Abstract
Background and Aims
Plant-parasitic nematodes are able to sense and respond to gradients of chemical signals. How pH and inorganic salts in the rhizosphere affect nematode accumulation and host-seeking is poorly understood. We investigate the response of different groups of plant-parasitic nematodes to pH and salt concentration gradients.
Methods
Responses of infective juveniles (J2) of the economically important plant-parasitic nematodes, soybean cyst nematodes (SCN; Heterodera glycines) and root-knot nematodes (RKN; Meloidogyne incognita and M. hapla) to pH and salt gradients were assessed using Pluronic F-127 gel-based assays. Microelectrodes were utilized to measure pH and ion concentration gradients in the gel.
Key Results
Differences were found between the three nematode species in response to acid, base and salts. For SCN, maximum nematode accumulation was between pH range 4.98–5.46 in an acid gradient, while the preferred alkaline pH ranges were 8.40–8.78 and 9.52–9.99. The preferred Cl− concentration for SCN attraction was 171–256 mM. RKN showed weak attraction to base and salt at low J2 concentration but increasing attraction at a greater nematode concentration.
Conclusions
The pH and inorganic salts affect nematode behavior, accumulation, and survival. These findings provide new considerations for strategies to manage plant-parasitic nematodes under field conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant-parasitic nematodes (PPN) are responsible for substantial reductions in crop yield and quality globally with root-knot nematodes (RKN, Meloidogyne spp.) and cyst nematodes (CN, Heterodera and Globodera spp.) widely considered to be the two most damaging and economically important groups (Jones et al. 2013). Many RKN species including the tropical species Meloidogyne incognita and the temperate climate species M. hapla have very broad host ranges spanning over 1000 plant species (Jones et al. 2013). Cyst nematodes generally have a narrow host range but can be very damaging to the affected crop. For example, soybean cyst nematode (SCN, H. glycines) is one of the most damaging pests of soybean (Glycine max) (Wrather and Koenning 2006, 2009). Both nematode groups are biotrophic and sedentary endoparasites, and both hatch from eggs as second stage juveniles (J2), which constitute the infective stage. These non-feeding J2 must locate an appropriate host entry site, penetrate the root, and establish a permanent feeding site inside the host root that will serve as a nutrition source for the extended biotrophic development and reproduction (Jones and Goto 2011; Sobczak and Golinowski 2011).
During the early stages of plant-nematode interaction, semiochemicals secreted by the host root and other signals from the rhizosphere environment (Bais et al. 2006; Rasmann et al. 2012) direct nematode behaviors, host location and infection (Perry 1997; Perry and Moens 2011). The broad host range for RKN and narrow host range for SCN suggest that chemical cues produced by the host and perceived by these nematode groups may be different. Wang et al. (2018) compared the behaviors and attraction of H. glycines and M. incognita to three sources of root chemicals (intact root tips, root extracts, and exudates collected from root systems) from three plant species (marigold, pepper and soybean) and found that M. incognita was attracted to root tips of each plant species even though marigold is not a host, but H. glycines was only attracted to its host, soybean. In contrast, root exudates and root extracts from all three plant species attracted H. glycines but were repellent to M. incognita. In a different study, Liu et al. (2019) found that H. glycines displayed strong chemotaxis to root exudates of soybean and little attraction to nonhosts cotton and soybean whereas M. incognita was attracted to exudates of cotton and soybean, but not peanut, which is not a host. The different responses of root exudates to SCN between the two tests (Liu et al. 2019; Wang et al. 2018) might derive from the assay methods or the composition of the exudates. Root exudates comprise a multitude of primary and secondary metabolites which likely include host specific and general attractants as well as repellents (cf., Čepulytė et al. 2018). The balance of attractants and repellents depends on plant age and condition and informs the J2 regarding the suitability of the host to sustain a long term biotrophic interaction. For example, roots of Arabidopsis mutants defective in the ethylene response pathway show increased attractiveness to RKN compared to wild type (Fudali et al. 2013) and SCN (Hu et al. 2017). Lauric acid isolated from crown daisy root exudates attracts M. incognita at low concentrations, but at higher concentrations, it is a repellent (Dong et al. 2014). These and other studies support the complexity of the interplay between host exudates and nematode response.
In addition to host-specific semiochemicals, nematodes also perceive and respond to gradients of other components in the soil or rhizosphere including physical and chemical factors, such as temperature, pH, redox potential, and CO2 (Diez and Dusenbery 1989; Wang et al. 2009a, 2010). These responses have been found to differ among PPN species. For example, the reniform nematode Rotylenchulus reniformis is attracted by 0.5 m MgCl2, NH4Cl, NaCl, Na2SO4, NaC2H3O2, Mg(C2H3O2)2 and MgSO4, while those compounds were not attractants for RKN M. javanica (Riddle and Bird 1985). However, Castro et al. (1990) found inorganic salts of the ions K+, NH4 +, Cs+, NO3 −, and Cl− at 0.01 m are strongly repellent to J2 of M. incognita and cations Li+, Na+ and Rb+ at 0.01 m elicited little or no response. Papademeriou and Bone (1983) reported dosage-dependent attraction of SCN to ZnSO4, ZnCl2, CaSO4 and MgCl2. Nitrate salts have also been reported to be dosage dependent attractants for RKN and SCN (Beeman et al. 2016; Hida et al. 2015; Hosoi et al. 2017). Masler et al. (2017) found that CaCl2 was 15-fold more attractive to H. glycines than to M. incognita. Other studies found that CH3COONa and CH3COOK attracted citrus nematode Tylenchulus semipenetrans but Na2CO3 and NaHCO3 were attractants for the RKN M. javanica (Abou-Setta and Duncan 1998; Ali et al. 2011). Interpretation of these studies has been limited by inability to directly view the response of the nematodes due to the opaque medium or to measure accurately the gradient concentrations during the response.
Gels of the block copolymer Pluronic F-127 (PF-127) have proved to be useful for investigating the behavior of PPN J2. Nematode move freely in this highly transparent, non-toxic gel allowing monitoring of behavior in three dimensions rather than in two dimensions as on the surface of an agar gel (Wang et al. 2009b). A 23% gel of PF-127 is liquid at 15ºC but semi-solid at 20ºC or higher allowing nematodes to be mixed with gel under low temperature and nematode behavior and chemotaxis to then be observed in real time at ambient temperature (Wang et al. 2009a, b, 2010).
When RKN are uniformly dispersed in PF-127 gel, they aggregate into tight clumps after 1–2 days (Wang et al. 2009b). This behavior varies with isolate and occurs more rapidly at high nematode density (Wang et al. 2009b, 2010).
Stable chemical gradients can be easily produced in PF-127 gel (Wang et al. 2009a). By placing a chemical dispenser containing an acidic solution in the gel, we previously demonstrated that a stable radial pH gradient is produced in the gel centered at the dispenser openings (Wang et al. 2009a). Using a pH indicator, we showed that the gradient is stable for over 24 h, and the steepness of the gradient depends on the size of the dispenser opening. When an acetic acid solution was used to form a gradient in PF-127 gel containing uniformly distributed J2 of RKN, nematodes accumulated in a ring around the dispenser opening. By using a microelectrode, the pH range at which the nematodes accumulated was measured as 4.5–5.4 (Wang et al. 2009a). These results suggested that PF-127 gel would be broadly applicable for examining the responses of PPN to chemical gradients. Our preliminary studies indicated that alkaline amino acids resulted in strong accumulation for both RKN and SCN (unpublished data), but it was not clear whether this accumulation was caused by alkaline pH or specific attraction. The free living nematodes Caenorhabditis elegans is attracted to alkaline pH up to 10, but strongly avoids pH > 10 (Murayama and Maruyama 2013; Sassa et al. 2013). Here we utilize PF-127 medium to examine SCN response to acidic and alkaline pH gradients. We also examine how RKN responds to alkaline pH gradients.
As discussed above, a variety of responses, sometimes conflicting, have been reported for RKN and SCN to various salts. In the soil, inorganic salts (e.g. Na+, Cl−, CO32−, SO42−) exist naturally and the concentration can be altered by the application of fertilizers. The investigation of soil environment factors pH and salts as attractants or repellents to RKN and SCN and the preferred attraction concentration, the comparison of the chemotaxis of RKN with SCN will not only shed more light on host-seeking mechanism but also provide insight for making new effective control strategies. Here we utilize the advantages of PF-127 gel to more precisely interrogate the response of PPN to these compounds.
Materials and methods
Nematode cultures
For SCN culture, HG Type 2.5.7 (SCN race 5) was maintained on susceptible soybean cv. ‘Dongsheng 1’ in pots filled with a 1:1 ratio of autoclaved sand and soil under glasshouse conditions at 22–28 °C with a 16/8 light/dark cycle (Hua et al. 2018). At 35 days after inoculation, cysts were collected from plant roots and soil and crushed to release eggs (Hua et al. 2018). The collected eggs were surface sterilized with 10% bleach for 1.5 min and then were rinsed three or four times with sterile water. Eggs were hatched at 28 °C for 3–5 days and J2 were collected for chemotaxis assay.
For RKN culture, M. incognita was originally isolated and identified from tomato plants (Li et al. 2016). M. hapla was originally provided by Dr. Yuxi Duan from Shenyang Agricultural University and confirmed with species-specific primers in our lab (Li et al. 2016). All cultures were maintained on tomato cv. Zhongshu 4 at 22–28 °C in the glasshouse. Nematode eggs were extracted using NaOCl (Hussey and Barker 1973) from infested tomato roots at 40–60 days post inoculations and hatched in the incubator at 28 °C for 3–4 days and J2 were collected for chemotaxis assays.
Pluronic gel preparation and attraction assays
PF-127 gel (NF Prill Poloxamer 407, BASF, Mt Olive, NJ, USA) at 23% w/v in 10 mM Tris-MES (morpholino-ethanesulfonic acid) (Sigma-Aldrich) was prepared as previously described (Wang et al. 2009b). The attraction assay followed the method by Wang et al. (2009a). Twenty ml of PF-127 solution containing 6000 freshly hatched J2 was poured into 100 mm diameter Petri dish (Jiangsu Kangjian Medical Apparatus Co., Ltd., China) at 15 °C. Acetic acid (HAc) (Tianjin Fuyu Fine Chemical Co., Ltd, China) solutions were used to determine nematode chemotactic behavior to acidic pH (Wang et al. 2009a). Two “chemical dispensers”, each containing approximately 100 µl test solution in 23% PF gel, were placed horizontally anti-parallel and 40 mm apart in the center of the plate and 30 mm away from the edge of the plate. The chemical dispensers were prepared by cutting 5 mm from the small end and 20 mm from the large end of 50 mm-long, 200-µl pipette tips (Jet Biofil, Guangzhou, China). Sodium hydroxide (NaOH) (Tianjin Dalu Chemical Company, China) was used for determining nematode response to alkaline pH gradients. For these assays, the chemical dispensers were prepared by cutting 20 mm from the large end of the 200-µl pipette tips (Jet Biofil, Guangzhou, China) and no cut from the small end. One chemical dispenser containing 100 µl test solution in 23% PF-127 gel was placed into each plate. The small opening was touching the inside edge of the plate leaving enough space for chemical gradient formation around the large opening.
To evaluate nematode response to salts, NaCl and other inorganic salts (MgCl2, KCl, KNO3, MgSO4 and Na2SO4) (Sinopharm Chemical Reagent Co., Ltd, China) were used. Ten ml of 23% PF-127 solution without 10 mM Tris-MES containing 3000 freshly hatched J2 was poured into a 60 mm diameter Petri dish. One chemical dispenser containing approximately 50 µl test solution in 23% PF-127 was placed into the Petri dish with the small opening touching to the inside rim of the plate. The chemical dispenser was prepared by cutting 10 mm from the small opening and 25 mm from the large opening of a 200-µl pipette tip. The dispenser was placed into the plate with the small end touching the inside edge of the plate leaving enough space for chemical gradient formation around the large opening.
Control samples were prepared with sterile deionized water in 23% PF-127. All the plates were incubated at 26 °C. The attraction and repulsion were observed microscopically at 5 and 24 h. Three plates were included in each experiment and each experiment was repeated two or three times. Nematodes around pipette tips were captured with an OLYMPUS SZX-16 dissecting microscope by using Cellsens Standard Image Software (Olympus Corporation, Japan). The broader views of the nematode distribution patterns in the petri dish were taken using a digital camera.
Nematode mortality assay
To assess nematode mortality in response to acidic and alkaline pH and inorganic salt solutions, approximately 100 J2 were added into each well of a 6-well tissue culture plate containing 1 ml of test solution. Sterile deionized water was used as control. The shape and activity of nematodes were observed under OLY MPUS SZX-16 dissecting microscope (Olympus Corporation, Japan) at 2 and 24 h. Nematodes that were straight rods and did not move when touched with Ultrafine Single Deer Hair (Ted Pella, Inc., USA) were considered to be dead. Separate samples were used for 2 h- and 24 h- mortality counting. The percent mortality was calculated as the number of dead J2/the total number of J2 in the well x 100. The pH of solutions was measured with PHSJ-3F lab pH meter (Shanghai INESA Scientific Instrument Co., Ltd, China). Three replications were carried out for each test and the experiment was repeated twice.
pH measurements in the gel
PHSJ-3F lab pH meter (Shanghai INESA Scientific Instrument Co., Ltd, China) was used to measure the pH value by direct insertion of the PHR-146 Micro Combination pH electrode (Lazar Research Laboratories, Inc., USA) into gel at the boundaries of the halo of nematode aggregation area at 24 h after assay initiation (Wang et al. 2009a). More than 50 readings were made from three plates and the average value was considered to be the preferred pH for nematode aggregation. Three-point standardization method (pH 7, pH 4 and pH 10) was used for the pH meter calibration based on the manufacturer’s instructions. Each measurement was repeated at least twice.
Chloride ion concentration measurement
The concentration of chloride ions (Cl− ) in gel was measured with a 62 ORP hand-held meter (Jenco Instrument Inc., San Diego, CA, USA) by direct insertion of a LIS-146CLCM micro chloride electrode (Lazar Research Laboratories, Inc., Los Angeles, CA, USA) into gel at the boundaries of the halo of nematode aggregation. NaCl solutions (10− 3, 10− 2, 10− 1 and 1 m) were used as standards to draw calibration curves according to the manufacturer’s instruction. The experiment for the Cl− measurements was repeated at least twice.
Data analysis
Data were subjected to analysis of variance using SPSS One-Way ANOVA (IBM, Armonk, New York, USA). The Student’s t test (P < 0.05) was used to evaluate the significant difference.
Results
Aggregation of Heterodera glycines in acidic pH gradients
The responses of H. glycines (HG Type 2.5.7) to acetic acid gradients formed in PF-127 gel by dispensers containing three concentrations (0.17, 0.34, or 0.85 m) of acetic acid at 5 and 24 h post exposure are shown in Fig. 1. Nematode accumulation at both openings of the dispenser was detected as early as 1 h after the assay start (not shown) and clear accumulation patterns were seen at both the large and small openings at 5 h (Fig. 1a). Stable accumulation was still observed at 24 h (Fig. 1b, c). Nematodes accumulated around the small opening of dispensers containing 0.85 m acetic acid and at large openings with each of the three concentrations (Fig. 1c). The halo formation indicated that, as previously noted for M. hapla (Wang et al. 2009a), H. glycines accumulates in a preferred pH range.
Accumulation of Heterodera glycines (HG Type 2.5.7) in acetic acid gradients. At the start of the experiment nematodes were uniformly dispersed in a 100 mm diameter Petri dish containing 20 ml PF-127 gel with 300 J2/ml. Dispensers containing 0.17, 0.34, or 0.85 m acetic acid as indicated at the top of the image was then added to the plates. Nematode accumulation around the small opening of dispensers is shown at 5 h (a) and at 24 h (b) post exposure. Lower magnification views of nematode accumulation patterns around the large opening of dispensers at 24 h are shown in Panel c. Scale bar = 1 mm in a and b. Scale bar = 1 cm in c
The pH values at the inner and outer boundaries of the halo of nematode accumulation area for each of the three concentrations of acetic acid were 4.98 ± SD 0.02 and 5.46 ± 0.03, respectively. No accumulation of nematodes was observed in control (not shown).
Aggregation of Heterodera glycines in alkaline pH gradients
When subjected to gradients formed by dispensers containing three different NaOH concentrations (0.1, 0.5 and 1 m), H. glycines J2 were observed to accumulate around the larger opening of dispensers at 5 h. At 24 h two halos with different diameters were apparent, and the halo diameters were greater for higher concentrations of NaOH (Fig. 2), indicating that J2 accumulate at two different concentration ranges of NaOH. No accumulation of nematodes was observed for control dispensers.
Accumulation patterns of Heterodera glycines in NaOH gradients at 24 h after the assay starting in PF-127 gel. Assays were carried out in a 100 mm diameter Petri dish containing 20 ml PF-127 gel with 300 J2/ml. Nematode accumulation patterns around the large opening of dispensers at 24 h are shown with three concentrations of 0.1 m (a), 0.5 m (b), and 1 m (c). Scale bar = 1 cm
The pH at the boundaries of the regions of accumulation were assessed at 24 h using a pH microelectrode. For the inner circle, the pH values at the inner and outer boundary were 9.99 ± 0.03 and 9.52 ± 0.05, respectively. For the outer circle, the pH values at the inner and outer boundary were 8.78 ± 0.02 and 8.40 ± 0.01, respectively.
Aggregation of Heterodera glycines in inorganic salt gradients
The response of H. glycines was monitored in gradients formed by dispensers with two different NaCl concentrations (1 and 2 m). At 5 h post exposure, nematode strong accumulation with clumping was observed at the large opening of the dispenser with 1 m NaCl, and a single halo containing clumps of J2 was formed around the large opening of the dispenser containing 2 m NaCl (Fig. 3a). Nematodes continued to accumulate and clump together at (1 m NaCl) or around (2 m NaCl) the opening of the disperser at 24 h (Fig. 3b). Accumulation inside the dispenser close to the large opening was also observed at 24 h for both 1 m NaCl and 2 m NaCl (Fig. 3b).
Accumulation patterns of Heterodera glycines in NaCl gradients. Assays were carried out in 60-mm diameter Petri dishes containing 10 ml PF-127 gel with 300 J2/ml and photographed at 5 h (a) and 24 h (b) post exposure. Dispensers contain 1 and 2 m NaCl or no salt (CK) as indicated at the top of the three columns. Scale bar = 1 mm
The Cl− concentrations flanking the halo where J2 accumulated were measured at 5 h by using microelectrodes and standard curves. The Cl− concentrations at the inner and outer boundary of the halo were 256 mM ± SD 10 and 171 mM ± SD 3, respectively.
The responses of H. glycines to gradients formed by dispensers containing a 1 m concentration of the inorganic salts KCl, KNO3, MgCl2, MgSO4, or Na2SO4 were tested. An obvious accumulation inside the dispenser was observed for all tested salts at 5 h (Fig. 4a, c) and 24 h (Fig. 4b, d). Tighter nematode aggregations (clumps) were formed at 24 h than at 5 h (Fig. 4). Close examination revealed that approximately 60–70% nematodes inside the dispensers for Na2SO4 at 5 h (Fig. 4c) and for MgSO4 at 24 h and almost all individuals for KCl and Na2SO4 at 24 h were straight and did not move suggesting that they were dead (Fig. 4d). No obvious accumulation was observed for control (Fig. 4a, b).
Migration of Heterodera glycines in inorganic salt gradients. Assays were carried out in a 60-mm diameter Petri dish containing 10 ml PF-127 gel with 300 J2/ml at 5 h (a, c) and 24 h (b, d) post exposure. Dispensers contain 1 m salts (KCl, KNO3, MgCl2, MgSO4 and Na2SO4) or no salt (CK) as indicated at the top of the columns. Scale bar = 1 mm
Aggregation of Meloidogyne spp. in alkaline pH and NaCl gradients
We used the dispenser gradient assay to investigate the response of M. incognita and M. hapla to NaOH and NaCl (Fig. 5). For assays in which the initial concentration of nematodes in the gel was 300 J2/ml, a faint halo observed around the large opening of the dispenser containing 1 m NaOH at 24 h post exposure of M. hapla (Fig. 5b), but no accumulation was observed for M. incognita. However, when an initial nematode concentration of 3000 J2/ml was used, two clear halos of nematode accumulation were seen around the large opening of the dispenser containing 1 m NaOH for both species (Fig. 5c-e, l, m). For both nematode species, the areas of accumulation included tight clumps of individuals. The pH values flanking the accumulation halos were 8.53 ± 0.15 and 8.97 ± 0.10 for the outer halo and 9.50 ± 0.12 and 10.1 ± 0.16 in the inner halo. These pH ranges are similar to those found for SCN.
Response of Meloidogyne hapla (Mh) (a-i) and M. incognita (Mi) (j-m) to 1 m NaOH and 2 m NaCl gradients. Responses are shown at 24 h after the starting assay. Scale bars for a-c and i-l = 1 cm and other scale bars = 1 mm. The NaOH test utilized 100 mm diameter Petri dishes containing 20 ml PF-127 gel with 300 J2/ml or 3000J2/ml nematodes as indicated. The NaCl test utilized 60-mm diameter Petri dishes containing 10 ml PF-127 gel with the indicated nematode concentrations. The arrows point to enlarged sections. The large white spots in panels e, h, j and m are tight aggregates of nematodes
M. incognita had no response to gradients formed with 2 m NaCl at either nematode concentration (Fig. 5k). M. hapla also did not show a response at 300 J2/ml (Fig. 5g), but a distinct halo punctuated by tight aggregates was present around the large opening of the dispenser at 3000 J2/ml (Fig. 5h and i). No accumulation around the large opening of the dispenser was seen for controls (Fig. 5a, f and j) even though nematode ‘balls’ were found randomly in the plate with control in Fig. 5j.
Nematode mortality in response to acidic pH, alkaline pH and salts
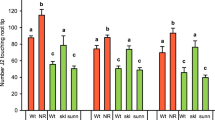
Mortality assays with different concentrations of HAc showed 21% SCN mortality at 0.0001 m (0.1 mM) HAc (pH 4.84) and 84% mortality at 0.5 mM HAc (pH 4.56) at 2 h, but up to 70% and 100% mortality at 24 h, respectively. The RKN species M. incognita and M. hapla did not show a significant increase in mortality at pH 4.84 compared to control and only 11 and 15% mortality, respectively, at pH 4.56 at 2 h (Table 1). Further, there was no significant difference in mortality between 2 and 24 h at each tested concentrations for both M. incognita and M. hapla (Table 1), indicating that RKN can tolerate lower pH than SCN. All species tested largely survived treatment with 10 mM NaOH (pH12.21) after 2 h, but 50 mM NaOH (pH12.81) proved lethal. There was no significant difference in mortality for NaOH or NaCl treatments between RKN and SCN (Table 1) and also between 2 and 24 h except M. incognita response to 10 and 100 mM NaCl (Table 1). Comparison of mortality of H. glycines to increasing concentrations of inorganic salts revealed that 10 and 100 mM Na2SO4, KCl and MgSO4 were significantly more toxic than the same concentrations of NaCl (Table 1). At 24 h, 10 mM MgSO4 and Na2SO4 and 100 mM Na2SO4, KCl and MgSO4 caused higher nematode mortality than those at 2 h and Na2SO4 displayed up to 94% mortality (Table 1). At 0.5 or 1 m concentrations of all salts tested, most or all nematodes were dead after 2 h (Table 1).
Discussion
In an acidic pH gradient, we found that J2 of H. glycines accumulated between pH 5.0 and 5.5. This range is similar to, but narrower than, the pH range of 4.5–5.5 that was previously found for RKN accumulation (Wang et al. 2009a). Correspondingly, SCN mortality (84% at 2 h and 100% at 24 h) at pH 4.5 was much higher than that found for RKN species (11–15% at 2 or 24 h) (Table 1). In previous studies using pH indicators, the surface of the root at the zone of elongation of growing seedling roots has been found to be the most acidic with pH less than 5 for maize (Mulkey and Evans 1981; Peters and Felle 1999), tomato and Medicago truncatula (Wang et al. 2009a). RKN are strongly attracted to and invade the root primarily in the zone of elongation (Williamson and Gleason 2003). In contrast, SCN are able to penetrate a broader region of the root (Marhavý et al. 2019), and thus may not need to target the elongation zone. Our previous observations support this: most RKN J2 are attracted to the root tip within the terminal 1.5 mm (Wang et al. 2009b), but for SCN, the attraction region extends up to 5 mm (Hu et al. 2017). These findings are consistent with a role for acidic gradients as general plant cues in directing RKN and SCN to appropriate host entry sites. In our previous work we investigated the response of M. hapla to a range of acids in addition to acetic acid including strong acids (HCl, H2SO4, HClO4, methanesulfonic acid) and carboxylic acids (acetic, citric, formic, lactic, propionic and succinic). We found that all acids tested attracted the J2 and that salts of some organic acids, but not acetate, appeared to be specific attractants (Wang et al. 2009a). Here we examine the response of SCN to only acetic acid, but we suggest that PF-127 based assays provide a useful format to examine the response of SCN to gradients of specific organic acids as well as other exudate components to identify molecules that potentially contribute to host specificity (Sikder and Vestergård 2020; Sasse et al. 2018).
Several nematode species have been noted to accumulate at alkaline pH. For example, accumulation of entomopathogenic nematode (EPN), Neoaplectana carpocapsae was observed at pH 8.6 and 9.7 in basic pH gradients formed by NaOH in a background of 0.1 m NaCl (Pye and Burman 1981). The free living nematode C. elegans is also attracted by alkaline pH up to ~ 10 (Dusenbery 1974; Murayama et al. 2013; Ward 1973) but is strongly repelled by pH > 10.5 (Sassa et al. 2013). When exposed to an alkaline pH gradient, SCN accumulated at two preferred pH ranges (8.4–8.8; 9.5–10), and both RKN species tested displayed similar patterns of accumulation but only at higher nematode concentrations. Together these experiments suggest that response to mildly alkaline pH may be broadly conserved among nematode taxa. However, it is unclear why PPN accumulate at alkaline pH and why there are two preferred pH ranges for accumulation. The perception of and response to alkaline gradients has been best studied in the model nematode C. elegans. Murayama and Maruyama (2013) found that signal strengths from C. elegans sensory neurons ASE-left (ASEL) and ASH determine nematode chemotactic behaviors to alkaline pH and the competition between the two sensory neurons results in nematode behavior decision. A transmembrane receptor-type guanylyl cyclase (gcy), GCY-14, expressed in ASEL gustatory neuron in C. elegans was identified to modulate the sensing of extracellular alkalinity (Murayama et al. 2013). A G-protein α subunit, GOA-1, plays an important role in avoiding strongly alkaline pH in C. elegans (Sassa and Maruyama 2013). It may be that the interplay between sensory neurons and receptors is responsible for the two preferred pH ranges for PPN, but this sophisticated type of analysis is not currently feasible for PPN. Both RKN and SCN are surprisingly tolerant of alkaline pH and survive at pH 12.2 in our mortality assay (Table 1).
Previous reports indicated that SCN J2 are attracted to inorganic salts MgCl2, KNO3 and NH4NO3, but did not respond to 0.5 m KCl, Na2SO4, or ZnSO4 using microfluidic chips (chemical chip) (Beeman et al. 2016). Hosoi et al. (2017) reported 0.5 m KNO3 and nitrate analogs attracted SCN using agar plugs for compound delivery. SCN J2 had no significant response to Na2SO4 and NaCl but showed attraction to MgCl2 with agar tests (Papademeriou and Bone 1983). Strong repellency of M. incognita J2 to Cl− was found in Castro et al. (1990). In our assay we found that SCN J2 were attracted to the gradients of all tested salt gradients, NaCl, KCl, KNO3, MgCl2, MgSO4 and Na2SO4 (Figs. 3 and 4), M. hapla at higher density showed attraction to 2 m NaCl and M. incognita had no response to NaCl (Fig. 5) using Pluronic gel system. RKN and SCN responses to acid, base and salts in this and previous studies are summarized in Table 2. In most cases results were consistent between studies, but there were some differences. Response differences for same nematode species between studies could be due to response variation between nematode isolates or to the assay system used for the study.
Using a microelectrode, we determined that the SCN accumulated at chloride ion concentrations between 171 and 256 mM at the 5 h time point. Nematode accumulation patterns were different among tested salts at 5 and 24 h (Figs. 3 and 4). Nematodes mainly accumulated at and within big opening of the dispenser for 1 m NaCl, but within the dispenser for other 1 m salts, KCl, KNO3, Na2SO4, MgCl2 or MgSO4. Further, nematodes clumped together tightly and looked very active with bent shapes with 1 m NaCl, but they accumulated together loosely and most appeared to be dead with 1 m Na2SO4. Interestingly, 60 to 70% of the J2 inside the dispenser containing 1 m Na2SO4 appeared to be dead at 5 h after assay start but nematodes within the dispenser were active for 1 m KNO3 (Fig. 4). At the 24 h time point, the number of nematodes inside the dispenser had increased further and almost all nematodes inside the dispenser containing 1 m Na2SO4, and 1 m KCl appeared to be dead (Fig. 4). These results demonstrate that both anions and cations play roles for nematode accumulation. Mortality assays at 2 and 24 h confirmed that at 0.01 or 0.1 m Na2SO4, KCl and MgSO4 were significantly more toxic to SCN J2 than was NaCl. Together this indicates that in our assay system, SCN J2 are attracted to lethal concentrations of Na2SO4, KCl and MgSO4. As mentioned above, in assays carried out with nematode concentrations of 300 J2/ml, neither M. incognita or M. hapla responded to gradients formed with 2 m NaCl. However at higher nematode density, M. hapla, but not M. incognita, accumulated in a halo around the dispenser opening (Fig. 5).
Accumulation of RKN at favored concentrations of salt or base was associated with the presence of tight clumps of nematodes (Fig. 5). Our previous studies (Wang et al. 2009b) revealed that the rate of RKN clumping after dispersal in PF-127 gel increased with nematode density. We suggested that this may be due to inter-nematode communication. Nematodes produce a class of compounds called ascarosides that act as pheromones, playing key roles in social interaction or chemical communication among nematode populations for both free-living nematodes and parasitic nematodes (Braendle 2012; Choe et al. 2012; Manosalva et al. 2015). We also noted indications of tight clumping behavior in the response of SCN to salt gradients (Fig. 3) suggests that social interactions may also lead to clumping for this species. Isolates of C. elegans also differ in aggregation in response to food and multiple other signals (de Bono and Bargmann 1998; de Bono et al. 2002; Ding et al. 2019, 2020). Considerable work has been carried out to characterize the neurons, molecular receptors, and signal integration leading to these behaviors in this model organism (cf., de Bono and Maricq 2005; Macosko et al. 2009; Ortiz et al. 2009). For plant-parasitic nematodes, less is known as these organisms are much less tractable and available tools are limited. However, genetic analysis of segregation of clumping in M. hapla indicates that inheritance of clumping/non-clumping may involve a single genetic locus (Wang et al. 2010). In addition, recent experiments using RNAi have demonstrated that homologs of several C. elegans chemosensory genes have roles in behavior and chemotaxis in M. incognita (Shivakumara et al. 2019).
PF-127 gel has the advantage that it is highly transparent and semisolid, which allows nematodes to move in three-dimensions. The formation of stable chemical gradients in the gel together with the use of microelectrodes have provided detailed information on the preferred pH and ion concentrations for nematode accumulation. These observations provide new insights into the complexity of behavior of PPN in the opaque soil environment such as the importance of density-dependent social interactions in their response. A long range goal is to apply the knowledge we gained here to manage PPN through disrupting nematode host-seeking by modifying soil pH and/or salt dosages. For example, the finding that SCN goes to toxic levels of salts suggests bait and kill strategies could be developed.
References
Abou-Setta MM, Duncan LW (1998) Attraction of Tylenchulus semipenetrans and Meloidogyne javanica to salts in vitro. Nematropica 28:49–59
Ali JG, Alborn HT, Stelinski LL (2011) Constitutive and induced subterranean plant volatiles attract both entomopathogenic and plant parasitic nematodes. J Ecol 99:26–35. https://doi.org/10.1111/j.1365-2745.2010.01758.x
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:233–266. https://doi.org/10.1146/annurev.arplant.57.032905.105159
Beeman AQ, Njus ZL, Pandey S, Tylka GL (2016) Chip technologies for screening chemical and biological agents against plant-parasitic nematodes. Phytopathology 106:1563–1571. https://doi.org/10.1094/PHYTO-06-16-0224-R
Braendle C (2012) Pheromones: evolving language of chemical communication in nematodes. Curr Biol 22:R294–R296. https://doi.org/10.1016/j.cub.2012.03.035
Castro CE, Belser NO, Mckinney HE, Thomason IJ (1990) Strong repellency of the root knot nematode, Meloidogyne incognita by specific inorganic ions. J Chem Ecol 16:1199–1205. https://doi.org/10.1007/BF01021019
Čepulytė R, Danquah WB, Bruening G, Williamson M (2018) Potent attractant for root-knot nematodes in exudates from seedling root tips of two host species. Sci Rep 8:10847. https://doi.org/10.1038/s41598-018-29165-4
Choe A, von Reuss SH, Kogan D, Gasser RB, Platzer EG, Schroeder FC, Sternberg PW (2012) Ascaroside signaling is widely conserved among nematodes. Curr Biol 22:772–780. https://doi.org/10.1016/j.cub.2012.03.024
de Bono M, Bargmann CI (1998) Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell 94:679–689. https://doi.org/10.1016/S0092-8674(00)81609-8
de Bono M, Maricq AV (2005) Neuronal substrates of complex behaviors in C. elegans. Annu Rev Neurosci 28:451–501. https://doi.org/10.1146/annurev.neuro.27.070203.144259
de Bono M, Tobin DM, Davis MW, Avery L, Bargmann CI (2002) Social feeding in Caenorhabditis elegans is induced by neurons that detect aversive stimuli. Nature 419:899–903. https://doi.org/10.1038/nature01169
Diez JA, Dusenbery DB (1989) Repellent of root-knot nematodes from exudate of host roots. J Chem Ecol 15:2445–2455. https://doi.org/10.1007/BF01020375
Ding SS, Schuacher LJ, Javer AE, Endres RG, Brown AEX (2019) Shared behavioral mechanisms underlie C. elegans aggregation and swarming. eLife 8:e43318. https://doi.org/10.7554/eLife.43318
Ding SS, Romenskyy M, Sarkisyan KS, Brown AEX (2020) Measuring Caenorhabditis elegans spatial foraging and food intake using bioluminescent bacteria. Genetics 214:577–587. https://doi.org/10.1534/genetics.119.302804
Dong L, Li X, Huang L, Gao Y, Zhong L, Zheng Y, Zuo Y (2014) Lauric acid in crown daisy root exudate potently regulates root-knot nematode chemotaxis and disrupts Mi-flp-18 expression to block infection. J Exp Bot 65:131–141. https://doi.org/10.1093/jxb/ert356
Dusenbery DB (1974) Analysis of chemotaxis in the nematode Caenorhabditis elegans by countercurrent separation. J Exp Zool 188:41–47. https://doi.org/10.1002/jez.1401880105
Fudali SL, Wang C, Williamson VM (2013) Ethylene signaling pathway modulates attractiveness of host roots to the root-knot nematode Meloidogyne hapla. Mol Plant Microbe In 26:75–86. https://doi.org/10.1038/srep41282
Hida H, Nishiyama H, Sawa S, Higashiyama T, Arata H (2015) Chemotaxis assay of plant-parasitic nematodes on a gel-filled microchanneldevice. Sens Actuat B 221:1483–1491. https://doi.org/10.1016/j.snb.2015.07.081
Hosoi A, Katsuyama T, Sasaki Y, Kondo T, Yajima S, Ito S (2017) Nitrate analogs as attractants for soybean cyst nematode. Biosci Biotech Bioch 81:1542–1547. https://doi.org/10.1080/09168451.2017.1332980
Hu Y, You J, Li C, Williamson VM, Wang C (2017) Ethylene response pathway modulates attractiveness of plant roots to soybean cyst nematode Heterodera glycines. Sci Rep 7:41282. https://doi.org/10.1038/srep41282
Hua C, Li C, Hu Y, Mao Y, You J, Wang M, Chen J, Tian Z, Wang C (2018) Identification of HG types of soybean cyst nematode Heterodera glycines and resistance screening on soybean genotypes in northeast China. J Nematol 50:41–50. https://doi.org/10.21307/jofnem-2018-007
Hussey RS, Barker KR (1973) A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis Rep 57:1025–1028
Jones MGK, Goto DB (2011) Root-knot nematodes and giant cells. In: Jones J, Gheysen G, Fenoll C (eds) Genomics and molecular genetics of plant-nematode interactions. Springer, Dordrecht, pp 83–100
Jones JT, Haegeman A, Danchin EG, Gaur HS, Helder J, Jones MG, Kikuchi T, Manzanilla-López R, Palomares-Rius JE, Wesemael WM, Perry RN (2013) Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol 14:946–961. https://doi.org/10.1111/mpp.12057
Li C, Hu Y, Wang C (2016) Identification of species and races of root-knot nematodes in greenhouse from Daqing city in Heilongjiang Province. Soils Crops 5:105–109. https://doi.org/10.11689/j.issn.2095-2961.2016.02.006
Liu W, Jones AL, Gosse HN, Lawrence KS, Park S-W (2019) Validation of the chemotaxis of plant parasitic nematodes toward host root exudates. J Nematol 51:1–10. https://doi.org/10.21307/jofnem-2019-063
Macosko EZ, Pokala N, Feinberg EH, Chalasani SH, Butcher RA, Clardy J, Bargmann CI (2009) A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature 458:1171–1175. https://doi.org/10.1038/nature07886
Manosalva P, Manohar M, von Reuss SH, Chen S, Koch A, Kaplan F, Choe A, Micikas RJ, Wang X, Kogel KH, Sternberg PW, Williamson VM, Schroeder FC, Klessig DF (2015) Conserved nematode signalling molecules elicit plant defenses and pathogen resistance. Nat Commun 6:7795. https://doi.org/10.1038/ncomms8795
Marhavý P, Kurenda A, Siddique S, Tendon DV, Zhou F, Holbein J, Shahim HM, Grundler FMW, Farmer EE, Geldner N (2019) Single cell damage elicits regional, nematode-restricting ethylene responses in roots. EMBO J 38:e100972. https://doi.org/10.15252/embj.2018100972
Masler EP, Rogers ST, Hooks CRR (2017) Behavioral differences of Heterodera glycines and Meloidogyne incognita infective juveniles exposed to root extracts in vitro. Nematology 19:175–183. https://doi.org/10.1163/15685411-00003038
Mulkey TJ, Evans ML (1981) Geotropism in corn roots: evidence for its mediation by differential acid efflux. Science 212:70–71. https://doi.org/10.1126/science.212.4490.70
Murayama T, Maruyama IN (2013) Decision making in C. elegans chemotaxis to alkaline pH. Commun Integr Biol 6:e26633. https://doi.org/10.4161/cib.26633
Murayama T, Takayama J, Fujiwara M, Maruyama IN (2013) Environmental alkalinity sensing mediated by the transmembrane guanylyl cyclase GCY-14 in C. elegans. Curr Biol 23:1007–1012. https://doi.org/10.1016/j.cub.2013.04.052
Ortiz CO, Faumont S, Takayama J, Ahmed HK, Goldsmith AD, Pocock R, McCormick KE, Kunimoto H, Iino Y, Lockery S, Hobert O (2009) Lateralized gustatory behavior of C. elegans is controlled by specific receptor-type guanylyl cyclases. Curr Biol 19:996–1004. https://doi.org/10.1016/j.cub.2009.05.043
Papademetriou MK, Bone LW (1983) Chemotaxis of larval soybean cyst nematode, Heterodera glycines race 3, to root leachates and ions. J Chem Ecol 9:387–396. https://doi.org/10.1007/BF00988457
Perry RN (1997) Plant signals in nematode hatching and attraction. In: Fenoll C, Grundler FMW, Ohl SA (eds) Cellular and molecular aspects of plant–nematode interactions. Kluwer Academic Press, Dordrecht, pp 38–50
Perry RN, Moens M (2011) Introduction to plant-parasitic nematodes; modes of parasitism. In: Jones J, Gheysen G, Fenoll C (eds) Genomics and molecular genetics of plant-nematode interactions. Springer, Dordrecht, pp 3–20
Peters WS, Felle HH (1999) The correlation of profiles of surface pH and elongation growth in maize roots. Plant Physiol 121:905–912. https://doi.org/10.1104/pp.121.3.905
Pye AE, Burman M (1981) Neoaplectana carpocapsae: nematode accumulations on chemical and bacterial gradients. Exp Parasitol 51:13–20. https://doi.org/10.1016/0014-4894(81)90037-0
Rasmann S, Ali JG, Helder J, van der Putten WH (2012) Ecology and evolution of soil nematode chemotaxis. J Chem Ecol 38:615–628. https://doi.org/10.1007/s10886-012-0118-6
Riddle DL, Bird AF (1985) Responses of the plant parasitic nematodes Rotylenchulus reniformis, Anguina agrostis and Meloidogyne javanica to chemical attractants. Parasitology 91:185–195. https://doi.org/10.1017/S0031182000056626
Sassa T, Maruyama IN (2013) A G-protein α subunit, GOA-1, plays a role in C. elegans avoidance behavior of strongly alkaline pH. Commun Integr Biol 6:e26668. https://doi.org/10.4161/cib.26668
Sassa T, Murayama T, Maruyama IN (2013) Strongly alkaline pH avoidance mediated by ASH sensory neurons in C. elegans. Neurosci Lett 555:248–252. https://doi.org/10.1016/j.neulet.2013.06.001
Sasse J, Martinoia E, Northen T (2018) Feed your friends: do plant exudates shape the root microbiome? Trends Plant Sci 23:25–41. https://doi.org/10.1016/j.tplants.2017.09.003
Shivakumara TN, Dutta TK, Chaudhary S, von Reuss SH, Williamson VM, Rao U (2019) Homologs of Caenorhabditis elegans chemosensory genes have roles in behavior and chemotaxis in the root-knot nematode Meloidogyne incognita. Mol Plant Microbe Interact 32:876–887. https://doi.org/10.1094/MPMI-08-18-0226-R
Sikder MM, Vestergård M (2020) Impacts of root metabolites on soil nematodes. Front Plant Sci 10:1792. https://doi.org/10.3389/fpls.2019.01792
Sobczak M, Golinowski W (2011) Cyst nematodes and syncytia. In: Jones J, Gheysen G, Fenoll C (eds) Genomics and molecular genetics of plant-nematode interactions. Springer, Dordrecht, pp 61–68
Wang C, Bruening G, Williamson VM (2009a) Determination of preferred pH for root-knot nematode aggregation using pluronic F-127 gel. J Chem Ecol 35:1242–1125. https://doi.org/10.1007/s10886-009-9703-8
Wang C, Williamson VM, Lower S (2009b) Application of pluronic gel to the study of root-knot nematode behaviour. Nematology 11:453–464. https://doi.org/10.1163/156854109x447024
Wang C, Lower S, Thomas VP, Williamson VM (2010) Root-knot nematodes exhibit strain-specific clumping behavior that is inherited as a simple genetic trait. PLoS One 5:e15148. https://doi.org/10.1371/journal.pone.0015148
Wang C, Masler EP, Rogers ST (2018) Responses of Heterodera glycines and Meloidogyne incognita infective juveniles to root tissues, root exudates, and root extracts from three plant species. Plant Dis 102:1733–1740. https://doi.org/10.1094/PDIS-09-17-1445-RE
Ward S (1973) Chemotaxis by the nematode Caenorhabditis elegans: identification of attractants and analysis of the response by use of mutants. Proc Natl Acad Sci USA 70:817–821. https://doi.org/10.1073/pnas.70.3.817
Williamson VM, Gleason CA (2003) Plant-nematode interactions. Curr Opin Plant Biol 6:327–333. https://doi.org/10.1016/s1369-5266(03)00059-1
Wrather JA, Koenning SR (2006) Estimates of disease effects on soybean yields in the United States 2003 to 2005. J Nematol 38:173–180
Wrather JA, Koenning SR (2009) Effects of diseases on soybean yields in the United States 1996 to 2007. Plant Health Progress. https://doi.org/10.1094/PHP-2009-0401-01-RS
Acknowledgements
This project was supported by National Natural Science Foundation of China (31772139, 31471749) and Key Projects of Natural Science Foundation of Heilongjiang Province of China (ZD2017006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ulrike Mathesius
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hua, C., Li, C., Jiang, Y. et al. Response of soybean cyst nematode (Heterodera glycines) and root-knot nematodes (Meloidogyne spp.) to gradients of pH and inorganic salts. Plant Soil 455, 305–318 (2020). https://doi.org/10.1007/s11104-020-04677-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04677-z