB, Dutta B, Davis RF, Timper P, Noe JP, Dickson DW (2016) Evidence for a disease complex between Pythium aphanidermatum and root-knot nematodes in cucumber. Plant Health Prog 17(3):200–201. https://doi.org/10.1094/php-br-16-0036
Oka Y (2020) From old-generation to next-generation nematicides. Agronomy 10(9). https://doi.org/10.3390/agronomy10091387
Philbrick AN, Adhikari TB, Louws FJ, Gorny AM (2020) Meloidogyne enterolobii, a major threat to tomato production: current status and future prospects for its management. Front Plant Sci 11:1773. https://doi.org/10.3389/fpls.2020.606395
Poinar GO (1990) Taxonomy and biology of Steinernematidae and Heterorhabditidae. In: Gaugler R, Kaya HK (eds). Entomopathogenic nematodes in biological control. Boca Raton, Florida, pp 23–60
Ralmi NHAA, Khandaker MM, Mat N (2016) Occurrence and control of root knot nematode in crops: a review. Aust J Crop Sci 10(12):1649–1654. https://doi.org/10.21475/ajcs.2016.10.12.p7444
Rasmann S, Ali JG, Helder J, Putten WHvd (2012) Ecology and evolution of soil nematode chemotaxis. J Chem Ecol. https://doi.org/10.1007/s10886-012-0118-6
Rasmann S, Kollner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, Gershenzon J, Turlings TCJ (2005) Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434(7034):732–737. https://doi.org/10.1038/nature03451
Rasmann S, Turlings TC (2007) Simultaneous feeding by aboveground and belowground herbivores attenuates plant-mediated attraction of their respective natural enemies. Ecol Lett 10(10):926–936. https://doi.org/10.1111/j.1461-0248.2007.01084.x
Ruan WB, Shapiro-Ilan D, Lewis EE, Kaplan F, Alborn H, Gu XH, Schliekelman P (2018) Movement patterns in entomopathogenic nematodes: continuous vs. temporal. J Invertebr Pathol 151:137–143. https://doi.org/10.1016/j.jip.2017.11.010
Ruan WB, Zhan LL, Xiao W, Chen S (2012) An improved method for quantification of Heterodera glycines in plant tissues. Nematropica 42(2):237–244. https://doi.org/10.1007/s10725-005-5089-y
Sayedain FS, Ahmadzadeh M, Fattah-Hosseini S, Bode HB (2021) Soil application of entomopathogenic nematodes suppresses the root-knot nematode Meloidogyne javanica in cucumber. J Plant Dis Prot 128(1):215–223. https://doi.org/10.1007/s41348-020-00367-1
Searle SR, Speed FM, Milliken GA (1980) Population marginal means in the linear model: an alternative to least squares means. Am Stat 34(4):216–221. https://doi.org/10.1080/00031305.1980.10483031
Shapiro-Ilan D, Hazir S, Glazer I (2020) Advances in use of entomopathogenic nematodes in integrated pest management. In: Kogan M, Heinrichs EA (eds) Integrated management of insect pests: current and future developments. Burleigh Dodds Science Publication, Cambridge, pp 1–30
Shukla N, Yadav R, Kaur P, Rasmussen S, Goel S, Agarwal M, Jagannath A, Gupta R, Kumar A (2018) Transcriptome analysis of root-knot nematode (Meloidogyne incognita)-infected tomato (Solanum lycopersicum) roots reveals complex gene expression profiles and metabolic networks of both host and nematode during susceptible and resistance responses. Mol Plant Pathol 19(3):615–633. https://doi.org/10.1111/mpp.12547
Tonelli M, Penaflor MFGV, Leite LG, Silva WD, Martins F, Bento JMS (2016) Attraction of entomopathogenic nematodes to sugarcane root volatiles under herbivory by a sap-sucking insect. Chemoecology 26(2):59–66. https://doi.org/10.1007/s00049-016-0207-z
Trudgill DL, Blok VC (2001) Apomictic, polyphagous root-knot nematodes: exceptionally successful and damaging biotrophic root pathogens. Annu Rev Phytopathol 39:53–77. https://doi.org/10.1146/annurev.phyto.39.1.53
Tsai AYL, Iwamoto Y, Tsumuraya Y, Oota M, Konishi T, Ito S, Kotake T, Ishikawa H, Sawa S (2021) Root-knot nematode chemotaxis is positively regulated by l-galactose sidechains of mucilage carbohydrate rhamnogalacturonan-I. Sci Adv 7(27). https://doi.org/10.1126/sciadv.abh4182
Verdejo-Lucas S, Gomez P, Talavera M (2019) Pathogenicity of Meloidogyne incognita and M. javanica on recombinant inbred lines from a crossing of Cucurbita pepo subsp. pepo × C. pepo subsp. ovifera. Plant Pathol 68(6):1225–1232. https://doi.org/10.1111/ppa.13025
Wang CL, Lower S, Williamson VM (2009) Application of Pluronic gel to the study of root-knot nematode behaviour. Nematology 11:453–464. https://doi.org/10.1163/156854109x447024
White GF (1927) A method for obtaining infective nematode larvae from cultures. Science 66:302–303. https://doi.org/10.1126/science.66.1709.302-a
Zhalnina K, Louie KB, Hao Z, Mansoori N, da Rocha UN, Shi SJ, Cho HJ, Karaoz U, Loque D, Bowen BP, Firestone MK, Northen TR, Brodie EL (2018) Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat Microbiol 3(4):470–480. https://doi.org/10.1038/s41564-018-0129-3
Zhang X, Machado RA, Doan CV, Arce CC, Hu L, Robert CA (2019) Entomopathogenic nematodes increase predation success by inducing cadaver volatiles that attract healthy herbivores. eLife 8. https://doi.org/10.7554/eLife.46668
Zhen S, Li Y, Hou Y, Gu X, Zhang L, Ruan W, Shapiro-Ilan D (2018) Enhanced entomopathogenic nematode yield and fitness via addition of pulverized insect powder to solid media. J Nematol 50(4):495–506. https://doi.org/10.21307/jofnem-2018-050
Acknowledgements
This work was jointly supported by the financial support of National Science Foundation (32171637), National Key R&D Program of China (2017YFE013040 and 2019YFE0120400), Tianjin National Science Foundation (19JCZDJC34400) and Major Science and Technology Project of China National Tobacco Corporation (110202001034, LS-03), Fundamental Research Funds for the Central Public Welfare Research Institutes (TKS20220507).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Jan Jansa.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Li, Y., Wei, X. et al. Direct antagonistic effect of entomopathogenic nematodes and their symbiotic bacteria on root-knot nematodes migration toward tomato roots. Plant Soil 484, 441–455 (2023). https://doi.org/10.1007/s11104-022-05808-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05808-4