Abstract
Aims
To evaluate the efficiency of acidified (to pH 5.5) and dried digestate solids as a fertilization product in a pot experiment with ryegrass.
Methods
The NH4-N pool of the solids was labelled with 15N prior to acidification and drying to estimate plant N uptake efficiency. The effect of digestate solids on plant growth and N and P uptake was compared to the effect of different mineral N-P fertilization combinations.
Results
Non-acidified dried digestate solids showed inferior N fertilization performance compared to their raw and acidified counterparts, and higher drying temperature reduced solids N mineralization. More than 40% of the NH4-N in the raw, raw acidified and acidified dried solids treatments was recovered by the ryegrass over 5-months, similar to recovery with medium mineral N rates, resulting in mineral fertilizer equivalency of 32–41%. Digestate solids treatment altered the water extractable P of the solids, but less so the ryegrass P uptake, mainly due to the buffer capacity of the soil. However, ryegrass P uptake in non-acidified dried solids treatments was probably inhibited by poor growth due to low N availability.
Conclusions
Acidification of digestate solids prior to drying may be an important treatment to maintain the solids fertilizing value while enhancing its manageability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anaerobic digestion (AD) is a well-established technology for the treatment of organic waste and the production of bio-methane as a renewable source of energy. The digestate produced from AD, has been commonly applied to soil as fertilizer or soil conditioner due to its’ rich nutrient content (Möller and Müller 2012). However, the increase in AD plants will result in large amounts of available digestate that can lead to disposal problems since most countries have various legislative restrictions on fertilizer and organic waste application to agricultural land. In addition, the environmental risks associated with storage and land application of organic waste emphasizes the need for digestate treatment to minimize such risks and to facilitate the transport and appropriate distribution of surplus digestate to areas with nutrient shortages (Jensen 2013).
Digestate from a biogas plant often undergoes a mechanical liquid-solid separation treatment where, the liquid fraction may be used in close proximity to their production, while the solids from the digestate (SD) may be stored and exported. However, the relatively low concentration of nutrients and carbon of solids strongly limit long distance transport (Möller and Müller 2012) and extra treatment of SD is generally needed. Among the existing technologies for SD treatment, drying is popular due to the significant reduction of the mass/volume-ratio and high dry matter (DM) content (up to 95%) of the resulting solids (Bennamoun 2012). However, thermal treatments often result in large NH3 volatilization from the solids (Deng et al. 2009) with negative environmental impact and the dried product is often depleted in inorganic nitrogen (Ninorg) with direct consequences on its short-term N fertilizing value (Chiyoka et al. 2014). Such low amount of available plant nutrients after drying may be a significant limitation for recycling SD back to the agricultural system. On the other hand, other studies suggest that the drying induced conditioning of the organic matter, increases the mineralization rate of the organic nitrogen (Norg) in the dried organic fertilizers, which largely compensates any ammonium-N (NH4-N) losses during the thermal treatment (Smith and Durham 2002).
Inorganic nitrogen losses during drying of digestate solids may be largely avoided by acidifying the solids to pH 5.5 (Pantelopoulos et al. 2016a), but the acid treatment of digestate solids may alter not only the pH but also the overall composition of the digestate solids and consequently their behavior following soil application (Fangueiro et al. 2013; Sommer et al. 2014). Fangueiro et al. (2009) reported increased N mineralization rates from the solid fraction of acidified pig slurry relatively to solids from untreated slurry. However, any associated effects on the actual plant nutrient availability is limited.
Furthermore, SD obtained by centrifugation contains most of the P initially present in the digestate and may provide an adequate replacement of mineral P fertilizers (Hjorth et al. 2010). Nevertheless, it has been shown that animal manure exposure to high temperatures (350 °C- 700 °C) decrease the soluble P content in the final ash or biochar product (Cantrell et al. 2012). Reports on temperatures appropriate for drying (e.g. 70 °C – 160 °C) are scarce and often contradictory in relation to the effect of drying on P solubility. Sistani et al. (2001) have shown that drying of broiler manure at 65 °C decreased the water extractable (WEP) content in the dried product however, when manure was dried at 160 °C there was no significant effect. In contrast, Dail et al. (2007) reported an increase of WEP in dried poultry manure, up to 92% compared to the fresh material. Moreover, Vadas and Kleinman (2006) also showed that thermal drying at temperatures between 40 and 80 °C increased the WEP in animal manures. In conclusion, the evidence for impacts of manure drying on soil WEP is conflicting. Furthermore, studies concerning the agronomic impact of dried SD at moderate temperatures with respect to their plant P availability are non-existent.
Therefore, while a considerable amount of literature addresses the N and P fertilizing value of digestate effluents of AD, little is known about the behavior of the treated SD in soil and their potential for mineral N and P fertilizer substitution in crop production. Thus, the overall objective of this study was to evaluate the yield response and N and P uptake of ryegrass when fertilized with SD treated by acidification and drying at different temperatures. It was hypothesized that:
-
1)
Drying of the SD will result in loss of ammonium-N (NH4-N), but will enhance N mineralization and plant N uptake rates after soil application of the dried SD, compared with the initial, raw digestate solids
-
2)
Higher drying temperatures will induce more pronounced effects on the increase of N mineralization and plant N uptake rates of the dried SD
-
3)
Drying of the SD will decrease the soluble fraction of P in the solids and the plant P uptake, with higher drying temperatures resulting in lower P availability
-
4)
Acidification retains a higher proportion of ammonium-N, but will also increase the organic N mineralization rates and N plant uptake after soil application of the acidified SD compared to non-acidified SD
-
5)
Acidification will increase the concentration of the soluble fraction of P and will induce higher P plant uptake rates in comparison with the non-acidified treatments
Materials and methods
Digestate solids
Dewatered solids from digestate (SD) were collected fresh from Mørso biogas plant, Denmark. Solids were sampled at the end of the solids carrying belt, after decanter centrifugation of the digestate, within a period of 45 min. Solids were sealed in 5-L plastic containers and kept frozen (−18 °C) until further use. Before initiation of the experiment, the necessary amount of solid was thawed at room temperature (24 h).
Prior to further treatment, the NH4-N pool of the solids was labelled with 15NH4 by applying 0.02 ml g−1 solids (wet weight basis (w.w)) (15NH4)2SO4-solution (98 atom% excess, corresponding to 0.92 g NH4-N addition kg−1 solids), and subsequently thorough mixing to evenly distribute the solution on the solids surface. The resulting 15N enrichment was between 6.25 and 10 atom% 15N excess in the solids ammonium pool. The increase of the 15N enrichment of the solids would allow following the fate and distinguishing the plant uptake of original solids ammonium-N from the N subsequently mineralized from soil and solids organic-N. Subsequently, acidification of the solids to pH 5.5 was performed with direct application of concentrated sulphuric acid (17.5 μl H2SO4 g−1 solids (dry matter basis (DM)) to the solids surface and mixing to achieve homogenous distribution of the acid. Drying of acidified as well as non-acidified solids was then performed in a conductive oven at either 70 °C or 160 °C, as described in detail by Pantelopoulos et al. (2016a). Briefly, 50 g (w.w) were placed in aluminum foil containers (250 ml) and dried until solids dry matter content reached approximately 85% (w.w.). Dried solids and parent materials were kept frozen (−18 °C) until further use.
Experimental setup
The soil used in the present study was collected from the upper soil layer (0–15 cm) of an agricultural field in Vila Real, Portugal. The soil has a sandy loam texture (8.8% clay, 11.8% silt and 79.4% sand) with pH (H2O) 5.7, 0.58% C, 0.99% OM, 1.02 mg kg−1 NH4-N, 9.7 mg kg−1 NO3-N, 6.2 and 159 mg kg−1 lactate extractable P and K (pH 3.5–3.7), respectively (Egner et al. 1960). The water holding capacity of the soil (WHC) was 24% (weight:weight) as estimated by the soil saturation method. Soil was air-dried (max. 3% moisture content), sieved through 5 mm mesh and pre-incubated for two weeks in the greenhouse with the moisture level maintained at 50% of the WHC.
After the end of pre-incubation period, in the SD treatments (see Table 1 for details) 2.7 kg soil (DM) were mixed with 2.7 g (DM) SD kg−1 soil, equivalent to 8 tons (DM) ha−1 or on average 287 (±26) kg N ha−1 and 172 (±0.5) kg P ha−1 (considering the uppermost 20 cm of the soil and a soil density of 1.5 g cm−3) as it is common for agricultural practices in Portugal. In the mineral fertilized reference treatments, mineral N fertilizer (15NH4NO3 with 10 atom% excess 15N) was applied at the beginning of the experiment at two rates N1 (110 mg N pot−1) and N2 (220 mg N pot−1), corresponding to area-equivalent application rates of 120 and 240 kg N ha−1, respectively, if assuming an incorporation depth of 20 cm. Furthermore, each mineral N fertilizing rate was combined with three inorganic P (applied as superphosphate) fertilization rates equivalent to 0 (P0), 40 (P1) and 80 (P2) mg P pot−1, equivalent to application rates of 0, 45, 90 kg P ha−1 (Table 1). Soil without external addition of N and P served as a control treatment (N0P0). In this paper, the terms N(x) and P(x) will also be used, to abbreviate all the three levels of N and P addition in soil, respectively. All treatments received 220 mg K pot−1 (as KCL) as basal fertilization (equivalent to 250 kg K ha−1). Each treatment was replicated 4 times, resulting in a total of 52 pots. Soil was packed at a density close to 1.49 g cm−3 and water was added to reach 65% WHC moisture conditions. In each pot, 100 seeds of ryegrass were sown one week after application of the solids to pots. Pots were regularly weighted and irrigated with distilled water to maintain stable moisture conditions. No leachate was produced, and hence no nutrients were lost via leaching.
A first pre-cut was performed 10 days after plant emergence to standardize the starting point of the plants’ growth and the harvested biomass was left in the soil surface of the respective pots. The ryegrass aerial biomass was cut when ryegrass showed signs of physiological maturity at 35, 64 and 130 days after plant emergence, however, plants were collected slightly pre-mature at the second cut due to signs of pest insect infection in the greenhouse. All aboveground biomass above 20 mm from the soil surface was cut, and dry matter was determined after oven-drying at 45 °C for 96 h. The dry biomass was milled and analyzed for total N and 15N atom% excess by elemental analyses and isotope ratio mass spectrometry. Total P and other elements were determined by ICP-OES after microwave digestion of biomass samples with nitric acid (HNO3) and hydrogen peroxide (H2O2) (Hansen et al. 2013).
A parallel incubation was established with the same treatments to monitor the evolution of water extractable P (WEP) in the soil without the influence of plants. Pre-incubated soil (50 g DM) was mixed with the different treatments, in triplicate, at rates equivalent to the pot experiment. Soil was gently compacted to 1.49 g cm−3 and moisture content was raised to 65% WHC as previously described. Plastic lids were loosely attached to the containers to ensure the aeration of the soil and minimize water evaporation. Samples were incubated in the same greenhouse where planted pots were placed. Soil moisture was regularly checked by weighing, and addition of distilled water when necessary, to maintain stable moisture conditions. After 1, 48 and 130 days, a triplicate set of soil samples from each treatment was removed and WEP was determined, after air drying of the samples at room temperature. The determination of WEP in the digestate solid treatments and in the soil samples was performed after water extraction (1:60, sample (DM): distilled water), shaken on a rotary shaker for 1 h and subsequent filtration through a 0.42 μm syringe filter. Filtrates were kept refrigerated (4 °C) for less than 12 h until inorganic orthophosphate P concentration was determined by flow injection analysis with the Ammonium Molybdate method. Similarly, at the end of the plant trial at day 130, bulk soil samples from the planted pots were extracted and analyzed for WEP.
Calculations and statistics
Nitrogen and phosphorus uptake in above ground plant material at time of harvest (NU, PU) was calculated by multiplying shoot dry matter yields (g DM pot−1) with N and P concentration (mg N g−1 DM) of harvested biomass respectively. Apparent N recovery (ANR) and Apparent P recovery (APR) were calculated as:
The N derived from 15NH4-labelled fertilizer (\( {Ndff}_{15_{NH4}} \)mg N pot−1) in the solids or mineral reference treatments was estimated similar to (Nannen et al. 2010) as follows:
where a, b, c and d denote the atom% 15N–excess in (a) the applied treatment material (SD or mineral N fertilizer) and (b) unlabeled control soil, as well as 15N–excess in the harvested biomass in (c) the SD or mineral N applied treatment and (d) nonfertilized (unlabeled) treatment, and NUtreatment is the total plant N uptake (mg N pot−1) in the respective treatment.
Recovery proportion of 15N–labelled NH4-N (R15NH4-N) from the applied treatments was calculated by
where, f is the amount of 15NH4-N (mg N pot−1) applied with each treatment.
The nitrogen mineral fertilizer equivalence (N-MFE) of digestate solids treatments was calculated according to Muñoz et al. (2004) by regressing the N uptake from ryegrass treated with SD treatments against the linear N uptake in ryegrass treatments received increasing mineral N and P fertilizer levels.
To reveal the effect of the applied treatments and ryegrass cut times on plant biomass yields, total N concentration in harvested biomass, the amount of nitrogen derived from the labelled 15NH4-N pool of the treatments, the R15NH4-N recovery and ANR, a two-way analysis of variance (ANOVAs) was conducted. Assumption of homogenous variance of different groups was tested using Levene’s test. The significance of the differences was estimated using the Tukey post-hoc test (HSD). Statistical analysis was performed with SPSS 23.0 software package for Windows (IBM Inc., Chicago, IL).
Results
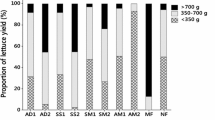
Ryegrass yields obtained within each cut and summed total values (Fig.1), varied significantly with treatment (P < 0.01) and cut time (P < 0.01) and the interaction Treatment x Cut time had also a significant effect (P < 0.01) (Table 2). The highest total yields were observed for N2P1 and N2P2 and the lowest in soil amended with raw solids dried at 160 °C (D160). At the first cut, ryegrass yields in soil amended with raw (R) and acidified digestate solids treatments (RA, AD70 and AD160) were generally comparable with treatments receiving equivalent amount of mineral N and P fertilization. At second and third cut, treatments received the highest level of mineral N, irrespectively of the level of P applied (N2Px) gradually outperformed the yields of the respective organically fertilized treatments and the other mineral fertilizer treatments. Thermal treatment of non-acidified SD had a significant impact on ryegrass yields (Fig. 1) and dried non-acidified SD (D70 and D160) resulted in plant yields close to the unamended control treatment (N0P0) and significantly depressed relative to all other fertilized treatments. Non-acidified SD dried at 70 °C (D70) led to significantly (P < 0.01) higher shoot yields (first cut and total) compared to SD dried at 160 °C (D160) though. However, when acidified, the drying only reduced ryegrass yield significantly when comparing RA and AD70 treatments (a 14% reduction), whereas AD160 was not significantly lower than RA. In minerally fertilized (NxPx) treatments, plant growth increased when N and P application rates increased, with P not being limiting at low N rate, but at the highest N rate, P application gave a significant increase in total yield.
Ryegrass yield (dry matter, DM) at each cut (35, 64 and 130 days after plant emergence, respectively) and accumulated total yield in the different treatments amended with digestate solids and reference mineral fertilizers (N, P), for abbreviations refer to Table 1. Error bars represent standard error of the mean (n = 4). Treatments with different letters are significantly different (P < 0.05) according to Tukey test (HSD)
All treatments, except D160, resulted in a significant increase of ryegrass N uptake compared to the control treatment (N0P0) (Fig.2). The highest ryegrass N uptake (and N concentration values, suppl. Mat Tab S1) values were observed at the first cut and significantly decreased at later cuts. Among SD treatments, accumulated total N uptake declined in the order RA = R > AD70 = AD160 > D70 > D160. Within each cut and accumulated, plant N uptake from R and all acidified SD treatments, was comparable to that of low N mineral treatments (N1Px). Overall, N uptake in both individual cuts and total were generally greater with higher N application rates (N2Px). A further increase in N uptake with increased P fertilization rates was only significant at the high N (N2Px) application rates.
Total N uptake and uptake of N derived from the 15N labelled ammonium pool of the respective fertilizer (Ndff15 NH4-N) (black part of bars) in ryegrass shoots amended with digestate solids and reference mineral fertilizers (N, P), for abbreviations refer to Table 1. Error bars represent standard error of the mean (n = 4). Treatments with different letters are significantly different (P < 0.05) according to Tukey test (HSD)
Uptake of amendment-derived NH4-N (indicated by the black part of the bars in Fig. 2) varied significantly (P < 0.01) between treatments (Table 2). Drying decreased the amendment-derived NH4-N uptake in all cases, with average reduction of 97% for D160 and 93% for D70 compared to the R treatment. Furthermore, non-acidified SD treatments, showed reduced amendment-derived NH4-N plant uptake compared with to their acidified counterparts, by 53% between the raw solids and 96% between the dried digestate solids treatment. The effect of drying on ryegrass uptake of amendment derived NH4-N was less pronounced in the case of the acidified solids where, the associated reduction was on average 20% for AD70 and 26% for AD160 compared to the raw acidified solids. In the mineral fertilizers treatments, higher N application rates resulted in higher amendment-derived NH4-N while the effect of P application rate was only significant between the null (P0) and the P1, with not significant effect between the P1 and P2 application rates.
Treatments had a significant effect (P < 0.01, Table 2) on both the specific recovery of 15NH4-N as well as the apparent total N recovery (ANR) from labelled solids and NxPx treatments (Fig.3). Thermal drying of SD significantly decreased N recovery from the labelled 15NH4-N pool of the solids (Fig.3, left), irrespectively of any chemical pre-treatment of the solids. Drying decreased the 15NH4-N recovery from acidified solids by 8% and by 37% in the case of non-acidified treatments while no significant difference were observed between solids dried at different temperatures. The respective N recovery from the mineral reference amendments showed a steady increase, concomitant with the increase in N and P application rates with an exception in the case of NxP0 treatments, where no significant differences in 15NH4-N recovery were detected between high and low N application rates.
Cumulative recovery of 15NH4-N (left) and apparent N recovery (ANR, difference method, right) from labelled solids and mineral reference amendments in ryegrass shoots amended with digestate solids and reference mineral fertilizers (N, P), for abbreviations refer to Table 1. Error bars represent standard error of the mean (n = 4). Treatments with different letters are significantly different (P < 0.05) according to Tukey test (HSD)
Results of Apparent Nitrogen Recovery (ANR) (Fig. 3, right) followed similar trends between treatments to those described before for recovery from the labelled 15NH4-N pool. Overall, the lowest ANR was detected for the dried non-acidified treatments where solids dried at the highest temperature of 160 °C led to an ANR of −2% and solids dried at 70 °C resulted in an ANR of 4%. The results highlighted the significant drying induced decrease (P < 0.01) of ANR in non-acidified treatments compared to the respective raw solids. Acidification of SD increased the ANR compared to the raw non-acidified counterparts while, drying of acidified solids decreased the ANR of the solids by 7% and 6% at the lowest and highest drying temperatures respectively. ANR values for the mineral treatments clearly outperformed the respective values from digestate solids and the highest ANR values were observed for the N2P2 treatment, where ANR was 79%. The results revealed a clear effect of P on N recovery with similar values of ANR in N1P0 and N2P0, significantly lower than the remaining mineral treatments.
The concentration of water extractable phosphorus (WEP, Fig. 4) in soil increased after the application of raw and treated SD and P containing mineral fertilizers, compared to control (N0P0) and (NxP0) treatment. The highest level of WEP at day 1 was found in the AD160 treatment with 14.5 mg P kg−1 soil, while the lowest values were observed in N1P0 (4.2 mg P kg−1) and N2P0 (5.5 mg P kg−1 soil) treatments, with no significant difference from the control soil. During the incubation trial, WEP values of digestate solids treatments and NxP2 showed an average reduction of 40% in WEP by day 130 while NxP1 treatments showed largely stable WEP concentrations throughout the incubation. No clear effect of either acidification nor drying of SD were observed on WEP content after soil application, even if acidified SD dried at 160 °C tended to have higher values compared to the other organic treatments until day 48. Nevertheless, at the end of the incubation, the absence of any significant differences between treatments indicates that the soil reached an equilibrium state as in the soil sampled after ryegrass biomass harvest at day 130 from the pot trial.
Water extractable phosphorus (WEP) concentration in incubated soil and residual WEP in pots' soil amended with digestate solids and reference mineral fertilizers (N, P), for abbreviations refer to Table 1. Error bars represent standard error of the mean (n = 3 for incubated soil and n = 4 for soil from the pot trial). Treatments with different letters are significantly different (P < 0.05) according to Tukey test (HSD)
Cumulative P uptake in the ryegrass plants ranged between 3.6 and 15.8 mg P pot−1 (DM) for the control and N2P2 treatments respectively (Fig. 5). Considering non-acidified solids, thermal treatment significantly decreased the cumulative P uptake from plants in levels similar to ryegrass amended with solely N fertilizer (NxP0) (P < 0.01). Furthermore, the highest drying temperature appeared to further depress plant P uptake compared to non-acidified solids dried at 70 °C. The effect of acidification on plant P uptake from digestate solids was evident only in the case of dried solids, where AD70 showed significantly higher P uptake compared to D70 while AD160 showed almost double cumulative P uptake compared to D160. Mineral reference treatments showed a gradual increase in ryegrass P uptake concomitant with the increase in N and P application rates.
Cumulative phosphorus uptake and apparent phosphorus recovery (APR) in ryegrass (shoots) amended with digestate solids and reference mineral fertilizers (N, P), for abbreviations refer to Table 1. Error bars represent standard error of the mean (n = 4). Treatments with different letters are significantly different (P < 0.05) according to Tukey test (HSD)
Furthermore, Fig. 5 provides an overview of the apparent P recovery (APR) from the different treatments utilized in the study. The highest values of APR were observed for the mineral reference treatments and peaked at 22% APR for the N2P1 treatment. Generally, for the mineral treatments, lower P application rates (P1) resulted in higher APR values than the higher rate (P2), within each N application rate. Among the digestate solids treatments, Fig. 5 highlights the significantly lower APR values of D70 and D160 compared with the rest of the organic treatments.
The different treatments applied to SD significantly altered their nitrogen mineral fertilizer equivalence (N-MFE) (Fig. 6). Raw acidified digestate solids (RA) showed the highest N-MFE value of 40.8% whereas the lower value of −4.3% was observed for non-acidified digestate solids dried at the highest temperature (D160). Drying generally decreased the N-MFE of digestate solids compared to their raw counterparts however, the effect of drying temperature was significant only in the case of non-acidified solids with D160 showing significant lower N-MFE compared to D70. Acidification increased the N-MFE of raw solids by 16% and acidified dried solids showed similar N-MFE values to the raw non-acidified solids (R).
Nitrogen mineral fertilizer equivalence (N-MFE, relative efficiency of the total N in solids to substitute mineral fertiliser N) of raw digestate solids (R), raw acidified digestate solids (RA), digestate solids and acidified solids dried at 160 °C and 70 °C (D160, D70, AD160, AD70, respectively). Error bars represent standard error of the mean (n = 4). Treatments with different letters are significantly different (P < 0.05) according to Tukey test (HSD)
Discussion
Effect of SD treatment on plant growth and N availability
Ryegrass biomass trends
The positive response of ryegrass growth to higher N fertilization rates through mineral fertilizers in the current study, indicates the soils’ low N supply capacity as the primary limiting factor for plant growth (Cordovil et al. 2007; Gunnarsson et al. 2010) (Fig.1). Moreover, the decrease of ryegrass average yields per day from 1st to 3rd cut observed here in the solids amended treatments, are in line with previous studies that have assessed the fertilizing value of a single application of organic fertilizers (Grigatti et al. 2011; 2014). The significant decline in shoot biomass yields during the 2nd growing cycle (from day 35 to 64) of ryegrass compared to the rest of the harvests, may also be partly attributed to the restricted and lower solar radiation during winter season as well as to temporal N unavailability due to N immobilization (de la Fuente et al. 2013). It should be further noticed that the 2nd harvest was performed before the plants reached physiological maturity, to avoid the risk of a pest insect incident in the greenhouse.
Ryegrass utilized 55% to 79% of the N applied with synthetic fertilizers, in line with previous results (Cavalli et al. 2014; Gutser et al. 2005). The significantly lower N recoveries from mineral treatments with N but not P (NxP0) may be attributed to P deficiency (Chaudhary et al. 2008) or to higher N allocation to the roots. Indeed, the low P concentration of the soil used here was highlighted by the positive response of ryegrass growth to P fertilization when comparing yield differences between N2P0 and N2P1 or N2P2 treatments (Fig.1).
Effect of drying
Digestate solids acidification prior to drying, led to contrasting effects on ryegrass biomass yield after soil application of the dried solids. Ryegrass amended with dried non-acidified solids, showed significantly lower yields compared to the other organic and mineral treatments (Fig. 1). On average, 46% and 58% reduction in DM yields was observed for the D70 and D160 respectively, compared to raw solids (R), in agreement with Chiyoka et al. (2014) who reported 45% reduction in plant biomass yields after fertilization with pelletized digestate solids compared to untreated solids. In the current study, the observed differences in biomass yields between solids dried at different temperatures were similar to those reported by Moritsuka et al. (2006) who found that increased drying temperature of bio-solids significantly reduced plant growth. In our study, this can be partly attributed to differences in the initial NH4-N (Table. 1) content and/or differences in the mineralization pattern between D70 and D160 as observed by Pantelopoulos et al. (2016b).
Drying of non-acidified solids, resulted in significant NH4-N losses (mean loss 80%) due to temperature-induced enhancement of NH3-N volatilization during drying (Table 1 and Pantelopoulos et al. 2016a). Therefore, the lower plant growth observed in treatments with dried non-acidified SD can be attributed to the smaller mineral nitrogen pools of those treatments compared to R (Table 1). Short-term plant N uptake relies mostly on the mineral N content of the fertilizer, explaining the lower nitrogen content and nitrogen uptake from ryegrass amended with dried non-acidified SD after the first two cuts, relatively to R (Table 2 and Fig.2). Similarly, earlier studies have linked the short-term fertilizing value of bio-solids to their mineral nitrogen content (Gutser et al. 2005; Smith and Tibbett 2004). Furthermore, the drying temperature of non-acidified SD also affected nitrogen uptake, more pronouncedly during the first cut (Fig.2). Plant tissue analysis from ryegrass amended with D160 showed N concentration lower than in D70 treatments (Table 2) and significantly lower than the critical threshold of 28–32 mg N g−1 proposed for optimal growth (Reuter and Robinson 1997). In contrast, earlier findings suggest that the initial NH4-N content of dewatered and dried bio-solids had no significant contribution to the plant available nitrogen (Cogger et al. 2004), mainly because the largest proportion of total N in those studies was in the organic form. Indeed, higher Norg mineralization from heat-dried solids has been reported previously, potentially explaining the higher N fertilizing value of dried bio-solids reported elsewhere (Smith and Durham 2002). However, the NH4-N:Ntot ratio of the raw SD used here, was close to 30% indicating a smaller and potentially more stable organic N pool.Chiyoka et al. (2014) report a similar decrease in NH4-N recovery from pelletized digestate solids relative to the untreated solids. The significantly higher 15NH4-N recovery in R compared to the respective dried treatments in all three cuts performed (Figs. 2 and 3) may be attributed to NH4-N losses during drying, but could also be caused by higher N immobilization in the dried treatments. Indeed, several studies reported that drying increases the dissolved organic carbon in manures, which may trigger growth of the soil microbial community and result in an increased N immobilization (Bolan et al. 1996; Peters and Jensen 2011).
The significantly lower non-labelled N uptake in the case of dried non-acidified solids compared to their raw counterpart (Fig.2, grey part of the bars), may be attributed to differences in the Norg mineralization between those treatments, which may have resulted in less mineralized Ninorg being taken up by plants (Flavel and Murphy 2006). This is likely to be related to the low initial Ninorg content of the dried solids, which along with the depletion of soil mineral N pool by plant uptake may have retarded the Norg mineralization from D70, and D160 compared to the R treatment (Henriksen and Breland 1999). However, the D70 treatment provided larger amount of unlabeled N to ryegrass, indicating higher Norg mineralization relative to D160, and that Norg mineralization was affected by drying temperature. This is in good agreement with previous studies, which demonstrated that lower drying temperatures promote the conditioning of the organic matter, and enhance Norg mineralization (Moritsuka et al. 2006). Moreover, the negative ANR value for D160 highlights the strong immobilization induced by the dried, NH4-N poor material, as already observed in previous studies (Pantelopoulos et al. 2016b).
Drying of acidified solids decreased the ryegrass yields compared to ryegrass amended with RA. However, the magnitude of the decrease in plant yields was less pronounced and only detectable in the first cut. Acidification retained the largest proportion of solids’ initial NH4-N content in the dried products irrespectively of the drying temperature. Therefore, the decreased plant growth and uptake of N derived from the 15NH4-N pool of the non acidified solids, may be largely explained by the Ninorg losses during the drying treatment (Table 1 and Pantelopoulos et al. 2016a). Furthermore, drying might also in this case have increased the solubilization of solids’ carbon and promoted N immobilization in acidified dried solids compared to the RA, possibly explaining the small decrease in cumulative 15NH4-N and apparent N recoveries from AD70 and AD160 (Fig.3). Although higher drying temperatures of acidified solids resulted in reduced NH4-N content of AD160 compared to AD70, a previous aerobic incubation study of the same treatments (Pantelopoulos et al. 2016b) revealed an almost identical net N turnover in soil, indicating a similar potential plant N availability as confirmed in the present study.
Effect of acidification
Acidification of digestate solids had pronounced effect on ryegrass growth and all measured N availability indicators, mostly in the case of dried solids. The significantly higher NH4-N content of acidified dried solids compared to D70 and D160, largely explain the large differences observed in plant growth and N uptake between non-acidified and acidified treatments (Figs.1, 2). Furthermore, prior experimentation with the same solids treatments in incubations studies revealed, that for the first 4 months of incubation, the Ninorg concentration in soil amended with D70 and D160 were lower than the respective values of the control treatment (Pantelopoulos et al. 2016b). This highlights the strong N immobilization pattern of D70 and D160 treatments and may explain the significant lower 15NH4-N recovery and ANR in the non-acidified dried solids (Fig. 3).
Nevertheless, acidification did not influence the ryegrass growth and total plant N uptake significantly in the case of raw solids (Figs.1, 2). Direct application of sulphuric acid on the surface of SD particles increased the NH4-N content of the solids (Table 1), possibly due to chemical hydrolysis of the most labile Norg fraction of the solids (Pantelopoulos et al. 2016a). The observed increase of the NH4-N pool in RA compared to R may explain the higher ryegrass N uptake from the 15NH4-N pool of the acidified raw solids. However, the total N uptake from RA and R treatments did not differ significantly, indicating a possible higher Norg mineralization (production of unlabeled Ninorg) from the raw non-acidified solids. This is consistent with previous studies, which demonstrated that acidification decreased the gross N mineralization rates of acidified solids possibly due to an acid induced stabilization of the Norg pool of those solids treatments (Pantelopoulos et al. 2016b).
Effect of SD treatment on soil WEP and P availability
Ryegrass shoot P concentrations, within each cut, indicates the low soil level of plant available P in the control treatments without P application (NxP0, 1.01–2.90 mg P g−1 DM), whereas the mineral P application in the NxP1 or NxP2 and SD treatments enhanced shoot P concentrations (1.25–3.66 and 1.94–5.55 mg P g−1 DM, respectively; see also Table S1). Furthermore, data showed that increased P application rates with mineral P resulted in an increase of WEP in soil (Fig.4), though not proportional to the mineral P application (Table 1, corresponding to 15 (P1) or 30 (P2) mg P kg-1 soil) and declining over time, so at day 130 there was no significant difference in WEP between mineral P treatments. This may be attributed to the P sorption capacity, controlling P solubility in soil (Guppy et al. 2005; Siddique and Robinson 2003). Similar, Gasser et al. (2012) report only limited increase in WEP in soils amended with increased mineral P application rates, in the range of 0–100 mg P kg−1 soil. Furthermore, Griffin et al. (2003), assessed the effect of mineral P and manures on soil WEP and concluded that significant differences in WEP between treatments were very short term.
Effect of drying
Thermal treatment, increased the WEP in solids proportionally to the drying temperature (Table 1) and consequently, our third hypothesis that soluble P would decrease with drying was not confirmed in the present study. The evidence on the effects of drying is conflicting; contrary to our results, Chapuis et al. (2003) reported a decrease in WEP when dairy manures were dried (65 °C). However, Ajiboye et al. (2004) reported up to 25% increase in the WEP of dairy and hog manure following oven drying. They attributed this increase to the hydrolysis of NaHCO3 extractable organic P to WEP and desorption of P bound on solids surface. Furthermore, (Li et al. 2014) in a study assessing the effect of elevated drying temperature (80 °C- 240 °C) of sewage sludge on inorganic phosphorus content, reported that increased drying temperature resulted in more transformation of organic phosphorus to inorganic forms. The drying temperatures used in the current study, possibly had also resulted in the release of cellular P from microorganisms present in the solids (Toor 2006). However, Vadas and Kleinman (2006) discussed the conflicting results on effect of drying and partially attributed this to differences between the methods of WEP determination and particularly on the manure:water ratio used in each study.
Results of ryegrass P uptake and APR obtained here (Fig. 5) are in good agreement with previous studies dealing with ryegrass and thermally treated biosolids (Waldrip et al. 2011; Moritsuka et al. 2006). Despite the similar soil WEP content between non-acidified treatments (Fig. 4), P uptake and APR were significantly lower in the dried solids compared to R (Fig.5). Nevertheless, the ryegrass shoot P content did not show any significant reduction in tissue P concentrations (Table S1), indicating that ryegrass growth and ability to utilize the available soil P was controlled by the strong interaction with the low Ninorg content of the dried non-acidified solids.
Effect of acidification
Verifying the initial hypothesis (5), results showed that the WEP content in acidified digestate solids was significantly higher relatively to the respective non-acidified solids (Table 1). Similarly, in studies where acidification constituted a pre-treatment before mechanical separation of slurries, increased soluble phosphorus concentrations in acidified solid fraction have been reported (Sommer et al. 2014; Roboredo et al. 2012). The increased dissolution of P has been favorably correlated with pH values gradually approaching 5.5 (Hjorth et al. 2013). Earlier studies concerning the effect of acidification on P solubility have indicated that down to pH 6.7, struvite dissolution was responsible for the increased inorganic P (Pi) concentrations in manures while further increase in Pi at pH 5.5 was attributed to dissolution of Ca-phosphate minerals (Pagliari 2014). The acidification of the SD performed here may also have promoted partial hydrolysis of less soluble fraction of organic P (Po) to more labile forms (Condron and Newman 2011).
A direct comparison of plant P availability between dried non-acidified and acidified solids treatments would be hampered by their large differences in Ninorg content as described earlier. Nevertheless, there were only small differences in soil WEP among acidified treatments and raw non-acidified solids (Fig. 4), and their respective P uptake and APR were also similar (Fig. 5). Apart from soil P fixing capacity controlling P availability, the amount of soluble P in the soil-amendment mixture will be influenced by the soil’s pH (Dao and Cavigelli 2003; Jones and Oburger 2011). While the moderately acidic soil used here (pH: 5.7) has probably not promoted the further solubilization of P from the acidified solids (mean pH: 5.9), it may have had an impact on the P solubility from non-acidified digestate solids (pH range: 7.5–9.1) after their soil incorporation (Goloran et al. 2014).
Implications at farm scale
The results of this study indicate that acidification and drying had a significant impact on the performance of digestate solids as organic N and P fertilizers. Drying of solids will facilitate mass reduction and hence the transportability of the solids. However, in the case of non-acidified solids their low N-MFE (Fig.6) highlight the need for high nutrient compensation by additional mineral N fertilizers, to sustain adequate crop yields and utilize the P content of the solids appropriately. In the current study, the solid treatments application rates were based on equivalent dry matter application, but if applied on a plant available N basis, excessive application of D70 and D160 to meet crop N demands may lead to an over-application of P and therefore, dried non-acidified solids should better be utilized as P amendments only (Cogger et al. 2001).
Raw digestate solids and all acidified treatments showed values of N-MFE consistent with other studies concerning solid manures, with similar NH4-N content (Fig. 6) (Jensen 2013). Kai et al. (2008) reported up to 50% increase in the N-MFE of acidified pig slurry compared to non-acidified slurry when the treatments were surface applied to soil. This is due to the inhibition of NH3 volatilization induced by acidification. However, in cases where NH3 emission during application were minimized through soil incorporation, only a minor increase of N-MFE between non-acidified and acidified treatments was reported (Sørensen and Eriksen 2009). In the current study, dried acidified treatments showed N-MFE values similar to the R treatment, highlighting the capability of the combined acidification-drying treatment of the SD to produce a concentrated organic amendment without deterioration of its immediate fertilizing value. Moreover, in the current study, ryegrass amended with mineral fertilizers showed a mean cumulative S content of 3.9 mg S pot−1, significantly lower than the respective value of 11.4 mg S pot−1 in the digestate solids treatments (data not shown). This is mainly attributed to the S content of the organic treatments where, solids increased soil’s S content by 81.6 and 371 mg S pot−1 for the non-acidified and acidified solids respectively. Nevertheless, the S content of ryegrass aboveground harvested biomass did not show any significant differences between pots received acidified and non-acidified solids, with a mean accumulated ryegrass tissue content of 11.4 mg S pot−1, indicating that the excessive amount of S applied with the acidified treatments have not been utilized by the plants. Sulphuric acid as acidification agent, promotes the sulphate-S content of the solids which may further increase their nutrient and commercial value, depending on soil and crop type, as crop demand for sulphate-S typically needs to be supplied with the fertilizers. Nevertheless, the balance between S application with acidified treatments and plant uptake should be always considered, to avoid the risk of S leaching especially in soils with low S absorption capacity.
Conclusions
Drying of digestate solids promoted losses of NH4-N with no evidence of increased N mineralization induced by higher drying temperature. On the contrary, lower drying temperature appeared to promote the organic matter degradation contributing to higher amounts of available N compared to solids dried at higher temperature. Nevertheless, non-acidified dried solids were outperformed in terms of ryegrass growth and N uptake from the rest of the treatments, with results similar to control soil. Drying of non-acidified solids increased the soluble P content, concomitant with drying temperature. Nevertheless, plant P uptake was mostly related to the N availability of the different solids, since soil largely buffered any differences in the solubility of the solids P content induced by SD treatment. Acidification of solids preserved N for plant uptake during drying and decreased N mineralization of the solids, most evident in the short-term. All acidified treatments and the raw non-acidified solids showed ryegrass yields and N uptake similar to the low N applications rates of mineral fertilizers tested in this study while their N-MFE ranged from 32% to 41%, indicating the need for supplementation with available N in mineral fertilizers. As expected acidification increased the water-soluble fraction of P, however drying of acidified solids did not significantly alter P solubility. Moreover, P uptake values from treatments with high Ninorg content are similar to the respective P uptake at the high N and low P mineral treatments. Acidification and drying of digestate solids appears as an attractive method for the recycling of organic matter to the soil system and has been demonstrated that the nutrient efficiency and agronomic benefit of the acidified dried product could be similar to the raw solids. The relatively new concept of thermochemically-treated bio-wastes exposes a number of potential aspects for future research, including assessment of their agronomic value in the field scale with more complete soil sets and plant species. Furthermore, the effect of the digestate solids post-treatment on the soil microbial activities related to biochemical cycles and soil structures needs to be evaluated in the future. Moreover, the effect of acidification and drying on the solubility and availability of metals present in the digestate solids should be included in future research.
References
Ajiboye B, Akinremi OO, Racz GJ (2004) Laboratory characterization of phosphorus in fresh and oven-dried organic amendments. J Environ Qual 33:1062–1069. https://doi.org/10.2134/jeq2004.1062
Bennamoun L (2012) Solar drying of wastewater sludge: a review. Renew Sust Energ Rev 16:1061–1073. https://doi.org/10.1016/j.rser.2011.10.005
Bolan NS, Baskaran S, Thiagarajan S (1996) An evaluation of the methods of measurement of dissolved organic carbon in soils, manures, sludges, and stream water. Commun Soil Sci Plant 27:2723–2737. https://doi.org/10.1080/00103629609369735
Cantrell K, Hunt P, Uchimiya M et al (2012) Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour Technol 107:419–428. https://doi.org/10.1016/j.biortech.2011.11.084
Cavalli D, Cabassi G, Borrelli L et al (2014) Nitrogen fertiliser value of digested dairy cow slurry, its liquid and solid fractions, and of dairy cow slurry. Ital J Agron 9:71–78. https://doi.org/10.4081/ija.2014.567
Chapuis-Lardy L, Temminghoff EJM, Goede RGM (2003) Effects of different treatments of cattle slurry manure on water-extractable phosphorus. Njas - Wageningen J Life Sci 51:91–102. https://doi.org/10.1016/S1573-5214(03)80028-3
Chaudhary M, Adu-Gyamfi J, Saneoka H et al (2008) The effect of phosphorus deficiency on nutrient uptake, nitrogen fixation and photosynthetic rate in mashbean, mungbean and soybean. Acta Physiol Plant 30:537–544. https://doi.org/10.1007/s11738-008-0152-8
Chiyoka W, Zvomuya F, Hao X (2014) A bioassay of nitrogen availability in soils amended with solid digestate from anaerobically digested beef cattle feedlot manure. Soil Sci Soc Am J 78:1291–1300. https://doi.org/10.2136/sssaj2013.01.0030
Cogger CG, Bary AI, Fransen SC, Sullivan DM (2001) Seven years of biosolids versus inorganic nitrogen applications to tall fescue. J Environ Qual 30:2188–2194
Cogger CG, Bary AI, Sullivan DM, Myhre EA (2004) Biosolids processing effects on first-and second-year available nitrogen. Soil Sci Soc Am J 68:162–167
Condron L, Newman S (2011) Revisiting the fundamentals of phosphorus fractionation of sediments and soils. J Soils Sediments 11:830–840. https://doi.org/10.1007/s11368-011-0363-2
Cordovil C, Cabral F, Coutinho J (2007) Potential mineralization of nitrogen from organic wastes to ryegrass and wheat crops. Bioresour Technol 98:3265–3268. https://doi.org/10.1016/j.biortech.2006.07.014
Dail H, He Z, Erich S, Honeycutt W (2007) Effect of drying on phosphorus distribution in poultry manure. Commun Soil Sci Plant 38:1879–1895. https://doi.org/10.1080/00103620701435639
Dao TH, Cavigelli MA (2003) Mineralizable carbon, nitrogen, and water-extractable phosphorus release from stockpiled and composted manure and manure-amended soils. Agron J 95:405–413
de la Fuente C, Alburquerque JA, Clemente R, Bernal MP (2013) Soil C and N mineralisation and agricultural value of the products of an anaerobic digestion system. Biol Fertil Soils 49:313–322. https://doi.org/10.1007/s00374-012-0719-9
Deng W-Y, Yan J-H, Li X-D, Wang F, Zhu X-W, Lu S-Y, Cen K-F (2009) Emission characteristics of volatile compounds during sludges drying process. J Hazard Mater 162:186–192. https://doi.org/10.1016/j.jhazmat.2008.05.022
Egner H, Riehm H, Domingo WR (1960) Investigations of the chemical soil analysis as a basis for the evaluation of nutrient status in soil. II. Chemical extraction methods for phosphorus and potassium determination. K Lantbruks Høgsk Ann 26:199–215
Fangueiro D, Ribeiro H, Vasconcelos E, Coutinho J, Cabral F (2009) Treatment by acidification followed by solid-liquid separation affects slurry and slurry fractions composition and their potential of N mineralization. Bioresour Technol 100:4914–4917. https://doi.org/10.1016/j.biortech.2009.04.032
Fangueiro D, Surgy S, Coutinho J, Vasconcelos E (2013) Impact of cattle slurry acidification on carbon and nitronen dynamics during storage and after soil incorporation. J Plant Nutr Soil Sci 176:540–550. https://doi.org/10.1002/jpln.201200117
Flavel T, Murphy D (2006) Carbon and nitrogen mineralization rates after application of organic amendments to soil. J Environ Qual 35:183. https://doi.org/10.2134/jeq2005.0022
Gasser O, Chantigny A et al (2012) Plant-available and water-soluble phosphorus in soils amended with separated manure solids. J Environ Qual 41:1290. https://doi.org/10.2134/jeq2011.0290
Goloran JB, Phillips IR, Xu ZH et al (2014) Effects of amendments and fertilization on plant growth, nitrogen and phosphorus availability in rehabilitated highly alkaline bauxite-processing residue sand. Soil Use Manag 30:198–208. https://doi.org/10.1111/sum.12109
Griffin TS, Honeycutt CW, He Z (2003) Changes in soil phosphorus from manure application. Soil Sci Soc Am J 67:645–653. https://doi.org/10.2136/sssaj2003.6450
Grigatti M, Girolamo G, Chincarini R et al (2011) Potential nitrogen mineralization, plant utilization efficiency and soil CO2 emissions following the addition of anaerobic digested slurries. Biomass Bioenergy 35:4619–4629. https://doi.org/10.1016/j.biombioe.2011.09.007
Grigatti M, Cavani L, Marzadori C, Ciavatta C (2014) Recycling of dry-batch digestate as amendment: soil C and N dynamics and ryegrass nitrogen utilization efficiency. Waste Biomass Valorization 5:823–833. https://doi.org/10.1007/s12649-014-9302-y
Gunnarsson A, Bengtsson F, Caspersen S (2010) Use efficiency of nitrogen from biodigested plant material by ryegrass. J Plant Nutr Soil Sci 173:113–119. https://doi.org/10.1002/jpln.200800250
Guppy CN, Menzies NW, Moody PW, Blamey FPC (2005) Competitive sorption reactions between phosphorus and organic matter in soil: a review. Aust J Soil Res 43:189. https://doi.org/10.1071/SR04049
Gutser R, Ebertseder T, Weber A et al (2005) Short-term and residual availability of nitrogen after long-term application of organic fertilizers on arable land. J Plant Nutr Soil Sci 168:439–446. https://doi.org/10.1002/jpln.200520510
Hansen TH, de Bang TC, Laursen KH, Pedas P, Husted S, Schjoerring JK (2013) Multielement plant tissue analysis using ICP spectrometry. Methods Mol Biol 953:121–141. https://doi.org/10.1007/978-1-62703-152-3_8
Henriksen T, Breland T (1999) Nitrogen availability effects on carbon mineralization, fungal and bacterial growth, and enzyme activities during decomposition of wheat straw in soil. Soil Biol Biochem 31:1121–1134
Hjorth M, Christensen KV, Christensen ML, Sommer SG (2010) Solid—liquid separation of animal slurry in theory and practice. A review. Agron Sustain Dev 30:153–180. https://doi.org/10.1051/agro/2009010
Hjorth, M., Cocolo, G., Jonassen, K., Sommer, S.G., 2013. Acidifications effect on transformations in and composition of animal slurry. In: RAMIRAN 2013-15th International Conference
Jensen, L.S., 2013. Animal manure residue upgrading and nutrient recovery in biofertilisers. In: Animal manure recycling: treatment and management, Wiley, New York, pp 271–294
Jones DL, Oburger E (2011) Solubilization of phosphorus by soil microorganisms. In: Phosphorus in action. Springer, pp 169–198
Kai P, Jensen JE, Hansen MN, Sommer SG (2008) A whole-farm assessment of the efficacy of slurry acidification in reducing ammonia emissions. Eur J Agron 28:148–154
Li R, Yin J, Wang W et al (2014) Transformation of phosphorus during drying and roasting of sewage sludge. Waste Manag 34:1211–1216. https://doi.org/10.1016/j.wasman.2014.03.022
Möller K, Müller T (2012) Effects of anaerobic digestion on digestate nutrient availability and crop growth: a review. Eng Life Sci 12:242–257. https://doi.org/10.1002/elsc.201100085
Moritsuka N, Matsuoka K, Matsumoto S et al (2006) Effects of the application of heated sewage sludge on soil nutrient supply to plants. J Soil Sci Plant Nutr 52:528–539. https://doi.org/10.1111/j.1747-0765.2006.00062.x
Muñoz GR, Kelling KA, Powell JM, Speth PE (2004) Comparison of estimates of first-year dairy manure nitrogen availability or recovery using nitrogen-15 and other techniques. J Environ Qual 33:719–727. https://doi.org/10.2134/jeq2004.7190
Nannen D, Herrmann A, Loges R et al (2010) Recovery of mineral fertiliser N and slurry N in continuous silage maize using the 15N and difference methods. Nutr Cycl Agroecosyst 89:269–280. https://doi.org/10.1007/s10705-010-9392-2
Pagliari PH (2014) Variety and solubility of phosphorus forms in animal manure and their effects on soil test phosphorus. In: Applied manure and nutrient chemistry for sustainable agriculture and environment. Springer, pp. 141–161
Pantelopoulos A, Magid J, Jensen L (2016a) Thermal drying of the solid fraction from biogas digestate: effects of acidification, temperature and ventilation on nitrogen content. Waste Manage 48:218–226. https://doi.org/10.1016/j.wasman.2015.10.008
Pantelopoulos A, Magid J, Jensen LS (2016b) Net and gross nitrogen turnover in soil amended with acidified and differently dried solids from biogas digestate. Soil Sci Soc Am J 80:943. https://doi.org/10.2136/sssaj201603.0059
Peters K, Jensen LS (2011) Biochemical characteristics of solid fractions from animal slurry separation and their effects on C and N mineralisation in soil. Biology and Fertility of Soils 47(4):447–455. https://doi.org/10.1007/s00374-011-0550-8
Reuter DJ, Robinson, JB (1997). Plant analysis: an interpretation manual. Csiro Publicing
Roboredo M, Fangueiro D, Lage S, Coutinho J (2012) Phosphorus dynamics in soils amended with acidified pig slurry and derived solid fraction. Geoderma 189:328–333. https://doi.org/10.1016/j.geoderma.2012.06.030
Siddique M, Robinson S (2003) Phosphorus sorption and availability in soils amended with animal manures and sewage sludge. J Environ Qual 32:1114–1121. https://doi.org/10.2134/jeq2003.1114
Sistani KR, Rowe DE, Miles DM, May JD (2001) Effects of drying method and rearing temperature on broiler manure nutrient content. Commun Soil Sci Plant 32:2307–2316. https://doi.org/10.1081/CSS-120000285
Smith S, Durham E (2002) Nitrogen release and fertiliser value of thermally-dried biosolids. Water Environ J 16:121–126. https://doi.org/10.1111/j.1747-6593.2002.tb00382.x
Smith M, Tibbett M (2004) Nitrogen dynamics under Lolium Perenne after a single application of three different sewage sludge types from the same treatment stream. Bioresour Technol 91:233–241. https://doi.org/10.1016/S0960-8524(03)00205-0
Sommer S, Hjorth M, Leahy J et al (2014) Pig slurry characteristics, nutrient balance and biogas production as affected by separation and acidification. J Agric Sci 153:177–191. https://doi.org/10.1017/S0021859614000367
Sørensen P, Eriksen J (2009) Effects of slurry acidification with sulphuric acid combined with aeration on the turnover and plant availability of nitrogen. Agric Ecosyst Environ 131:240–246. https://doi.org/10.1016/j.agee.2009.01.024
Toor GS, Hunger S, Peak JD, Sims JT and Sparks DL 2006 Advances in the characterization of phosphorus in organic wastes: Environmental and agronomic applications. Adv Agron 89:1–72
Vadas PA, Kleinman P (2006) Effect of methodology in estimating and interpreting water-extractable phosphorus in animal manures. J Environ Qual 35:1151. https://doi.org/10.2134/jeq2005.0332
Waldrip H, He Z, Erich S (2011) Effects of poultry manure amendment on phosphorus uptake by ryegrass, soil phosphorus fractions and phosphatase activity. Biol Fertil Soils 47:407–418. https://doi.org/10.1007/s00374-011-0546-4
Acknowledgements
The research leading to these results received funding from the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme FP7/2007-2013/under REA grant agreement no. [289887]. The paper reflects only the authors’ view and the European Union is not liable for any use that may be made of the information contained therein.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ismail Cakmak
Electronic supplementary material
ESM 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Pantelopoulos, A., Magid, J., Jensen, L.S. et al. Nutrient uptake efficiency in ryegrass fertilized with dried digestate solids as affected by acidification and drying temperature. Plant Soil 421, 401–416 (2017). https://doi.org/10.1007/s11104-017-3463-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3463-y