Abstract
Cucumber (Cucumis sativus) cultivation in commercial greenhouses occupies an important section of vegetable production in Iran. Root-knot nematode Meloidogyne javanica considered the most destructive soil-borne pathogen in cucumber growing greenhouses. In this study, biocontrol activity of three species of entomopathogenic nematodes (EPNs, i.e., Steinernema carpocapsae, S. feltiae and Heterorhabditis bacteriophora) was determined on M. javanica infecting cucumber under growth chamber and greenhouse conditions. The aqueous suspension of infective juveniles (IJs) was used in five different inoculation times (i.e., 1 or 2 weeks pre-inoculation, simultaneously, and 1 or 2 weeks post-inoculation of the pathogenic nematode into the cucumber soil). Results showed that S. carpocapsae and H. bacteriophora were capable of decreasing all the pathogenicity indices (number of galls, eggs and egg masses) of M. javanica in growth chamber, as well as greenhouse conditions. The best application time for EPNs was determined as 1 week after post-inoculation of M. javanica into the soil. Although EPNs showed significant inhibition in 25 IJ/cm2 (3.8 IJ/cm3) of soil, the best biocontrol activity was observed in 125 IJ/cm2 (19.1 IJ/cm3). Furthermore, the highest reduction in pathogenicity indices was observed when EPNs-colonized cadavers were used as carrier of biocontrol agents. Significant increase in plant growth indices (e.g., fresh/dry weight of shoots/roots) was recorded for all treatments except S. feltiae. Altogether, our results provide a novel insight into the applicability of EPNs against the root-knot nematode M. javanica on cucumber. Further investigations are warranted to evaluate the commercial usability of the agents in cucumber growing greenhouses in Iran.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cucumber (Cucumis sativus) with producing 1.7 million tons in 2016 is one of the widely cultivated vegetables around the globe. Iran was ranked in fourth place in cucumber and gherkin production following China, Russia and Turkey (FAOSTAT 2017). Indeed, gourd plants (Cucurbitaceae) are increasingly grown on a large scale in Iran, in particular cucumber which is cultivated in both the greenhouse facilities and open areas where climatic conditions allow several harvests during the same year. Several biotic constrains were reported to affect cucumber production in Iran. Among the diseases caused by fungal and oomycete pathogens, Phytophthora root and stem rot and downy mildew are the most important ones (Esmaili-Shirazi and Banihashemi 2008). Further, bacterial diseases have been increasingly reported in the country (Sedighian et al. 2014). However, members of root-knot nematodes (Meloidogyne spp.) are considered the main constrain in both the field and greenhouse productions of cucumber in Iran. Although several Meloidogyne species were reported in Iran, M. javanica is the most destructive agent on annual crops in the country (Akhyani et al. 1984). As obligate plant parasites Meloidogyne spp. cause root galls, shoot chlorosis, stunted growth, water absorption disruption and nutrition deficiencies in the infested plants, leading to substantial yield losses (Hunt and Handoo 2009).
Regarding the day-to-day harvesting schedule which leads to limited options for use of conventional chemicals and pesticides in cucumber production, biotic pathogens and pests are considered as the main bottlenecks in the cucumber industry, especially where organic production is desired. On the other hand, special characteristics of cucumber cultivation under greenhouse facilities with ecologically controlled environment and the rapidity of cropping cycle create an excellent opportunity to deploy a biological control system in the greenhouse cucumber production. Several attempts were made to combat cucumber diseases and pests using biological control methods which are not only environmentally safer and sustainable, but also cheaper in a long-term prospect (Cao et al. 2011; Huang et al. 2012).
Among the promising biocontrol agents, soil-dwelling entomopathogenic nematodes (EPNs; families Steinernematidae and Heterorhabditidae) have frequently been used for biological control of insect pests and supposed to be applicable for plant parasitic nematodes as well (Bird and Bird 1986; Grewal et al. 1997; Pérez and Lewis 2002, 2004). EPNs are environmentally safe and have no side effect on free-living nematodes and other soil microbiota, which play an important role in cycling of organic materials (Somasekhar et al. 2002). Two native EPN isolates, S. rarum and H. bacteriophora decreased false root-knot nematode (Nacobbus aberrans) by 57 and 53%, respectively, on tomato plants (Caccia et al. 2013). Some studies have reported reducing plant parasitic nematode (PPN) populations by application of EPNs in the greenhouse and field trials (Bird and Bird 1986; Smitley et al. 1992; Grewal et al. 1997; Jagdale et al. 2002; Molina et al. 2007). However, the application of EPNs does not always reduce PPN populations (Fallon et al. 2002; Nyczepir et al. 2004; Shapiro-Ilan et al. 2006) and the results are divers. These reports indicated that the PPNs suppression by EPNs is impacted by EPN species, host plant, PPN species and application rate and time of EPNs (Pérez and Lewis 2004; Molina et al. 2007).
Infective juveniles (IJs) of EPNs that had emerged from insect cadaver had greater infectivity (Shapiro and Lewis 1999), more survival and dispersal capacity than IJs in aqueous suspension (Del Valle et al. 2013). Suppression of PPNs using EPN-infected insect cadaver has been investigated by Jagdale and Grewal (2008) and Del Valle et al. (2013), who reported applications of insect cadavers infected with S. carpocapsae and H. bacteriophora isolate Rama Caida can reduce Aphelenchoides fragaria and the number of M. incognita eggs, respectively.
The objective of the present study was to evaluate whether EPNs are capable of decreasing the infection rate of M. javanica on cucumber plants. We have analyzed the inhibitory effect of three widely used EPNs species against the root-knot nematode under different population density (25 vs. 125 infective juveniles (IJs)/cm2) and inoculation times (application of EPNs before, simultaneously, and after the pathogenic nematode). We have also evaluated two EPNs inoculation methods (i.e., aqueous suspension vs. insect cadavers colonized with EPNs) to the infested cucumber plants under greenhouse conditions. Altogether, our results provide novel insights into the applicability of EPNs against root-knot nematodes of cucumbers.
Materials and methods
Biological materials: collection, maintenance and reproduction
Root-knot nematodes were collected from symptomatic greenhouses tomato, eggplant and cucumber plants in Isfahan province, central Iran where severe outbreaks of the pathogen were recorded. The root-knot nematode was purified from a single egg mass and was propagated on tomato plants. Morphological characteristics of the nematodes were determined in the Nematology laboratory (Hunt and Handoo 2009). Molecular identification of the nematodes was performed using the M. javanica-specific primer pair OPAFjav (GGTGCGCGATTGAACTGAGC) and OPARjav (CAGGCCCTTCAGTGGAACTATAC) as described by Dong et al. (2001). DNA extraction was performed using the method described by Silva et al. (2000). The quality and quantity of DNAs were spectrophotometrically evaluated and adjusted to 50 ng µl−1 using Nanodrop® ND-100 (Nanodrop Technologies, Waltham, Massachusetts, USA) for further uses. For PCR reactions, Universal PCR Kit—Ampliqon® Taq DNA Polymerase Master Mix Red (Ampliqon A/S, Odense, Denmark)—was applied according to the manufacturer’s recommendations. For each strain, a 50 µl PCR, including 100 ng total DNA and 3 µl of each primer (10-pmol × µl−1), PCR conditions were as follows: 94 °C for 2.5 min, 64 °C for 0.5 min and 72 °C for 6 min using 37 cycles. PCR amplification products were electrophoretically fractionated on 1.2% agarose gel in TBE buffer 1X (0.09 M Tris–borate, and 2 mM EDTA). Gels were stained with ethidium bromide (0.5 μg/ml) and photographed under ultraviolet light. After verified identification by PCR, eggs of M. javanica were transferred into sterile soil containing a tomato seedling and maintained under greenhouse conditions for 45–60 days post-inoculation to obtain a high titer of pathogen population. The eggs were separated from egg masses with 1.5% NaOCl and stored for further use.
Three species of EPNs, i.e., Steinernema feltiae, S. carpocapsae and Heterorhabditis bacteriophora were obtained from Koppert B. V. Company (Berkel en Rodenrijs, The Netherlands). The quality of the formulated nematodes was checked under the stereomicroscope after separating them via a modified Berman funnel. The greater wax moth (Galleria mellonella) using the formulated diet (Eischen and Dietz 1990) was used as the host insect to reproduce EPNs. Third-stage juveniles of EPNs were harvested from the White traps (Kaya and Stock 1997) and stored in water at 16 °C and applied within 6 days of emergence. For long-term storage (about 8 months), infective juveniles of EPNs were transferred from the White traps to Falcon tubes (50 cc) containing thin sponges and tubes were stored horizontally at 8 °C.
To obtain colonized insect cadavers with EPNs, larvae were lined on a filter paper in Petri dishes, and they were exposed to 100 infective juveniles (IJs) of each of the EPN species. Petri dishes were incubated at 25 °C for 48 h, and infected cadavers were transferred to new Petri dishes lined with dry filter paper for a further 48 h of incubation to allow the development of typical signs of EPN infection (Del Valle et al. 2013).
Growth chamber experiments
Cucumber seeds (cv. Alfrid) were sown in paper cups (7-cm in diameter) containing a mixture of 150 g sterile perlite, soil and peat (ratio 1:1:1) and maintained in a growth chamber (23 °C and 14/10 h lights). Cucumber seedlings were inoculated with M. javanica eggs (500 eggs/cup) simultaneously with the infective juveniles of S. feltiae, S. carpocapsae, and H. bacteriophora which were suspended in sterile water and released to the soil of each cucumber plant at a rate of 25 IJs/cm2 or 3.8 IJs/cm3 (1000 IJs/cup). Inoculated plants were maintained at the same conditions as described above. Control plants were inoculated with M. javanica eggs (500 eggs/cup) in the same manner, except that sterile water was used instead of EPNs. The experiment was performed as a completely randomized design with eight replications (cups) per treatment. Four cups out of the eight replications per treatment were evaluated 15 days post-inoculation, and the second stage juveniles of root-knot nematode were counted using the acid fuchsin staining method. The remaining four cups were evaluated 35 days post-inoculation, and M. javanica eggs were counted. The entire experiment was repeated twice.
Greenhouse experiments
Estimation of the best application time for EPNs
Cucumber seeds were sown in plastic pots (18-cm in diameter) containing a mixture of 2 kg perlite, soil and peat (ratio 1:1:1) and maintained in a greenhouse (25 ± 3 °C and 14 h natural lights). The plants were watered when needed, and no further intervention was made during the experiment. Plants were inoculated using M. javanica eggs (5000 eggs/pot) as well as the larvae of two EPNs species (i.e., S. carpocapsae, H. bacteriophora) from which we obtained promising results in the growth chamber tests. Each treatment consisted of one EPN species which was applied in two population densities (i.e., 25 IJs/cm2 or 3.8 IJs/cm3 of soil = 6500 IJs/pot and 125 IJs/cm2 or 19.1 IJs/cm3 = 32,500 IJs/pot) as well as the M. javanica eggs. The EPN species were applied in five different times, i.e., 1 and 2 weeks pre- and post-inoculation of M. javanica eggs, as well as simultaneous with the pathogenic nematode. Control plants were inoculated with M. javanica eggs in the same manner, while sterile water was used instead of EPNs. No further intervention was made following the inoculation, and all the plants were incubated under greenhouse conditions as described above. The experiments were conducted as a completely randomized design with four replications per treatment. Cucumber plants were removed from the pots 8 weeks post-M. javanica inoculation, and the number of nematode eggs, egg masses and root galls was counted for each plant.
Estimation of the best inoculum form for EPNs
Following the estimation of the most appropriate inoculation time for EPNs, two different methods of EPNs inoculum preparation and inoculation method into the soil were evaluated. In the first method, G. mellonella cadavers which were colonized with infective juveniles of EPNs for 5 days were applied into the soil (simultaneous application of cadavers colonized with EPN and eggs of M. javanica), while in the second method EPNs larvae were re-suspended in sterile water and applied 1-week after inoculation of M. javanica eggs. Cucumber seeds were sown in paper cups as described above. Three weeks old seedlings were transferred into plastic pots (18-cm in diameter) containing a mixture of 2 kg perlite, soil and peat (ratio 1:1:1). While transferring the seedlings, three EPN-infested cadavers were placed in the corners of an imaginary triangle inside the soil, in a position in which one cadaver was placed in the bottom of the seedling, while the two remaining were placed on either sides of the root system, 2 cm below the soil surface. In the second inoculation method, infective juveniles of the EPNs which were re-suspended in sterile water were applied into the soil adjacent to the root system. The estimated population density of EPNs into the pots was adjusted to 25 IJs/cm2 or 3.8 IJs/cm3 in aqueous suspension. Simultaneously, M. javanica (5000 eggs/pot) was inoculated into the puts. Negative control plants were inoculated with insect cadavers without EPNs. The pots were watered at 2-day intervals to maintain the contents as close as possible to field capacity. The experiment was arranged in a completely randomized design in a greenhouse, and plants were maintained in ambient conditions up to 50 days post-inoculation as described above. Colonization indices of pathogenic nematode (i.e., number of eggs, egg masses and gall index) and plant growth parameters (i.e., fresh/dry weight of root/shoot system and plant/root height) were estimated in all the individual plants. Gall index (GI) was determined using a 1–6 scale (Marull and Pinochet 1991) as follows: 1 = no galls, 2 = 1–10 galls, 3 = 11–30 galls, 4 = 31–70 galls, 5 = 71–90 galls and 6 = more than 91 galls/root system. Egg masses were stained by dipping the roots in 0.01 Phloxine B for 20 min (Daykin and Hussey 1985) and then washed. The number of eggs per egg mass was determined by counting the eggs in eight egg masses randomly after shaking in 1.5% NaOCl for 2 min. The total number of eggs per root systems was calculated as the average number of eggs/egg mass × number of egg masses. Analysis of variance (ANOVA) for all the obtained data was performed using the SAS (v. 9.1) software. Furthermore, Duncan׳s multiple range test was employed for significant difference among treatments at P < 0.05.
Results
Root-knot nematodes extracted from the roots and galls of symptomatic cucumber plants were identified as M. javanica based on the morphological characteristics of cuticular markings in the perineal pattern of the mature females (Sayedain et al. 2013). Furthermore, the primer pair OPAFjav/OPARjav directed the amplification of a 670 bp DNA fragment in the genomic DNA of the plant parasitic nematode, confirming the specimen as M. javanica. The nematodes was multiplied on tomato plants and used in the following experiments.
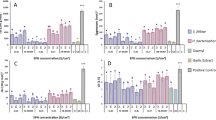
Root-knot nematode-infected cucumber plants inoculated with active juveniles of three EPNs species in the growth chamber showed a significant reduction in the number of juveniles in the root 15 days post-inoculation (F = 6.9; df = 3,12; P < 0.01, trial I) (F = 19.13; df = 3,12; P < 0.0001, trial II) (Fig. 1a) and number of eggs 35 days post-inoculation (F = 14.7; df = 3,12; P < 0.01, trial I) (F = 22.23; df = 3,12; P < 0.0001, trial II). Although the number of eggs in S. carpocapsae and H. bacteriophora treatments was significantly lower than in the control plants, the reduction was not significant in S. feltiae treatment (Fig. 1b, trial I). Treatment with S. feltiae in trial II decreased M. javanica egg recovered, but the number of eggs produced was significantly higher than S. carpocapsae and H. bacteriophora treatments (Fig. 1b, trial II). Regarding the unsatisfactory results of S. feltiae treatments on M. javanica control, we proceeded with S. carpocapsae and H. bacteriophora in the following experiment.
Effect of treatment with different EPN species (25 infective juveniles/cm2) on the number of Meloidogyne javanica inside cucumber roots. (A): number of juveniles of M. javanica inside roots and (B) number of M. javanica eggs/plant. S.c = Steinernema carpocapsae, S.f = Steinernema feltiae, H.b = Heterorhabditis bacteriopho. Mean values indicated by the same letter are not significantly different at P < 0.05 according to Duncan’s Multiple Range test
Best inoculation time of EPNs against M. javanica
In order to determine the best inoculation time of EPNs into the cucumber roots, greenhouse experiments were designed based on the results of the growth chamber experiment. The two EPN species H. bacteriophora and S. carpocapsae which effectively reduced M. javanica in the growth chamber tests also showed promising results in the greenhouse tests. For instance, 25 IJs/cm2 (3.8 IJ/cm3) of S. carpocapsae significantly decreased the number of M. javanica galls (F = 18.4; df = 5,18; P < 0.01), eggs (F = 21.6; df = 5,18; P < 0.01) and egg masses (Table 1). Besides, there were statistically significant differences among the inoculation times. Plants treated with 25 IJs/cm2 (3.8 IJ/cm3) of S. carpocapsae at 1 and 2 weeks pre-inoculation of M. javanica, as well as 1 week post-inoculation had a significantly lower number of root galls, egg masses and eggs (Table 1), while the lowest egg production was observed for the application of S. carpocapsae 1 week post-inoculation of cucumber plants with the M. javanica. Furthermore, at 125 IJs/cm2 (19.1 IJ/cm3) of S. carpocapsae in cucumber soil, numbers of galls (F = 29.6; df = 5,18; P < 0.01), eggs (F = 28.4; df = 5,18; P < 0.01) and egg masses were significantly reduced, and the lowest number of eggs was observed at 1 and 2 weeks, pre-inoculation and 1 week post-inoculation of M. javanica (Table 1).
As for H. bacteriophora, treatment of cucumber soil with 25 IJs/cm2 (3.8 IJ/cm3) significantly reduced the number of galls (F = 11.6; df = 5,18; P < 0.01), eggs (F = 20.4; df = 5,18; P < 0.01) and egg masses (Table 1). Although there was no significant difference in time of application at 1 and 2 weeks pre-inoculation and 1 week post-inoculation of M. javanica, the plants treated 1 week post-inoculation showed the lowest number of galls, egg masses and eggs in comparison with the control plants (Table 1). Finally, 125 IJs/cm2 (19.1 IJ/cm3) of H. bacteriophora significantly reduced the number of galls (F = 20.5; df = 5,18; P < 0.01), egg masses and eggs (F = 19.8; df = 5,18; P < 0.01) of M. javanica, while the lowest number of eggs was recorded for the application of H. bacteriophora 1 and 2 weeks pre-inoculation and 1 week post-inoculation of M. javanica (Table 1).
Best inoculum form of EPNs against M. javanica
A greenhouse test using the three EPN species was performed using two inoculum forms, i.e., colonized cadavers of G. mellonella versus active juveniles suspended in sterile water. Both the aqueous suspension and EPN-colonized cadavers led to a significant decrease in the gall index on cucumber roots, except for 25 IJs/cm2 (3.8 IJ/cm3) of aqueous suspension treatment of S. feltiae which was not statistically different from the control (Table 2). Similarly, the number of egg masses on cucumber roots was significantly reduced in all the treatments (F = 124.7; df = 7,24; P < 0.0001), except for the aqueous suspension of S. feltiae (Table 2). The lowest number of egg masses was observed in the plants treated with the EPN-colonized cadavers of S. carpocapsae (41.5 egg masses), H. bacteriophora (40.5 egg masses) and S. feltiae (42.5 egg masses), respectively. As for the egg numbers on cucumber roots inoculated with either inoculum form of EPNs, although a significant decrease was observed in the number of eggs in S. carpocapsae and H. bacteriophora treatments (4698 and 4920, respectively) compared to 12,906 eggs in the control plants, we did not observe a significant reduction in egg numbers in the S. feltiae treatment (F = 128.4; df = 7,24; P < 0.0001). The lowest number of eggs was observed following inoculation with EPN-colonized cadavers of S. carpocapsae (3361 eggs), H. bacteriophora (2470 eggs) and S. feltiae (3443 eggs), respectively (Table 2).
Application of EPNs on cucumber soil—in both the inoculum forms—resulted in a significant increase on the shoot fresh weight of cucumber plants compared to the control plants, except for S. feltiae (F = 3.5; df = 7,24; P < 0.009) (Table 2). Significant increases in the root fresh weight of cucumber plants were observed in both the application forms of S. carpocapsae (F = 3.3; df = 7,24; P < 0.013) (Table 2). The highest dry weight of cucumber roots was recorded following the inoculation of S. carpocapsae and H. bacteriophora aqueous suspensions (0.19 and 0.17 g, F = 8.8; df = 7,24; P < 0.0001). A significant increase in the shoot and root length was observed in all treatments except for EPN- colonized cadavers with S. feltiae (F = 3.3; df = 7,24; P < 0.012 and F = 3.7; df = 7,24; P < 0.066, respectively) (Table 2).
Discussion
In this study, we evaluated the biocontrol potential of three EPNs (i.e., H. bacteriophora, S. carpocapsae and S. feltiae) against the root-knot nematode M. javanica on cucumber in the growth chamber and under greenhouse conditions. Our results showed that H. bacteriophora and S. carpocapsae are capable of reducing nematode infestation in terms of number of galls, eggs and egg masses. Results also showed that both inoculation forms (i.e., aqueous suspensions vs. colonized cadavers of G. mellonella) were suitable inoculation methods under greenhouse conditions. To our knowledge, this is the first investigation on the applicability of EPNs to control M. javanica on cucumber plants.
Our data of the growth chamber experiment are in line with results by Fallon et al. (2002), showing that application of S. feltiae MG-14 and S. feltiae SN reduced M. javanica penetration on soybean after 3 days but did not affect M. javanica egg numbers recovered from tomato plant after 30 days. In contrast, Smitley et al. (1992) demonstrated that using H. bacteriophora did not reduce populations of M. rusticum in turf. Shapiro-Ilan et al. (2006) reported that despite the reduction in the number of egg masses in the S. riobrave treatment and increase in dry root weight in S. feltiae treatment, these two entomopathogenic nematodes could not control M. partityla in pecan. Furthermore, the application of S. riobrave and H. bacteriophora did not suppress Mesocriconema xenoplax in peach and pecan (Nyczepir et al. 2004).
Based on the infection indices observed on cucumber roots, the best application time of EPNs to the soil was 1 week post-inoculation of M. javanica. Our results were in congruence with those observed by Pérez and Lewis (2002) where the application of H. bacteriophora and S. feltiae (25 IJs/cm2 or 3.8 IJ/cm3) pre- or post-inoculation of M. incognita inhibited the penetration of the root-knot nematode and decreased the production of eggs on tomato plants. Although successful biocontrol activity was reported for S. feltiae on M. incognita in tomato plants, this EPN species was not able to decrease the number of eggs of M. hapla and M. javanica on peanut and tomato roots (Fallon et al. 2004; Pérez and Lewis 2004). Here, we could confirm that S. feltiae was also not effective against M. javanica on cucumber. However, it has been reported that the use of S. riobrave pre-inoculation with M. hapla had an inhibitory effect on the egg numbers (Pérez and Lewis 2004).
About how this interaction and suppression of plant-parasitic nematode, we believe that the parasitic behavior of M. javanica and invading the new roots above the root cap by J2 stage hatching from the egg, and on the other hand attracting S. carpocapsae to the root tips and staying there for some times (Nyczepir et al. 2004), can be a reason repel M. javanica (J2) and prevent of the root penetration. Moreover, Steinernema species can enter the roots and release their associated bacteria inside the root (Pérez and Lewis 2004), whereas the low penetration rate of S. feltiae MG-14 in tomato roots may have limited its potential to control M. javanica (Fallon et al. 2002). Suppression of root-knot nematodes by EPNs depends largely on the time of application, inoculum dosage, host plant, as well as the species of both the plant-parasitic nematode and EPN (Tsai and Yeh 1995). We found that application densities of 125 IJs/cm2 (19.1 IJs/cm3) would increase the biocontrol of M. javanica for all EPNs evaluated. Pérez and Lewis (2004) also found that application of 125 IJs/cm2 of H. bacteriophora co-inoculated with M. hapla in peanut reduced the egg production, while the same concentration in S. riobrave did not cause a better inhibitory effect on the pathogen. Although the application of S. carpocapsae, H. bacteriophora and S. feltiae as both aqueous suspension and colonized cadavers could reduce M. javanica infection, the colonized cadavers turned out to be more efficient in the biocontrol activity. Cadavers colonized with S. carpocapsae, H. bacteriophora and S. feltiae, respectively, resulted in a reduction of 72.9, 73.7 and 71.4% in the number of galls, and 73.9, 74.5 and 73.3% in the number of egg masses. In contrast, aqueous suspension of S. feltiae did not significantly decrease any of the measured indices of M. javanica on cucumber roots. Those data underline the importance of the inoculation method for the inhibitory activity of EPNs toward root-knot nematodes.
Application of colonized cadavers has the advantage of gradual releasing of EPNs juveniles in the soil, which depends on the availability of sufficient moisture and chemical activators around the cadavers (Koppenhöfer et al. 1997). Cadavers of G. mellonella were reported to support the stability and survival of the biocontrol agent in the soil environment. For instance, only the colonized cadavers of G. mellonella with H. bacteriophora—in comparison with several other insect species—were effective in reducing the number of root-knot nematode eggs in summer squash (Del Valle et al. 2013). Application of EPNs-colonized cadavers into the soil increased both the fresh and dry weight of cucumber shoots and roots. These results were in congruence with the results obtained in other successful applications of EPNs on annual crops, i.e., squash (Del Valle et al. 2013) and tomato (Kepenekci et al. 2016). The results published by Jagdale et al. (2002) and Molina et al. (2007) showed live and dead IJs of EPNs were toxic to plant-parasitic nematodes. However, Grewal et al. (1999) observed that only dead nematodes of S. feltiae and S. riobrave suppressed M. incognita penetration into tomato root. It seems that the controversial results reported in different studies were due in part to the differences in nematode inoculation methods and evaluating the particular stage of the plant-parasitic nematode life cycle. For instance, Molina et al. (2007) used three nematode application methods and evaluated gall index 9 weeks post-inoculation, while Grewal et al. (1999) have counted the number of J2 larva inside the roots 2, 4, 6 and 10 days post-inoculation. The observed biocontrol activity of EPNs against root-knot nematode is proposed to be attributed to the production of allelochemicals and ammonium by the symbiotic bacteria of EPNs (Grewal et al. 1999), induced systemic resistance in plants (Jagdale et al. 2009), competitive effects between the two groups of nematodes, and absorption of EPNs toward the root exudates (Tsai and Yeh 1995; Robinson 1995). In our study, with M. javanica the lower suppression using S. feltiae aqueous suspension may have been due to metabolites produced by its mutualistic bacteria because it was observed in another study conducted by the authors, that cell-free filtrate of X. nematophila and P. luminescens (symbiotic bacteria associated with S. carpocapsae and H. bacteriophora, respectively) was more effective than X. bovenii (symbiotic bacteria associated with S. feltiae) against M. javanica (J2) after 24 h (Sayedain et al. 2019). The interactions involved are highly complex, due to both the three organism system (host plant, plant-parasitic nematode and EPN) and the various soil factors. Natural products produced by symbiotic bacteria during the process of insect cadaver infection indicated nematicidal or repelling activity on plant-parasitic nematodes (Hu et al. 1999; Bi et al. 2018), and they are quantitatively and qualitatively different under in vivo and in vitro conditions (Webster et al. 2002).
In conclusion, this study provides for the first time novel insights into the applicability of EPNs for the biological control of root-knot nematode M. javanica on cucumber plants. Our results indicate that colonized cadavers of G. mellonella are capable of carrying sufficient IJs of EPNs to control of M. javanica on cucumber and improve growth under greenhouse conditions. However, further studies are warranted to evaluate the applicability of these EPNs in commercial greenhouses, and optimize the efficacy of insect cadaver to combat root-knot nematode. Moreover, since biocontrol agents are likely to pose physiological traits that are adapted to local climatic and ecological conditions, seeking endemic EPNs with biocontrol potential is highly recommended.
References
Akhyani A, Modjtahedi H, Naderi A (1984) Species and physiological races of root-knot nematodes in Iran. Iran J Plant Pathol 20:57–70
Bi Y, Gao C, Yu Z (2018) Rhabdopeptides from Xenorhabdus budapestensis SN84 and their nematicidal activities against Meloidogyne incognita. J Agric Food Chem 66(15):3833–3839. https://doi.org/10.1021/acs.jafc.8b00253
Bird AF, Bird J (1986) Observations on the use of insect parasitic nematodes as a means of biological control of root-knot nematodes. Int J Parasitol 16(5):511–516. https://doi.org/10.1016/0020-7519(86)90086-X
Caccia M, Lax P, Doucet M (2013) Effect of entomopathogenic nematodes on the plant-parasitic nematode Nacobbus aberrans. Biol Fertil Soils 49(1):105–109. https://doi.org/10.1007/s00374-012-0724-z
Cao Y, Zhenhua Z, Ning L, Yujuan Y, Xinyan Z, Biao S, Qirong S (2011) Bacillus Subtilis Sqr 9 can control Fusarium wilt in cucumber by colonizing plant roots. Biol Fertil Soils 47(5):495–506. https://doi.org/10.1007/s00374-011-0556-2
Daykin ME, Hussey RS (1985) Staining and histopathological techniques in nematology. In: Baker KR, Carter CC, Sasser JN (eds) An advanced treatise on Meloidogyne. Methodology, vol II. North Carolina State University Graphics, Raleigh, pp 39–48
Del Valle EE, Lax P, Rondan Dueñas J, Doucet ME (2013) Effects of insect cadavers infected by Heterorhabditis bacteriophora and Steinernema diaprepesi on Meloidogyne incognita parasitism in pepper and summer squash plants. Ciencia e investigación agraria 40(1):109–118. https://doi.org/10.7764/rcia.v40i1.456
Dong K, Dean RA, Fortnum BA, Lewis SA (2001) Development of PCR primer to identify species of root knot nematode: Meloidogyne arenaria, M. hapla, M. incognita and M. javanica. Nematropica 31:273–282
Eischen FA, Dietz A (1990) Improved culture techniques for mass rearing Galleria mellonella (Lepidoptera: Pyralidae). Entomol News 101(2):123–128
Esmaili-Shirazi E, Banihashemi Z (2008) The Role of Phytophthora melonis and P. drechsleri in Cucurbit Root Rot in Iran. Iran J Plant Pathol 44:54–72
Fallon DJ, Kaya HK, Gaugler R, Sipes BS (2002) Effects of etomopathiogenic nematodes on Meloidogyne javanica on tomatoes and soybeans. J Nematol 34(3):239–245
Fallon DJ, Kaya HK, Gaugler R, Sipes BS (2004) Effect of Steinernema feltiae-Xenorhabdus bovienii insect pathogen complex on Meloidogyne javanica. Nematology 6(5):671–680. https://doi.org/10.1163/1568541042843496
FAOSTAT (2017) Food and Agriculture Organization of the United Nations. FAOSTAT database. http://faostat.fao.org/
Grewal P, Martin W, Miller R, Lewis E (1997) Suppression of plant-parasitic nematode populations in turfgrass by application of entomopathogenic nematodes. Biocontrol Sci Tech 7(3):393–400. https://doi.org/10.1080/09583159730802
Grewal PS, Lewis EE, Venkatachari S (1999) Allelopathy: a possible mechanism of suppression of plant-parasitic nematodes by entomopathogenic nematodes. Nematology 1(7):735–743. https://doi.org/10.1163/156854199508766
Hu K, Li J, Webster J (1999) Nematicidal metabolites produced by Photorhabdus Luminescens (Enterobacteriaceae), bacterial symbiont of entomopathogenic nematodes. Nematology 1(5):457–469. https://doi.org/10.1163/156854199508469
Huang X, Nan Z, Xiaoyu Y, Xingming Y, Qirong S (2012) Biocontrol of Rhizoctonia solani damping-off disease in cucumber with Bacillus pumilus Sqr-N43. Microbiol Res 167(3):135–143. https://doi.org/10.1016/j.micres.2011.06.002
Hunt DJ, Handoo ZA (2009) Taxonomy, identification and principal species. In: Perry RN, Moens M, Starr JL (eds) Root-knot nematodes. CABI Publishing, London, pp 55–88
Jagdale GB, Grewal PS (2008) Influence of the entomopathogenic nematode Steinernema carpocapsae infected host cadavers or their extracts on the foliar nematode Aphelenchoides fragariae on Hosta in the greenhouse and laboratory. Biol Control 44(1):13–23. https://doi.org/10.1016/j.biocontrol.2007.07.001
Jagdale GB, Somasekhar N, Grewal PS, Klein MG (2002) Suppression of plant-parasitic nematodes by application of live and dead infective juveniles of an entomopathogenic nematode, Steinernema carpocapsae, on Boxwood (Buxus Spp.). Biol Control 24(1):42–49. https://doi.org/10.1016/S1049-9644(02)00004-X
Jagdale G, Kamoun S, Grewal P (2009) Entomopathogenic nematodes induce components of systemic resistance in plants: biochemical and molecular evidence. Biol Control 51(1):102–109. https://doi.org/10.1016/j.biocontrol.2009.06.009
Kaya HK, Stock SP (1997) Techniques in insect nematology. In: Lacey LA (ed) Manual of techniques in insect pathology. Academic Press, London, pp 281–324
Kepenekci I, Hazir S, Lewis EE (2016) Evaluation of entomopathogenic nematodes and the supernatants of the in vitro culture medium of their mutualistic bacteria for the control of the root-knot nematodes Meloidogyne incognita and M. arenaria. Pest Manag Sci 72(2):327–334. https://doi.org/10.1002/ps.3998
Koppenhöfer AM, Baur ME, Stock SP, Choo HY, Chinnasri B, Kaya HK (1997) Survival of entomopathogenic nematodes within host cadavers in dry soil. Appl Soil Ecol 6(3):231–240. https://doi.org/10.1016/S0929-1393(97)00018-8
Marull J, Pinochet J (1991) Host suitability of Prunus rootstocks to Meloidogyne species and Pratylenchus vulnus in Spain. Nematropica 21(2):185–195
Molina JP, Dolinski C, Souza RM, Lewis EE (2007) Effect of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) on Meloidogyne mayaguensis Rammah and Hirschmann (Tylenchida: Meloidoginidae) infection in tomato plants. J Nematol 39(4):338–342
Nyczepir AP, Shapiro-Ilan DI, Lewis EE, Handoo ZA (2004) Effect of entomopathogenic nematodes on Mesocriconema Xenoplax populations in peach and pecan. J Nematol 36(2):181–185
Pérez EE, Lewis EE (2002) Use of entomopathogenic nematodes to suppress Meloidogyne incognita on greenhouse tomatoes. J Nematol 34(2):171–174
Pérez EE, Lewis EE (2004) Suppression of Meloidogyne incognita and Meloidogyne hapla with entomopathogenic nematodes on greenhouse peanuts and tomatoes. Biol Control 30(2):336–341. https://doi.org/10.1016/j.biocontrol.2004.01.001
Robinson A (1995) Optimal release rates for attracting Meloidogyne incognita, Rotylenchulus reniformis, and other nematodes to carbon dioxide in sand. J Nematol 27(1):42–50
Sayedain FS, Olia M, Jaimand K (2013) Effect of initial density of Meloidogyne javanica on Salvia officinalis. J Med Plants By-Prod 2(1):13–16
Sayedain FS, Ahmadzadeh M, Talaei-Hasanlouei R, Olia M, Bode HB (2019) Nematicidal effect of cell-free culture filtrates of EPN-symbiotic bacteria on Meloidogyne javanica. Biol Control Pests Plant Dis 8(1):17–26. https://doi.org/10.22059/jbioc.2018.244323.212
Sedighian N, Shams-Bakhsh M, Osdaghi E, Khodaygan P (2014) Etiology and host range of bacterial leaf blight and necrosis of squash and muskmelon in Iran. J Plant Pathol 96:507–514. https://doi.org/10.4454/jpp.v96i3.3201
Shapiro DI, Lewis EE (1999) Comparison of entomopathogenic nematode infectivity from infected hosts versus aqueous suspension. Environ Entomol 28(5):907–911. https://doi.org/10.1093/ee/28.5.907
Shapiro-Ilan DI, Nyczepir AP, Lewis EE (2006) Entomopathogenic nematodes and bacteria applications for control of the pecan root-knot nematode, Meloidogyne partityla, in the greenhouse. J Nematol 38(4):449–454
Silva AT, Penna JCV, Goular LR, Santos MA, Arantes NE (2000) Genetic variability among and within races of Heterodera glycines ichinohe assessed by RAPD markers. Genet Mol Biol 23:323–329. https://doi.org/10.1590/S1415-47572000000200014
Smitley D, Warner F, Bird G (1992) Influence of irrigation and Heterorhabditis bacteriophora on plant-parasitic nematodes in turf. J Nematol 24(4S):637–641
Somasekhar N, Grewal PS, De Nardo EA, Stinner BR (2002) Non-target effects of entomopathogenic nematodes on the soil nematode community. J Appl Ecol 39(5):735–744
Tsai BY, Yeh HL (1995) Effect of Steinernema carpocapsae Weiser on the infectivity of Pratylenchus coffeae (Zimmermann) Filipjev & Schuurmans Stekhoven and Meloidogyne javanica (Treub) Chitwood. Plant Pathol Bull 4(3):106–110
Webster JM, Chen G, Hu K, Li J (2002) Bacterial metabolites. In: Gaugler R (ed) Entomopathogenic nematology. CABI, Wallingford, pp 99–114
Acknowledgements
Financial support for this study was provided by the University of Tehran. Also the authors would like to thank Gyah corporation for help at the beginning of this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Human and animal rights
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sayedain, F.S., Ahmadzadeh, M., Fattah-Hosseini, S. et al. Soil application of entomopathogenic nematodes suppresses the root-knot nematode Meloidogyne javanica in cucumber. J Plant Dis Prot 128, 215–223 (2021). https://doi.org/10.1007/s41348-020-00367-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-020-00367-1