Abstract
Few systems have been described in which herbivore-induced root volatiles mediate attraction of entomopathogenic nematodes (EPNs), and they only concern root damage inflicted by chewing insects. EPNs, especially Heterorhabditis indica and Steinernema carpocapsae, are potential biological control agents of sugarcane spittlebug (Mahanarva fimbriolata) populations. Here, we investigated the response of these two species of EPNs to sugarcane root volatiles damaged by M. fimbriolata nymphs in a belowground six-arm olfactometer. We also examined changes on root volatile profile in response to herbivory of sugarcane spittlebug nymphs. Results showed that both EPN species did not discriminate between odors of undamaged sugarcane and moistened sand (blank). However, when EPNs were exposed to odors of spittlebug-damaged and undamaged sugarcane roots, both species significantly preferred odors of spittlebug-damaged roots. Headspace collection followed by GC–MS analyses showed no qualitative difference (total of 11 compounds) between volatile profiles of spittlebug-damaged and undamaged sugarcane roots. In contrast to the previous studies involving feeding by root chewing insects, our root volatile analysis did not reveal any up-regulation resulting from sugarcane spittlebug damage, but the down-regulation of the terpenes dihydromyrcenol and β-isomethyl ionone when compared with the profile of undamaged sugarcane roots. Here, we propose alternative explanations for the EPN attraction to spittlebug-damaged roots as it is unlikely that reduced concentrations of the volatiles play a role in this interaction. Further studies are necessary to determine the key compounds of the root volatile emission to enhance biological control efficacy with EPNs against M. fimbriolata in sugarcane.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants defend themselves from herbivory by a broad spectrum of physical and chemical defenses that act directly on insect herbivores, or indirectly by emitting volatiles exploited by natural enemies as cues to locate their host/prey (Turlings et al. 1990; Dicke 1994; Turlings and Wäkers 2004). Attraction of parasitoids and predators to herbivore-induced plant volatiles (HIPVs) under attack of several feeding guilds of insect (e.g. chewing, sucking, mining, galling) has been intensively studied (Turlings et al. 1997; Van Poecke et al. 2001; Birkett et al. 2003; Zhu and Park 2005; Girling et al. 2006; Mumm and Hilker 2006; Tooker and Hanks 2006; De Boer et al. 2008). As a result, HIPVs have been regarded as a widespread defense mechanism in the plant kingdom, but their defensive role has been discussed as they also mediate interactions with other community members, attracting or repelling herbivore species (Bernasconi et al. 1998; Landolt et al. 1999; De Moraes et al. 2001; Carroll et al. 2006; Kaplan 2012).
Root tissue is also susceptible to herbivore attack by soil insects, which can compromise the uptake of nutrients and water. In a similar way to aboveground tritrophic interactions, roots under attack of soil herbivores emit a different volatile blend, which is exploited by soil-dwelling natural enemies, such as entomopathogenic nematodes (EPNs) (Rasmann et al. 2005), in host finding. These organisms penetrate into the insect, kill it with the aid of symbiotic bacteria, and feed on the proliferating bacteria as well as the decomposing insect cadaver (Strauch and Ehlers 1998).
In contrast to the vast knowledge on aboveground plant volatile-based interactions (reviewed by Mumm and Dicke 2010; Dicke and Baldwin 2010; Hare 2011), few systems have been described in which herbivore-induced root volatiles mediate the attraction of EPNs and little is known about the nature of these interactions. Recruitment of EPNs to plant root volatiles has been demonstrated in different plant species including crop plants, such as strawberry (Boff et al. 2002), corn (Rasmann et al. 2005), cabbage (Ferry et al. 2007), cotton (Rasmann and Turlings 2008) and citrus (Ali et al. 2010), and non-crop species, such as the conifer Thuja occidentalis L. (Van Tol et al. 2001) and common milkweed Asclepias syriaca L. (Rasmann et al. 2011). However, these studies focused on the root damage inflicted only by beetle larvae, which are chewing insects.
Physical injury inflicted by sap-sucking insects is almost imperceptible, unlike chewing insects that rapidly remove plant tissue and usually cause damage faster. Understanding of aboveground plant defenses indicates that plant recognition of herbivory is due not only to physical damage, but also to contact with insect-derived signals, the herbivore-associated molecular patterns (HAMPs) (Mithöfer and Boland 2008). As a result, sap sucking differently activates signal transduction pathways dependent on jasmonic acid (JA), salicylic acid (SA), and ethylene (ET) compared to chewing insect damage (Walling 2000; Thaler et al. 2012; Lu et al. 2014). The balance of these three main phytohormones along with others will determine the expression of appropriate and specific defenses against herbivores (Erb et al. 2012b). Therefore, plant volatile composition is usually distinct under attack of chewing or sap-sucking insects (Leitner et al. 2005; Gosset et al. 2009), resulting in different responses of community members (Van Poecke et al. 2003).
So far, no studies have examined induced root response under attack by soil-dwelling insects that pierce and suck phloem and xylem content, such as some species of aphids, leaf hoppers, stink bugs, and scales (Khan and Saxena 1984; Powel and Hardie 2002). Besides, according to the differences between the two plant parts (Erb et al. 2012a) and the fact that HIPV emission is tissue specific (Köllner et al. 2008), we cannot speculate about the mechanisms of recognition and response in roots based on the knowledge of aboveground-induced defenses.
Populations of the sugarcane spittlebug Mahanarva fimbriolata (Stål) (Hemiptera: Cercopidae) have greatly increased and become a serious pest in Brazilian sugarcane crops after prohibiting sugarcane harvest with the use of burning. Sugarcane spittlebug adults feed on and inject toxins into leaves, whereas nymphs live in the soil and specially suck the xylem content in the roots, blocking the transport of water and nutrients and causing a physiological disorder (Dinardo-Miranda et al. 2004; Garcia et al. 2007).
Since sugarcane is a perennial crop, not often perturbed, the use of EPNs to control the sugarcane spittlebug can be a potential management strategy (Southwood and Comins 1976). Particular strains of Heterorhabditis indica (Poinar, Jackson and Klein) (Rhabditida: Heterorhabditidae) and Steinernema carpocapsae (Weiser) (Rhabditida: Steinernematidae) have been selected as efficient biological control agents of sugarcane spittlebug populations (Leite et al. 2005). These species exhibit different foraging strategy: H. indica actively seeks out a host by crawling in the soil (cruiser), while S. carpocapsae is less active and usually waits for hosts passing by (ambusher) (Lewis 2002; Campbell et al. 2003; Lewis et al. 2006), although it can also behave like a cruiser (Wilson et al. 2012).
Here, we investigate the response of the two species of EPNs H. indica and S. carpocapsae of the same strain studied in Leite et al. (2005) to sugarcane root volatiles damaged by M. fimbriolata nymphs. We also examine changes in root volatile profile in response to sugarcane spittlebug herbivory. Knowledge on the cues used by herbivore natural enemies, such as EPNs, is of great relevance to develop integrated pest management techniques to enhance the biological control efficacy of soil-dwelling insects (Degenhardt et al. 2009; Hiltpold et al. 2010; Ali et al. 2012; Hiltpold and Turlings 2012).
Materials and methods
Plants
Sugarcane plants (Saccharum officinarum L. cultivar ‘SP 80-1842’) were grown in pots (200 ml) containing organic substrate (Golden-Mix®) and fertilizer (Osmocote®, 14-14-14 N-P-K) in an insect-free greenhouse from summer to autumn under natural light conditions (Piracicaba, SP, Brazil). All plants used in bioassays had four opened leaves and were approximately 25 to 30 days old.
Insects rearing
Nymphs of M. fimbriolata were collected in sugarcane crops in Piracicaba, SP, Brazil (22° 43′ 14″ to 22° 42′ 01″ S and 47° 38′ 46″ to 47° 36′ 49″ W) and reared on sugarcane plants under controlled conditions (25 ± 0.5 °C, 70 % RH, 14L:10D) (Garcia et al. 2007) for two to four generations. Briefly, M. fimbriolata adults were kept in cylindrical plastic cages containing sugarcane plants with wet cotton wool disks covering the soil, which served as a substrate for oviposition. After washing the cotton in running water over sieves, eggs were collected and maintained in Petri dishes on wet filter paper until hatching. Newly hatched nymphs were transferred to sugarcane roots and fed for 40 days until becoming adults.
Nematodes
Populations of EPNs H. indica and S. carpocapsae were obtained from the Laboratory of Biological Control (Instituto Biológico, Campinas, SP, Brazil) and multiplied in the greater wax moth Galleria mellonella (L.) at 25 ± 0.5 °C, according to the method described in Kaya and Stock (1997). Infective juveniles (IJs) emerging from caterpillars in White traps (White 1927) were collected in distilled water and transferred to pots (1 L) at volumes just enough to cover the bottom (shallow). The IJs were then stored at 15 °C for 4–6 days prior to use.
Olfactometer assays
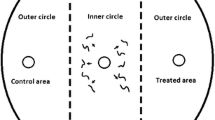
Nematode preference of both H. indica and S. carpocapsae toward odors emitted by damaged sugarcane roots (five fourth-to-fifth-instar spittlebug nymphs feeding on roots for 24 h and then removed just before assays) and undamaged sugarcane roots was assessed in a six-arm olfactometer, consisting of a central glass chamber (10 cm diameter, 8 cm depth) with six arms connected to side chambers (5 cm depth, 3 cm diameter) (Rasmann et al. 2005). Four days before bioassays, sugarcane plants were transferred to the olfactometer side chambers, and the remaining space was filled with a mixture of sterile sand and rock (2:1) and moistened with 10 % water (dry sand:water; g/g). Each side chamber containing wet soil and plants was weighed before being transferred to the greenhouse. To maintain the moisture content at approximately 10 % during the 4 days before the bioassay, the chambers were weighed daily and an appropriate volume of water was added to return it to its initial weight (Santos et al. 2014). Positions of chambers containing damaged sugarcane roots were interchanged with undamaged roots to avoid side bias. About 10,000 IJs were released into the central chamber, where they could freely choose among the chambers for over 24 h. After that, the olfactometer was disassembled, sand was collected from each detachable glass tube connecting the side chambers to the central arena, and nematodes were recovered in water by a modified Baermann funnel technique (Viglierchio and Schmitt 1983). After 24 h, 0.30 ml of water was collected to estimate the amounts of IJs using a McMaster counting slide. A total of eight replicates with more than 2000 recovered IJs were considered. Five and four replicates were discarded in bioassays with S. carpocapsae and H. indica, respectively.
Headspace collection and analysis
Roots of nine undamaged and damaged sugarcane by M. fimbriolata nymphs were harvested and flash frozen in N2(1) and stored at −30 °C prior to headspace collection (Rasmann et al. 2005; Rasmann and Turlings 2008). To collect root volatiles, 2 g of frozen roots was ground into a powder in liquid nitrogen and transferred to 500-ml tightly closed chambers with two exits: one connected to a glass column filled with 50 mg of adsorbent polymer (Hayesep-Q, 80/100 Mesh, Alltech Assoc.) and the other to a charcoal filter. A vacuum pump was connected to the adsorbent polymer column, creating an air flow for 8 h. Subsequently, columns were eluted with 300 µl of dichloromethane and concentrated under clean nitrogen air flow to 50 µl. Each sample received 10 µL of nonyl acetate (internal standard solution at 100 ng/µL). A 2-µl aliquot of each sample was injected into a gas chromatograph coupled to mass spectrometer (GC–MS, Varian 4000) equipped with HP5-MS capillary column (JeW Scientific, Folsom, CA; 30 m × 0.25 mm × 0.25 µm), using helium as the carrier gas. The column temperature was held at 40 °C for 5 min, increased to 150 °C (5 °C/min) and held for one min, and then raised (5 °C/min) until reaching a final temperature of 250 °C. Compound identifications were acquired by comparing the obtained mass spectra retention times with those of the NIST 98 library and authentic standards (Sigma-Aldrich, St. Louis, MO, USA), when available, as well as the Kovats index (KI) using n-alkane (C7–C30) standards (Table S1). Quantification was estimated based on the peak area relative to the amount of internal standard and corrected by fresh root tissue used for the volatile collection.
Statistical analysis
The normality and homogeneity of the number of nematodes recorded in the six-arm olfactometer assay and the relative amounts of each root volatile released by damaged or undamaged sugarcane roots were analyzed by Shapiro–Wilk and Bartlett tests. We adopted a general log-linear model (glm) (P < 0.05) and quasi-Poisson distribution for analyzing nematode choice assessed in olfactometer bioassay by one-way ANOVA. Relative amounts of volatiles were log- transformed, when required, to attend parametric assumptions. The composition of the volatile blend was analyzed by MANOVA (P < 0.05) and individual compounds were analyzed using Student’s test (P < 0.05) when data were normal, whereas non-normal data were analyzed by Welch test (P < 0.05). All analyses were performed using software package R 3.1.0 (R Development Core Team 2012).
Results
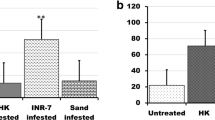
Infective juveniles of both EPN species equally chose undamaged sugarcane roots and blank (only moistened sand) (Fig. 1, glm, N = 8; S. carpocapsae: F (1, 14) = 0.08, P = 0.776; H. indica: F (1, 14) = 0.02, P = 0.882). However, they preferentially chose volatiles emitted by spittlebug-damaged over undamaged sugarcane roots (Fig. 1, glm, N = 8; S. carpocapsae: F (1, 14) = 6.29, P < 0.05; H. indica: F (1, 14) = 9.21, P < 0.01).
Response of entomopathogenic nematodes to sugarcane root volatiles released by plants damaged by Mahanarva fimbriolata, undamaged, or only moistened sand (Blank) in a six-arm olfactometer. a Steinernema carpocapsae. b Heterorhabditis indica. Pie charts in the right side of the figure show proportions of responsive (choice) and non-responsive (no choice) nematodes in the assay. Asterisks indicate significant difference between treatments according to one-way ANOVA (quasi-Poisson glm, *P < 0.05 and **P < 0.01)
Root volatile collection and analysis revealed that undamaged and spittlebug-damaged sugarcane roots emitted blends with similar composition (Fig. 2, MANOVA, N = 9, P = 0.39). Nevertheless, when compounds were examined individually, we found differences in amounts of dihydromyrcenol and β-isomethyl ionone, which were emitted at higher concentrations by undamaged sugarcane roots relative to spittlebug-damaged sugarcane root blend (Fig. 2, One-way ANOVA, N = 9; dihydromyrcenol: Welch’s test, t = 2.46, P = 0.036; β-isomethyl ionone: Student’s test, t = 2.26, P = 0.037).
Discussion
Our study confirms the previous findings showing that EPNs, irrespective of the foraging strategy (ambusher or cruiser), use herbivore-damaged root volatiles in host finding (Van Tol et al. 2001; Rasmann et al. 2005; Ali et al. 2010, 2011; Hiltpold et al. 2010). The most studied systems in the literature, corn and citrus, show that root feeding by beetle larvae mainly triggers the emission of terpenes, (E)-β-caryophyllene, and pregeijerene, which mediate the attraction of EPNs (Rasmann et al. 2005; Ali et al. 2011, 2012). In contrast to these studies, our root volatile analysis did not reveal any up-regulation resulting from sugarcane spittlebug damage, but the down-regulation of the terpenes dihydromyrcenol and β-isomethyl ionone when compared to the profile of undamaged sugarcane roots.
To collect sugarcane root volatiles, we performed a headspace collection with flash-frozen and ground root tissues, a method adapted from some classical studies in the literature (Rasmann et al. 2005; Rasmann and Turlings 2008). Ideally, root headspace collection would be performed without excising and grinding the roots and some researchers have developed such a method (Ali et al. 2011; Hiltpold et al. 2011; Eilers et al. 2015; Rostás et al. 2015). We tried collecting root headspace without grinding the tissue, but only collected detectable amounts in the GC–MS analysis using ground root tissue. Although our data did not likely reveal the exact belowground volatile emission in sugarcane since we collected volatiles from ruptured root cells, the method allowed us to compare the blend composition between the spittlebug-damaged and undamaged sugarcane roots. We found 11 volatile metabolites in the blends of both treatments. Besides dihydromyrcenol and β-isomethyl ionone, which were significantly reduced in the spittlebug-damaged sugarcane, we observed a trend of reduced levels of the terpenes linalool and geranylacetone, and the ester isoamyl benzoate in the spittlebug-damaged compared with undamaged roots (Fig. 2).
Feeding by hemipterans includes the secretion of salivary proteins that interact in the dynamics of induced plant defense (Sharma et al. 2014). Although attack by hemipterans on aerial plant parts often induces the emission of HIPVs (Du et al. 1998; Ninkovic et al. 2001; Birkett et al. 2003; Williams et al. 2005; Oluwafemi et al. 2011), some of the salivary components are involved in the strategies of manipulating plant defenses (Walling 2008; Felton et al. 2014). In some particular cases, feeding by sucking insects can either suppress or simply not elicit emission of HIPVs (Turlings et al. 1997; Schwartzberg et al. 2011). For example, the infestation of Philaenus spumarius (L.) (Hemiptera: Cercopidae), a spittlebug closely related to M. fimbriolata, does not elicit any volatile response in the late goldenrod (Solidago altissima L.) (Tooker et al. 2008), while herbivory by the tobacco budworm Heliothis virescens Fabricius (Lepidoptera: Noctuidae) does. The underlying mechanism for not eliciting or suppressing induced volatile response, as in the example of the late goldenrod and our study system, can result from the activation of the SA-signaling pathway, which can interact negatively with the JA-signaling pathway (Zhang et al. 2009; Ali and Agrawal 2014). Despite the suppressing effect on JA-related defenses, activation of SA-signaling by sap-sucking herbivory can induce the release of specific herbivore-induced plant volatiles, which are exploited by natural enemies (Zhang et al. 2013).
Sugarcane spittlebug nymphs seem to be a prey that can be easily found by natural enemies as they stay in the same spot sucking the xylem and phloem content for 30–40 days (Garcia et al. 2007). One would expect that lower volatile emission caused by sugarcane spittlebug feeding on sugarcane roots would be an adaptive strategy to becoming less detectable, therefore reducing chances of being found by natural enemies. Nonetheless, this hypothesis was not supported by our behavioral assays, which demonstrated that EPNs oriented themselves toward spittlebug-damaged over undamaged roots, despite the reduced emission of some components (Figs. 1 and 2).
EPNs possibly do not exploit reduction of the two volatiles, especially because they did not closely evolve with sugarcane plants. Therefore, we propose two alternative explanations, not mutually exclusive: (i) EPNs are guided by increased concentrations of volatiles emitted from spittlebug-damaged sugarcane roots; however, up-regulation of specific compounds was not detected by the root volatile collection method adopted here; (ii) EPNs are attracted to spittlebug-damaged sugarcane roots because of a synergistic effect between root volatiles and increased carbon dioxide (CO2) concentration in a similar way to what has been shown for the attraction of Heterorhabditis megidis (Poinar, Jackson and Klein) to (E)-β-caryophyllene and dimethyl disulfide (Turlings et al. 2012). Besides, we cannot discard the hypothesis that EPNs could have been attracted to residual odors left by spittlebug nymphs in damaged sugarcane roots, such as cues derived from the foam. Although we removed insects and foam, some could have been absorbed by the sand.
To the best of our knowledge, this is the first study that focused on belowground plant indirect defenses induced by sucking insects. Studying soil-dwelling insects is challenging and is one of the main reasons why belowground plant defenses have not been explored in detail as the aboveground environment. Unlike some EPN strains that do not respond to increased amounts of root volatiles (Anbesse and Ehlers 2013; Laznik and Trdan 2013), results show that our strains of H. indica and S. carpocapsae exploit changes on root volatile emission induced by spittlebug nymph feeding in host finding. However, it is unlikely that reduced concentrations of the terpenes dihydromyrcenol and β-isomethyl ionone in the blend potentially play a role on the discrimination of EPNs between spittlebug-damaged and undamaged sugarcane roots. As in any other study, it is possible that volatile compounds at undetectable amounts for the GC–MS analysis are exploited by EPNs. In contrast to previous studies involving feeding by root chewing insects (Rasmann et al. 2005; Ali et al. 2010), determining the key compound of the root volatile emission used as cues by EPNs to find a sucking insect, the sugarcane spittlebug, is a more complex task. As a result, further study is necessary before exploring root volatile emission to enhance biological control efficacy with EPNs against the spittlebug M. fimbriolata in sugarcane.
References
Ali JG, Agrawal AA (2014) Asymmetry of plant-mediated interactions between specialist aphids and caterpillars on two milkweeds. Funct Ecol 28:1404–1412. doi:10.1111/1365-2435.12271
Ali JG, Alborn HT, Stelinski LL (2010) Subterranean herbivore-induced volatiles released by citrus roots upon feeding by Diaprepes abbreviatus recruit entomopathogenic nematodes. J Chem Ecol 36:361–368. doi:10.1007/s10886-010-9773-7
Ali JG, Alborn HT, Stelinski LL (2011) Constitutive and induced subterranean plant volatiles attract both entomopathogenic and plant parasitic nematodes. J Ecol 99:26–35. doi:10.1111/j.1365-2745.2010.01758.x
Ali JG, Alborn HT, Campos-Herrera R, Kaplan F, Duncan LW, Rodriguez-Soana C, Koppenhöfer AM, Steilinski L (2012) Subterranean, herbivore-induced plant volatile increases biological control activity of multiple beneficial nematode species in distinct habitats. PLoS One 7(6):e38146. doi:10.1371/journal.pone.0038146
Anbesse S, Ehlers RU (2013) Heterorhabditis sp. not attracted to synthetic (E)-β-caryophyllene, a volatile emitted by roots upon feeding by corn rootworm. J Appl Entomol 137:88–96. doi:10.1111/j.1439-0418.2012.01753.x
Bernasconi ML, Turlings TCJ, Ambrosetti L, Bassetti P, Dorn S (1998) Herbivore-induced emissions of maize volatiles repel the corn-leaf aphid, Rhopalosiphum maidis. Entomol Exp Appl 87:133–142. doi:10.1046/j.1570-7458.1998.00315.x
Birkett MA, Chamberlain K, Guerrieri E, Pickett JA, Wadhams LJ, Yasuda T (2003) Volatiles from whitefly-infested plants elicit a host-locating response in the parasitoid, Encarsia formosa. J Chem Ecol 29:1589–1600. doi:10.1023/A:1024218729423
Boff MIC, Van Tol RHWM, Smits PH (2002) Behavioural response of Heterorhabditis megidis towards plant roots and insect larvae. Biocontrol 47:67–83. doi:10.1023/A:1014435627268
Campbell JF, Lewis EE, Stock SP, Nadler S, Kaya HK (2003) Evolution of host search strategies in entomopathogenic nematodes. J Nematol 35:142–145
Carroll MJ, Schmelz EA, Meagher RL, Teal PE (2006) Attraction of Spodoptera frugiperda larvae to volatiles from herbivore-damaged maize seedlings. J Chem Ecol 32:1911–1924. doi:10.1007/s10886-006-9117-9
De Boer JG, Hordijk CA, Posthumus MA, Dicke M (2008) Prey and non-prey arthropods sharing a host plant: effects on induced volatile emission and predator attraction. J Chem Ecol 34:281–290. doi:10.1007/s10886-007-9405-z
De Moraes CM, Mescher MC, Tumlinson JH (2001) Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410:577–580. doi:10.1038/35069058
Degenhardt J, Hiltpold I, Kölner TG, Frey M, Gierl A, Gershenzon J, Hibbard BE, Ellersieck MR, Turlings TCJ (2009) Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. Proc Natl Acad Sci USA 106:13213–13218. doi:10.1073/pnas.0906365106
Dicke M (1994) Local and systemic production of volatile herbivore induced terpenoides: their role in plant-carnivore mutualism. J Plant Physiol 143:465–472. doi:10.1016/S0176-1617(11)81808-0
Dicke M, Baldwin IT (2010) The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci 15:167–175. doi:10.1016/j.tplants.2009.12.002
Dinardo-Miranda LL, Vasconcelos ACM, Ferreira JMG, Garcia CA, Coelho AL, Gil MA (2004) Efficiency of Metarhizium anisopliae (Metsch) on sugarcane root froghopper Mahanarva fimbriolata (Stal) (Hemiptera: cercopidae). Neotrop Entomol 33:743–749. doi:10.1590/S1519-566X2004000600012
Du Y, Poppy GM, Powell W, Pickett JA, Wadhams LJ, Woodcock CM (1998) Identification of semiochemicals released during aphid feeding that attract parasitoid Aphidius ervi. J Chem Ecol 24:1355–1368. doi:10.1023/A:1021278816970
Eilers EJ, Pauls G, Rillig MC, Hansson BS, Hilker M, Reinecke A (2015) Novel set-up for low-disturbance sampling of volatile and non-volatile compounds from plant roots. J Chem Ecol 41:253–266. doi:10.1007/s10886-015-0559-9
Erb M, Glauser G, Robert CA (2012a) Induced immunity against belowground insect herbivores-activation of defenses in the absence of a jasmonate burst. J Chem Ecol 38:629–640. doi:10.1007/s10886-012-0107-9
Erb M, Meldau S, Howe GA (2012b) Role of phytohormones in insect-specific plant reactions. Trends Plant Sci 17:250–259. doi:10.1016/j.tplants.2012.01.003
Felton GW, Chung SH, Hernandez MGE, Louis J, Peiffer M, Tian D (2014) Herbivore oral secretions are the first line of protection against plant-induced defences. Annu Plant Rev 47:37–76. doi:10.1002/9781118829783.ch2
Ferry A, Dugravot S, Delattre T, Christides JP, Auger J, Bagnères AG, Poinsot D, Cortesero AM (2007) Identification of a widespread monomolecular odor differentially attractive to several Delia radicum ground-dwelling predators in the field. J Chem Ecol 33:2064–2077. doi:10.1007/s10886-007-9373-3
Garcia JF, Botelho PSM, Parra JRP (2007) Laboratory rearing technique of Mahanarva fimbriolata (Stål) (Hemiptera: cercopidae). Sci Agric 64:73–76. doi:10.1590/S0103-90162007000100011
Girling RD, Hassall M, Turner JG, Poppy GM (2006) Behavioural responses of the aphid parasitoid Diaeretiella rapae to volatiles from Arabidopsis thaliana induced by Myzus persicae. Entomol Exp Appl 120:1–9. doi:10.1111/j.1570-7458.2006.00423.x
Gosset V, Harmel N, Göbel C, Francis F, Haubruge E, Wathelet JP, du Jardin P, Feussner I, Fauconnier ML (2009) Attacks by a piercing-sucking insect (Myzus persicae Sultzer) or a chewing insect (Leptinotarsa decemlineata Say) on potato plants (Solanum tuberosum L.) induce differential changes in volatile compound release and oxylipin synthesis. J Exp Bot 60:1231–1240. doi:10.1093/jxb/erp015
Hare JD (2011) Ecological role of volatiles produced by plants in response to damage by herbivorous insects. Annu Rev Entomol 56:161–180. doi:10.1146/annurev-ento-120709-144753
Hiltpold I, Turlings TCJ (2012) Manipulation of chemically mediated interactions in agricultural soils to enhance the control of crops pests. J Chem Ecol 38:641–650. doi:10.1007/s10886-012-0131-9
Hiltpold I, Toepfer S, Kuhlmann U, Turlings TCJ (2010) How maize root volatiles affect the efficacy of entomopathogenic nematodes in controlling the western corn rootworm? Chemoecology 20:155–162. doi:10.1007/s00049-009-0034-6
Hiltpold I, Erb M, Robert CAM, Turlings TCJ (2011) Systemic root signalling in a belowground, volatile-mediated tritrophic interaction. Plant Cell Environ 34:1267–1275. doi:10.1111/j.1365-3040.2011.02327.x
Kaplan I (2012) Attracting carnivorous arthropods with plant volatiles: the future of biocontrol or playing with fire? Biol Control 60:77–89. doi:10.1016/j.biocontrol.2011.10.017
Kaya HK, Stock SP (1997) Techniques in insect nematology. In: Lacey LA (ed) Manual of Techniques in Insect Pathology. Elsiever, London, pp 281–324
Khan ZR, Saxena RC (1984) Electronically recorded waveforms associated with the feeding behavior of Sogatella furcifera (Homoptera: delphacidae) on susceptible and resistant rice varieties. J Econ Entomol 6:1479–1482. doi:10.1093/jee/77.6.1479
Köllner TG, Held M, Lenk C, Hiltpold I, Turlings TCJ, Gershenzon J, Degenhardt J (2008) A maize (E)-β -caryophyllene synthase implicated in indirect defense response against herbivores is not expressed in most American maize. Plant Cell 20:482–494. doi:10.1105/tpc.107.051672
Landolt PJ, Tumlinson JH, Alborn DH (1999) Attraction of Colorado potato beetle (Coleoptera: Chrysomelidae) to damaged and chemically induced potato plants. Environ Entomol 28:973–978. doi:10.1093/ee/28.6.973
Laznik Ž, Trdan S (2013) An investigation on the chemotactic responses of different entomopathogenic nematode strains to mechanically damaged maize root volatile compounds. Exp Parasitol 134:349–355. doi:10.1016/j.exppara.2013.03.030
Leite LG, Machado LA, Goulart RM, Tavares FM, Batista Filho LA (2005) Screening of entomopathogenic nematodes (Nemata: Rhabditida) and the efficiency of Heterorhabditis sp. against the sugar cane root spittlebug Mahanarva fimbriolata (Stål) (Hemiptera: Cercopidae). Neotrop Entomol 34:785–790. doi:10.1590/S1519-566X2005000500010
Leitner M, Boland W, Mithöfer A (2005) Direct and indirect defences induced by piercing-sucking and chewing herbivores in Medicago truncatula. New Phytol 167:597–606. doi:10.1111/j.1469-8137.2005.01426.x
Lewis EE (2002) Behavioral ecology. In: Gaugler R (ed) Entomopathogenic Nematology. CAB International, New York, pp 205–223
Lewis EE, Campbell JF, Griffin C, Kaya HK, Peters A (2006) Behavioral ecology of entomopathogenic nematodes. Biol Control 38:66–79. doi:10.1016/j.biocontrol.2005.11.007
Lu J, Li J, Ju H, Liu X, Erb M, Wang X, Lou Y (2014) Contrasting effects of ethylene biosynthesis on induced plant resistance against a chewing and a piercing-sucking herbivore in rice. Mol Plant 7:1670–1682. doi:10.1093/mp/ssu085
Mithöfer A, Boland W (2008) Recognition of herbivory-associated molecular patterns. Plant Physiol 146:825–831. doi:10.1104/pp.107.113118
Mumm R, Dicke M (2010) Variation in natural plant products and the attraction of bodyguards involved in indirect plant defense. Can J Zool 88:628–667. doi:10.1139/Z10-032
Mumm R, Hilker M (2006) Direct and indirect chemical defence of pine against folivorous insects. Trends Plant Sci 11:351–358. doi:10.1016/j.tplants.2006.05.007
Ninkovic V, Al Abassi S, Pettersson J (2001) The influence of aphid-induced plant volatiles on ladybird beetle searching behavior. Biol Control 21:191–195. doi:10.1006/bcon.2001.0935
Oluwafemi S, Bruce TJ, Pickett JA, Ton J, Birkett MA (2011) Behavioral responses of the leafhopper, Cicadulina storeyi China, a major vector of maize streak virus, to volatile cues from intact and leafhopper-damaged maize. J Chem Ecol 37:40–48. doi:10.1007/s10886-010-9891-2
Powel G, Hardie J (2002) Xylem ingestion by winged aphids. In: Proceedings of the 11th international symposium on insect-plant relationships 57:103–108. doi: 10.1007/978-94-017-2776-1_12
R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/
Rasmann S, Turlings TCJ (2008) First insights into specificity of belowground tritrophic interactions. Oikos 117:362–369. doi:10.1111/j.2007.0030-1299.16204.x
Rasmann S, Köllner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, Gershenzon J, Turlings TCJ (2005) Recruitment of entomopathogenic nematodes by insect damaged maize root. Nature 434:732–737. doi:10.1038/nature03451
Rasmann S, Erwin AC, Halitschke R, Agrawal AA (2011) Direct and indirect root defences of milkweed (Asclepias syriaca): trophic cascades, trade-offs and novel methods for studying subterranean herbivory. J Ecol 99:16–25. doi:10.1111/j.1365-2745.2010.01713.x
Rostás M, Cripps MG, Silcock P (2015) Aboveground endophyte affects root volatile emission and host plant selection of a belowground insect. Oecologia 177:487–497. doi:10.1007/s00442-014-3104-6
Santos F, Peñaflor MFGV, Paré PW, Sanches PA, Kamiya AC, Tonelli M, Nardi C, Bento JMS (2014) A novel interaction between plant-beneficial rhizobacteria and roots: colonization induces corn resistance against the root herbivore Diabrotica speciosa. PLoS One 9(11):e113280. doi:10.1371/journal.pone.0113280
Schwartzberg EG, Böröczky K, Tumlinson JH (2011) Pea aphids, Acyrthosiphon pisum, suppress induced plant volatiles in broad bean, Vicia Faba. J Chem Ecol 37:1055–1062. doi:10.1007/s10886-011-0006-5
Sharma A, Khan AN, Subrahmanyam S, Raman A, Taylor GS, Fletcher MJ (2014) Salivary proteins of plant-feeding hemipteroids-implication in phytophagy. B Entomol Res 104:117–136. doi:10.1017/S000748531300061
Southwood TRE, Comins HN (1976) A synoptic population model. J Anim Ecol 45:949–965. doi:10.2307/3591
Strauch O, Ehlers RU (1998) Food signal production of Photorhabdus luminescens inducing the recovery of entomopathogenic nematodes Heterorhabditis spp. in liquid culture. Appl Microbiol Biotech 50:369–374. doi:10.1007/s002530051306
Thaler JS, Humphrey PT, Whiteman NK (2012) Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci 17:260–270. doi:10.1016/j.tplants.2012.02.010
Tooker JF, Hanks LM (2006) Tritrophic interactions and reproductive fitness of the prairie perennial Silphium laciniatum Gillette (Asteraceae). Environ Entomol 35:537–545. doi:10.1603/0046-225X-35.2.537
Tooker JF, Rohr JR, Abrahamson WG, De Moraes CM (2008) Gall insects can avoid and alter indirect plant defenses. New Phytol 178:657–671. doi:10.1111/j.1469-8137.2008.02392.x
Turlings TCJ, Wäkers F (2004) Recruitment of predator and parasitoids by herbivore-injured plants. In: Cardé RT, Millar JG (eds) Advances in insect chemical ecology. Cambridge University Press, Cambridge, pp 21–74
Turlings TCJ, Tumlinson JH, Lewis WJ (1990) Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250:1251–1253. doi:10.1126/science.250.4985.1251
Turlings TCJ, Bernasconi M, Bertossa R, Bigler F, Caloz G, Dorn S (1997) The induction of volatile emissions in maize by three herbivore species with different feeding habits: possible consequences for their natural enemies. Biol Control 11:122–129. doi:10.1006/bcon.1997.0591
Turlings TCJ, Hiltpold I, Rasmann S (2012) The importance of root-produced volatiles as foraging cues for entomopathogenic nematodes. Plant Soil 358:51–60. doi:10.1007/s11104-012-1295-3
Van Poecke RMP, Posthumus MA, Dicke M (2001) Herbivore-induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid Cotesia rubecula: chemical, behavioral, and gene-expression analysis. J Chem Ecol 27:1911–1928. doi:10.1023/A:1012213116515
Van Poecke RMP, Roosjen M, Pumarino L, Dicke M (2003) Attraction of the specialist parasitoid Cotesia rubeculata to Arabidopsis thaliana infested by or non-host herbivore species. Entomol Exp Appl 1075:229–236. doi:10.1046/j.1570-7458.2003.00060.x
Van Tol RHM, Van der Sommen TC, Boff MIC, Van Bezooijen J, Sabelis MW, Smits PH (2001) Plants protect their roots by alerting the enemies of grubs. Ecol Lett 4:292–294. doi:10.1046/j.1461-0248.2001.00227.x
Viglierchio DR, Schmitt RV (1983) On the methodology of nematode extraction from field samples: funnel modifications. J Nematol 15:438–444
Walling LL (2000) The myriad plant responses to herbivores. J Plant Growth Regul 19:195–216. doi:10.1007/s003440000026
Walling LL (2008) Avoinding effective defenses: strategies employed by phloem-feeding insects. Plant Physiol 146:859–866. doi:10.1104/pp.107.113142
White GF (1927) A method for obtaining infective nematode larvae from cultures. Science 66:302–303. doi:10.1126/science.66.1709.302-a
Williams L, Rodriguez-Saona C, Paré PW, Crafts-Brandner SJ (2005) The piercing-sucking herbivores Lygus hesperus and Nezara viridula induce volatile emissions in plants. Arch Insect Biochem 58:84–96. doi:10.1002/arch.20035
Wilson MJ, Ehlers RU, Glazer I (2012) Entomopathogenic nematode foraging strategies–is Steinernema carpocapsae really an ambush forager? Nematology 14:389–394. doi:10.1163/156854111X617428
Zhang PJ, Zheng SJ, Van Loon JJ, Boland W, David A, Mumm R, Dicke M (2009) Whiteflies interfere with indirect plant defense against spider mites in Lima bean. Proc Natl Acad Sci 106:21202–21207. doi:10.1073/pnas.0907890106
Zhang PJ, Xu CX, Lu YB, Zhang JM, Liu YQ, David A, Boland W, Turlings TCJ (2013) Phloem-feeding whiteflies can fool their host plants, but note their parasioids. Funct Ecol 27:1304–1312. doi:10.1111/1365-2435.12132
Zhu J, Park KC (2005) Methyl salicylate, a soybean aphid-induced plant volatile attractive to the predator Coccinella septempunctata. J Chem Ecol 31:1733–1746. doi:10.1007/s10886-005-5923-8
Acknowledgments
The authors thank Juliano Troiano and Carlos Ribeiro for assistance on English writing and the financial support by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado de São Paulo (Grant 2013/05367-0) and Instituto Nacional de Ciência e Tecnologia-Semioquímicos na Agricultura.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by CAPES, FAPESP (Grant 2013/05367-0), and INCT Semioquímicos na Agricultura (Fapesp Proc. 2008/57701-2 and CNPq Proc. 573761/2008-6).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Liliane Ruess.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tonelli, M., Peñaflor, M.F.G.V., Leite, L.G. et al. Attraction of entomopathogenic nematodes to sugarcane root volatiles under herbivory by a sap-sucking insect. Chemoecology 26, 59–66 (2016). https://doi.org/10.1007/s00049-016-0207-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-016-0207-z