Abstract
Anaerobic digestion is currently considered a valuable technology for recycling of cattle slurry due to the production of biogas, a renewable source of energy. However, an appropriate management of the waste generated from anaerobic co-digestion of cattle slurry (digestate) is needed for the sustainability of the process. This paper shows the effects provoked on soil C and N mineralisation processes and on microbial biomass due to the addition of the digestate, the liquid and solid fractions obtained after separation of the digestate and the composted solid fraction of the digestate, in comparison with the effects of the non-digested cattle slurry. Composting was a very effective way of recycling the digestate, producing highly stable (mineralised-C reached only 3% of the added total organic-C) and hygienised material with an elevated fertilising potential (the net-N mineralisation accounted for 2% of total-N from compost). The agricultural use of cattle slurry, the digestate and the separated fractions should take into account their effects on soil C and N cycles: high decomposition rate (ranging from 25% to 50% of total organic-C) and partial N immobilisation in the soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cattle slurry represents nowadays an important resource for biogas production in the EU-27. Considering that the annual animal manure (cattle and pig) production is about 1,284 × 106 Mg (Holm-Nielsen et al. 2009), a biogas production potential of 18.5 Mtoe (million tons of oil equivalent) has been estimated. This alternative has potential financial benefits lying in biomass-to-energy schemes, which generate revenue from the energy produced, and often provide a by-product that could be potentially used as an agricultural fertiliser. Therefore, co-digestion of cattle slurry with other biomass substrates—such as agricultural wastes—seems to be a sustainable technology for producing biogas as a renewable source of energy, offering advantages for the management of these organic wastes and for mitigation of greenhouse gas emissions (Nielsen et al. 2002).
However, the sustainability of biogas production may depend on an appropriate end-use of the digested material obtained, which should be treated, disposed of, or re-used in a proper way, avoiding any possible negative environmental impact. It has been demonstrated that direct soil application of digestate can lead to benefits for crops, if integrated into good agricultural practices (Al Seadi 2002; Fuchs et al. 2008; Holm-Nielsen et al. 2009). Digested materials present some advantages for their use as soil amendments in comparison with untreated wastes, such as greater microbial stability and hygiene and a higher amount of N present as ammonium (Al Seadi 2002; Alburquerque et al. 2012a; Holm-Nielsen et al. 2009). Nevertheless, anaerobic digestate can produce disgusting odours, and its viscosity (Smet et al. 1998) and high humidity make its handling and application to soils difficult (Tchobanoglous et al. 2002). Also, the presence of high concentrations of easily degradable organic matter in the digestate could lead to an excessive increase in soil microbial activity (Alburquerque et al. 2012b) and cause detrimental effects on the plant–soil system such as phytotoxicity (e.g. toxic intermediate degradation products), anaerobic soil conditions, etc. Consequently, the anaerobic digestate may require further processing to enhance its potential fertilising value and applicability as a soil conditioner. Various treatment options for reducing the amount of water such as composting, physico-chemical treatments and mechanical or thermal dewatering of digestate have been previously discussed (Rehl and Müller 2011).
Separation of liquid and solid fractions of the digestate is a desirable upstream operation in the treatment process since dewatering the solid fraction reduces the cost of transport and facilitates its addition to soil (Holm-Nielsen et al. 2009). Additionally, the solid fraction can be transformed by composting, which final product would represent an attractive source of stable organic matter and nutrients for agricultural soils (Atallah et al. 1995). The composting of the solid fraction of the digestate can constitute a feasible method not only to handling these materials but also to improve the quality of the end-product, reducing odours emission by decreasing the concentration of volatile compounds, moisture content, potential phytotoxicity and also contributes to the elimination of pathogens (Bustamante et al. 2012). Moreover, the separated liquid fraction, which has lower amount of suspended solids, can be directly used as an agricultural fertiliser due to its high NH +4 –N concentration (Morris and Lathwell 2004). Further refining of the liquid fraction into concentrated fertilisers, or receiving post-treatments to obtain clean water suitable for other uses, could be of interest as well (Holm-Nielsen et al. 2009).
The increasing interest in Mediterranean countries in the use of organic materials derived from anaerobic digestion (raw digestate, liquid and solid fractions, etc.) as soil fertilisers contrasts with the fact that their effects on soil properties are largely unknown. Digestates from animal manures may hold elevated contents of metals (Cu and Zn) and salts since they are present in high concentrations in the raw materials (especially in pig slurries; Moral et al. 2005), as well as certain microbial instability, constituting a potential risk of metal accumulation in soil, salinisation or phytotoxic effects, N-immobilisation, etc. (Alburquerque et al. 2012a; McLachlan et al. 2004; Poggi-Varaldo et al. 1999). Therefore, the aims of the present paper were to elucidate the effects on soil of materials associated to cattle slurry anaerobic digestion, and to establish the most appropriate strategy for cattle slurry management based on the potential use as soil fertilisers and amendments of the (by)products generated by the different treatment processes (anaerobic co-digestion of cattle slurry, anaerobic digestion followed by liquid–solid separation of digestate, or composting of the solid fraction) and of untreated slurry. For that, the impact of the addition of these materials on both soil-C and soil-N cycles related parameters like microbial biomass C and N, and C and N mineralisation in soil, and on soil physico-chemical properties (pH, EC and available phosphorous) was evaluated in aerobic incubation experiments.
Materials and methods
Incubation experiments
The organic materials used in the present experiment were obtained from a dairy farm located in Requena (Valencia, Spain). Raw cattle slurry (CS) and anaerobically digested material or digestate (D) were sampled directly from an on-farm biogas plant. Briefly, the plant, with a total utilisable volume of 3,000 m3, produces 500 kW h day−1 of electrical power by co-digesting cattle slurry (84.1% fresh weight), cattle manure (4.3% fresh weight) and maize–oat silage (11.6% fresh weight). The separated solid (SD) and liquid (LD) fractions of the digestate were collected from a mechanical screen separator (screw press) installed after the storage tank. The screw press showed a removal efficiency for DM of 32%. The compost (CM) was obtained after composting the solid digestate fraction without bulking agent addition during 131 days (Bustamante et al. 2012). The organic materials were kept at <4°C for 2–3 days and processed quickly to prevent any chemical or biological alteration. Their main characteristics are shown in Table 1.
A calcareous sandy loam agricultural soil (14.8% clay, 22.3% silt and 62.9% sand) from La Alberca (Murcia, Spain) was taken from the superficial top 20 cm, air-dried and sieved to 2 mm before use. Its main characteristics were 24% CaCO3, pH 8.3, electrical conductivity (EC) 0.3 dS m−1, 24.3 g kg−1 organic matter (OM), 14.1 g kg−1 total organic-C (TOC) and 1.85 g kg−1 total nitrogen (TN).
The liquid materials (CS, D and LD) were thoroughly mixed with the soil in a proportion of 4 ml of fresh material per 100 g of dry soil (equivalent to a field application of 96 m3 ha−1), while the solid ones (SD and CM) were incorporated to the soil at a rate of 2 g of dry material per 100 g of dry soil (equivalent to 48 Mg ha−1), leading to organic C addition rates of 2.9, 3.3, 1.9, 20.1 and 15.6 Mg ha−1 for CS, D, LD, SD and CM, respectively. The above application rates were selected in order to avoid excessively low inputs of organic-C to the soil with the liquid samples, which could limit the accuracy of the C-mineralisation study. Unamended soil (S) was used as a control.
Soil–organic amendment mixtures were incubated under aerobic conditions in darkness at 26 ± 0.5°C for 56 days. Soil moisture was maintained at 60% of the water-holding capacity during incubation, with deionised water. Two parallel sets of incubations were carried out:
-
1.
A destructive set of samples (7 g of dry soil and the equivalent proportions of the amendments) to determine soil physico-chemical properties, following the methodology described by Alburquerque et al. (2012b). Briefly, a total of 44 replicate samples were run: at days 0, 2, 7, 14, 28, 42 and 56, three replicates per treatment for the analysis of inorganic-N (NH +4 –N and NO −3 –N), and two more to evaluate soil pH were taken; at days 0, 7 and 56 of incubation, three extra replicates per treatment were removed for the determination of available-P concentrations.
-
2.
A non-destructive set of samples (20 g of dry soil and the equivalent proportions of the amendments) for the determination of C-mineralisation (CO2–C evolved from the soil) and microbial biomass-C (BC) and -N (BN) concentrations (Alburquerque et al. 2012b). Evolved CO2–C was measured periodically (after 2, 4, 7, 10, 14, 17, 21, 28, 35, 42, 49 and 56 days of incubation) by titration of the NaOH solution placed in a small beaker inside the incubation vessels with 0.1 M HCl in an excess of BaCl2 to precipitate carbonates. Vessels without soil were used as blanks. The mineralisation of the organic-C from the amendments (Cm) was calculated as the difference between the CO2–C evolved in the amended soils and that produced in the unamended control soil, and was expressed as a percentage of the TOC added with the amendments. Both BC and BN concentrations were determined after 7 and 56 days of incubation.
Analytical methods
Moisture content of the solid and liquid organic materials was evaluated after drying to constant weight at 105°C while the OM content was determined by loss on ignition at 500°C for 24 h. Both TOC and TN concentrations were measured in freeze-dried samples using an automatic elemental microanalyser (EuroVector, Milan, Italy).
For the liquid materials (D, CS and LD), electrical conductivity (EC) and pH were measured directly after sample homogenisation. Ammonium was extracted by steam distillation of fresh samples alkalised with MgO, trapped in boric acid and titrated with HCl (Bremner and Keeney 1965). The 5-day biochemical oxygen demand (BOD5) is a reliable and commonly used method to calculate readily biodegradable OM (Alburquerque et al. 2012a), and was determined using the method and equipment supplied by WTW (Oxitop IS6) based on pressure measurement in a sealed system. Water-soluble organic C (WSC) was determined using an automatic analyser for liquid samples (TOC-V CSN+TNM-1 Analyser, Shimadzu) after sample filtration (0.45-μm pore diameter) according to method 5310B (APHA 2005). In the solid materials (SD and CM), WSC, EC and pH were measured after freeze-drying and grinding (<0.5 mm) in 1:10 water extracts (w/v). Ammonium concentration was analysed in SD as described before for liquid samples and in CM in 2 M KCl extracts (1:5 w/v) by the salicylate method (Kempers and Zweers 1986) using sodium dichloroisocyanurate as the chlorine source. The pathogens Escherichia coli and Salmonella spp. were determined in the organic materials as proposed by Chroni et al. (2009) and USEPA (2005), respectively.
Soil TOC and TN concentrations were determined by an automatic microanalyser; organic matter (OM) contents were calculated by multiplying TOC concentrations by 1.72 (Nelson and Sommers 1982). The CaCO3 content was measured with a Bernard calcimeter. A two-step sequential extraction procedure was carried out for soil inorganic-N evaluation during the incubation experiments: (1) ultrapure water (1:5 w/v) for 2 h: water-soluble NH +4 –N and NO −3 –N and (2) 2 M KCl (1:5 w/v) for 2 h: exchangeable NH +4 –N forms in the soil. Nitrate concentrations in soil extracts were measured using a nitrate-ion selective electrode (USEPA 2007) and NH +4 –N concentrations were evaluated as proposed by Kempers and Zweers (1986). Soil BC and BN concentrations were determined after a fumigation-extraction procedure (Brookes et al. 1985; Vance et al. 1987) in an automatic analyser for liquid samples, and calculated according to Wu et al. (1990). All values refer to oven-dried soil weight (105°C for 24 h). Soil pH and EC throughout the incubation experiment were determined in 1:2.5 and 1:5 (w/v) soil/water extracts, respectively. Available-P concentrations in soil were measured colorimetrically after NaHCO3 extraction (Watanabe and Olsen 1965).
Statistical analyses
Basic statistical analyses of data (ANOVA) were calculated using SPSS 18.0 software for Windows. The normal distribution of the data was checked by the Shapiro–Wilk’s test; when data failed this test, they were adjusted to a normal distribution through log transformation.
Data concerning C-mineralisation in soil were fitted to kinetic functions by the non-linear least-square technique (Marquardt–Levenberg algorithm), using Sigma-Plot 10.0 software (Systat Software Inc). The statistical significance of the curve-fitting, residual mean square (RMS) and F values were also calculated.
Results and discussion
Agrochemical characteristics of the tested materials
In the present study, all the liquid materials (CS, D and LD) were close to neutral or slightly alkaline, which has been commonly reported for both cattle slurry and digestates (e.g. Alburquerque et al. 2012a; Sommer and Olesen 1991). The separated solid fraction of the digestate (SD) showed a strong alkaline character (pH 8.9), which may affect soil pH and nutrient availability for plants if used as soil amendment. In contrast, CM showed a pH value of 6.9, slightly lower than typical values of compost (Bustamante et al. 2012). Elemental sulphur was added to SD for composting in order to reduce NH3 emissions during the process; its gradual oxidation and nitrification processes may account for the relatively low pH values observed in this material. Raw cattle slurry (CS), D and LD showed high EC as soluble salts remain mainly in the liquid fraction after separation processes of livestock manures (Burton 2007). Compost (CM) showed higher EC than SD (Table 1) due to increased ions concentration as a consequence of the mineralisation processes that occur during composting and to the mass loss of the pile (Paredes et al. 2000).
Degradation of labile organic matter during the anaerobic digestion resulted in lower BOD5 and WSC values in D than in CS (Table 1). Contrastingly, organic matter, TOC and dry matter were not reduced in D since a lignocellulosic material was added to CS as co-substrate for co-digestion (maize–oat silage at 11.6% fresh weight). Compost showed lower concentration of OM, TOC and WSC than SD, associated to the degradation of the organic compounds throughout composting.
High concentrations of NH +4 –N were found in CS, D, LD and SD. The production of NH +4 –N during the anaerobic process, derived from the breakdown of urea and other organic constituents (mainly aminoacids and proteins), enriches digestates in such N-form, which remain almost entirely in the liquid fraction after separation (Table 1) (Jørgensen and Jensen 2009). Ammonium-N from SD decreased during composting due to several processes such as NH3 volatilisation under high temperature and alkaline pH, microbial immobilisation and nitrification (NO −3 –N was 3.16 g kg−1 and <0.05 g kg−1 in CM and SD, respectively). Heavy metal concentrations in the tested organic materials were lower than the limits established by the Spanish legislation for fertiliser products prepared from organic materials (Cd < 0.01, Cr < 0.3, Ni < 0.5 and Pb < 3 mg l−1 for liquid materials, and Cd < 0.1, Cr < 10, Ni < 10 and Pb < 20 mg kg−1 for solid materials; BOE 2011).
Composting was the only process that allowed the sanitation of the product. Salmonella spp. was present in all materials with the exception of compost. Anaerobic digestion reduced the presence of E. coli from 7.9 × 104 MPN g−1 in cattle slurry to 4.0 × 102 MPN g−1 in digestate, but composting reduced it further to <10 MPN g−1 in the final compost, after the thermophilic temperatures were reached during the process (Bustamante et al. 2012).
Organic carbon mineralisation
The amount of CO2–C evolved throughout the incubation experiment showed a similar pattern in the soil amended with the three liquid materials (D, CS and LD). After 2 days of incubation, the amount of CO2–C evolved (expressed as milligrams of C per kilogram per day) from the amended soils was higher than that from the control soil (S), especially in the CS treatment (Fig. 1a), as this material contained a higher amount of biodegradable organic compounds than D and LD (higher BOD5d and WSC concentrations; Table 1). Control soil (S) showed certain initial mineralisation, which can be attributed to the degradation of the organic matter of this agricultural soil or to the storage conditions of the soil (4°C) previous to incubation. Highly biodegradable organic matter of the CS is mainly transformed to biogas during anaerobic digestion, leading to more stabilised organic matter content in D and LD (more resistant to microbial degradation in the soil). The CO2–C production rates decreased rapidly after the first week of incubation reaching nearly constant values from day 21 to 56 (<20 mg C kg−1 day−1; Fig. 1a), similar to those obtained in the control soil (S), as the easily mineralisable OM sources were exhausted. Similar dynamics have been described in agricultural soils amended with liquid organic wastes, such as pig slurry, due to the presence of a high concentration of easily degradable organic C in these materials (Saviozzi et al. 1993).
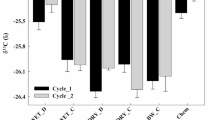
Daily production of CO2–C from the soil (mean value ± standard deviation; where absent, bars fall within symbols) (a), and cumulative mineralised-C from amended samples; dots are experimental data (mean value ± standard deviation, n = 3) and lines represent the curve-fitting (b). S control soil, CS cattle slurry, D digestate, LD liquid fraction of digestate, SD solid fraction of digestate, CM compost
The addition of organic-C rich solid materials (SD and CM) to soil also resulted in a rapid increase in the production of CO2–C compared to non-treated soil (Fig. 1a). But the dynamics of C-mineralisation were very different when soils were treated with SD or with CM. Control and compost-treated samples had their maximum values of CO2–C emission on the first measuring day, followed by a rapid decrease in the mineralisation rates (Fig. 1a). Contrastingly, the SD-treated soil samples reached the highest C-mineralisation rate during the first week of incubation (Fig. 1a). Compost contains stable, partially humified OM, very resistant to microbial degradation, while SD presents a great amount of easily biodegradable OM with low microbiological stability (Bernal et al. 1998a).
The amount of CO2–C evolved from the soils after 56 days of incubation decreased significantly (P < 0.001) in the order (mg CO2–C kg−1; mean ± standard deviation, n = 3): SD (2,704 ± 38) > CS (1,133 ± 41) > D (948 ± 23) > LD (871 ± 15) > CM (735 ± 23) > S (542 ± 6). Expressing such results as the %TOC of the organic materials mineralised reflected the different biodegradability of the OM present in these residues (Fig. 1b), assuming no priming effect in soil (Sinha et al. 1977). Thus, at the end of the incubation experiment (56 days), CM showed the lowest percentage of mineralised-C (3% of the added TOC), followed by SD (26%), D (30%), LD (42%) and CS (50%). Carbon mineralisation of CS and D was similar to that observed for cattle slurries and digestates in similar experiments with agricultural soils: 49–60% of TOC for cattle slurry (Sørensen 1998) and 30–34% of TOC for stable digestates (Alburquerque et al. 2012b).
Lower percentages of C-mineralisation of the solid materials (especially CM) compared to the liquid materials are a consequence of the higher proportion of solid OM of lignocellulosic character contained in CM and SD and the maturity degree of the OM after composting (Bernal et al. 1998a; Saviozzi et al. 1993). This may account also for the lower mineralisation of the D treatment compared to CS and LD.
Data from C-mineralisation in soil were fitted to a first-order kinetic model, where potentially mineralisable-C (C0) ranged from 2.7% to 47.6% (Table 2). The highest value of C0 was found in CS and SD for liquid and solid materials, respectively, although the TOC in SD seems to be slowly degraded (lowest rate constant; Table 2). As C0 indicates the maximum amount of mineralisable substrate by soil microorganisms, the proportion of TOC from the waste that can remain in the soil for a long time can be calculated. From a practical point of view, conservation of C in soil has been expressed as kilograms of C per megagram of dry material for solids (CM and SD) and kilograms of C per cubic meter of fresh material for liquids (CS, LD and D). Compost would increase soil organic C levels to a greater extent than SD, reaching 315 and 298 kg C Mg−1, respectively. Even if losses of TOC as CO2–C due to composting of SD were considered (22.4% of TOC), CO2–C emissions from CM (CO2–C evolved during composting + CO2–C emissions from the compost-amended soil) would be lower than those coming from the soil amended with SD (103 and 121 kg C Mg−1 for CM and SD, respectively).
Contrastingly, liquid materials would increase soil C in only 12, 16 and 24 kg C m−3 for CS, LD and D, respectively. Thus, if the main concern is to increase soil organic matter levels for soil protection, composting and using mature compost would always be recommended over soil application of fresh or anaerobically transformed wastes (Bernal et al. 1998a).
Nitrogen transformation in soil
The addition of the liquid materials (CS, D and LD) led to similar transformation dynamics of the inorganic–N (NH +4 –N and NO −3 –N) in the soil (Fig. 2): during the first week of incubation, the concentration of inorganic-N decreased in the soil. Such initial decrease in inorganic-N concentration could be due to microbial immobilisation of NH +4 –N since a concomitant strong CO2–C production was found in those soils (Figs. 1a and 2). Indeed, rapid immobilisation of NH +4 –N by soil microorganisms in the presence of easily degradable organic-C as an energy source has been previously reported (Bernal et al. 1998b; de la Fuente et al. 2010; Jørgensen and Jensen 2009; Kirchmann and Lundvall 1993). Other processes, such as fixation of NH +4 –N in soil clay (Nieder et al. 2011), or even NH3 volatilisation, may also contribute to the rapid decrease of NH +4 –N concentration observed in the soils. Nevertheless, the experimental procedure used has been proven to produce negligible N losses by ammonia volatilisation, and the low clay content of the soil may cause low NH +4 –N fixation as a non-exchangeable form in the soil (de la Fuente et al. 2010).
After the immobilisation period, inorganic-N concentration increased, indicating a partial re-mineralisation of the immobilised-N, with a fast NO −3 –N production in the soil (Fig. 2). A similar behaviour was found by Alburquerque et al. (2012b), who evaluated different digestates as soil amendments in an incubation experiment. These authors showed a rapid oxidation of the NH +4 –N applied by the digestates after 2 weeks of N immobilisation in the soil. At the end of the incubation time (56 days), the nitrification conversion (100 × [(NO −3 –N56d − NO −3 –N0d)soil+organic material − (NO −3 –N56d − NO −3 –N0d)soil]/TN added) accounted for 55% of TN for D and LD, and 41% of TN for CS.
Regarding the solid materials (SD and CM), the greatest decrease of inorganic-N concentration occurred in the soil with SD, the less stable material, which had also led to the highest CO2–C production in the soil (Figs. 1a and 2). Nitrification could be expected in the SD-treated soil; although the majority of NH +4 –N remains in the liquid fraction after separation a considerable amount of NH +4 –N is retained in the solid fraction (Table 1) due to its elevated moisture content (Möller et al. 2002). However, NO −3 –N formation was only slightly and temporarily observed in the soil treated with SD during the first week of incubation (Fig. 2). Microbial N-immobilisation can be considered as the main factor responsible for the following decrease of inorganic-N concentration in the SD-treated soil (Fig. 2). Despite the preferential microbial immobilisation of NH +4 over NO −3 , the latter could have been also used as a N source by soil microorganisms in the absence of ammonium in SD treated soil (Myrold and Posavatz 2007). Moreover, N losses by denitrification cannot be ruled out in the soils treated with SD, which supplied high amounts of highly labile OM to the soil and produced intense microbial respiration (CO2–C production, Fig. 1). This high respiration rate during the first days of incubation could have reduced oxygen concentration in the amended soil, leading to N losses by denitrification (Clemens and Huschka 2001; Dendooven et al. 1998).
Contrastingly, CM did not produce any inorganic-N decrease in soil, and even a slow organic-N mineralisation occurred as inorganic-N was produced with time (Fig. 2). The net-N mineralisation from CM (calculated as 100 × [(inorg-N56d – inorg-N0d)soil+compost − (inorg-N56d – inorg-N0d)soil]/TN added) accounted for 2% of total-N from compost (3.7% of organic-N). Compost addition resulted in high concentrations of inorganic-N (mainly NO −3 –N) in the soil throughout the incubation experiment (Fig. 2). This is related to the elevated concentration of NO −3 –N in CM (Table 1), coming from the nitrification of NH +4 that took place during the composting process (Bustamante et al. 2012). This is of interest regarding its potential use in agriculture, as it allows an easy calculation of the necessary application rate for supplying the correct amount of N according to the crop requirement (Bernal et al. 1998b).
From an agricultural point of view, the N-fertiliser value of the organic materials was assessed in this experiment as kg of NO −3 –N produced in soil per megagram of dry material for solids (CM and SD) or per cubic meter of fresh material for liquids (CS, LD and D), as NO −3 is the main form of N taken up by plants from the soil. Compost reached the greatest N-fertiliser value of the organic materials evaluated, accounting for 9.9 kg NO3–N Mg−1 while SD only provides 2.1 kg NO3–N Mg−1. In the liquid materials, the fertiliser value was interestingly greater in the anaerobically digested slurry than in the untransformed waste (4.1, 4.9 and 5.0 kg NO3–N m−3 in CS, LD and D, respectively).
Microbial and physico-chemical properties
As expected, the addition of easily available OM with CS led to rapid increases in the microbial BC and BN concentrations in soil with respect to the S treatment, showing the highest concentrations at day 7 (Table 3). Nevertheless, mineralisation of the most labile OM throughout the incubation resulted in non-statistically significant differences between CS, D, LD and S after 56 days of incubation (Table 3), suggesting that microbial biomass of the amended soils tended to return to its initial equilibrium.
Both solid materials (CM and SD) provoked a similar increase as CS in BC and BN concentrations compared to the control soil after 7 days of incubation (Table 3), remaining almost constant in CM-treated soils during the experiment. However, in SD BC concentrations drastically increased in the long term (56 days), and this treatment also showed the highest respiration rate (CO2–C) both at day 7 and 56 (Table 3). These findings are in agreement with the high immobilisation of N found in the soil receiving SD, suggesting an important effect on growth, composition and activity of soil microflora supported by the high metabolic quotient (CO2–C/BC) of this treatment (Anderson and Domsch 1990; Barajas-Aceves 2005; Clemente et al. 2007; Table 3). Inorganic-N immobilisation in SD-amended soils is also supported by a significant increase in BN concentrations in the soil treated with SD compared to the rest of the treatments at both sampling times. This is in good agreement with a previous study conducted by de la Fuente et al. (2010), who recorded an important inorganic-N immobilisation with a concomitant increase of BN concentrations in a pig slurry-treated soil after 56 days of incubation.
Soil pH increased immediately after CS, D and LD addition to soil (Fig. 3), but it rapidly decreased as a consequence of nitrification processes, showing similar pH values (around 8.3) to that found in S (8.4) at day 56. Compost provoked a slight decrease on soil pH with respect to S throughout the incubation experiment, whereas SD increased soil pH compared to S (Fig. 3). Frequently, optimal soil pH for crop production is within the interval 5.5–8.0, which suggests that the suitability of SD for agricultural purposes could be limited. With the exception of SD, all treatments led to an increase in soil EC values at the end of the experiment, following the sequence (dS m−1): CM (0.64) > LD (0.45) ≈ D (0.42) > CS (0.37) > control (0.30) ≈ SD (0.29). This fact was provoked by the relative high EC of some organic materials and the lack of leaching. Even though the observed soil EC values do not have a restriction for agriculture use of these materials, their application must be controlled to avoid an excessive salt accumulation in soil.
Precipitation and fixation of P in the soil due to the alkalinity caused in soils amended with SD could partially explain the great decrease of the available-P concentration found over time (Table 4). Plant available-P reduction by microbial immobilisation cannot be discounted in the SD treatment, as microorganisms require P for their growth and activity (Wu et al. 2007). Contrastingly, the lower pH of the soil amended with CM could explain the higher concentration of available P in these soils than in the rest of the treatments at all sampling times (Table 4). This is in good agreement with Preusch et al. (2002), who reported similar water-extractable P concentrations in soils amended with composted poultry litter and fresh litter.
To evaluate the P-fertiliser potential of the amendments, the concentration of available P in the soils after 56 days was expressed as kilograms of P2O5 per dry megagram (for CM and SD) or cubic meter (for CS, D and LD). The three liquid amendments showed similar results (3.2, 3.4 and 3.7 kg P2O5 m−3 for CS, D and LD, respectively), while CM potential (12.1 kg P2O5 Mg−1) was clearly higher than that of SD (7.3 kg P2O5 Mg−1), mirroring the results obtained for N-fertiliser potential.
Conclusions
Our study showed that direct application of digestate produced little benefits in the short term due to the rapid mineralisation of the organic matter added with this material. Therefore, the present study suggests that solid–liquid separation of co-digested cattle slurry followed by composting of the solid fraction and soil application of the liquid phase is the most suitable strategy based on agricultural purposes. In this context, the advantages of composting the solid fraction of a digestate are the microbial stability and sanitisation, as well as the easier handling and possible distribution of the material. Compost provided the greatest P and N fertiliser potential as it did not produce any immobilisation of these nutrients and net N-mineralisation occurred. This is of great interest regarding its use in agriculture with respect to the rest of the materials tested, as it allows calculation of the application rate needed to supply the appropriate amount of N and P to the soil according to the crop requirement. These results can help to establish the most suitable agronomic use and to detect potential negative impacts of the application to soil of organic materials coming from anaerobic digestion processes.
References
Alburquerque JA, de la Fuente C, Ferrer-Costa A, Carrasco L, Cegarra J, Abad M, Bernal MP (2012a) Assessment of the fertiliser potential of digestates from farm and agroindustrial residues. Biomass Bioenerg 40:181–189
Alburquerque JA, de la Fuente C, Bernal MP (2012b) Chemical properties of anaerobic digestates affecting C and N dynamics in amended soils. Agric Ecosyst Environ. doi:10.1016/j.agee.2011.03.007
Al Seadi T (2002) Good practice in quality management of AD residues from biogas production. IEA Bioenergy, Task 24—Energy from Biological Conversion of Organic Waste, January 2002 (available at: http://web.sdu.dk/ bio/pdf/manage.pdf).
Anderson TH, Domsch KH (1990) Application of eco-physiological quotients (qCO2 and qD) on microbial biomass from soils of different cropping histories. Soil Biol Biochem 22:251–255
APHA (2005) Standard methods for the examination of water & wastewater (21st ed.). American Public Health Association, American Water Works Association and Water Environment Federation, Washington, DC, USA.
Atallah T, Andreux F, Chone T, Gras F (1995) Effect of storage and composting on the properties and degradability of cattle manure. Agric Ecosyst Environ 54:203–213
Barajas-Aceves M (2005) Comparison of different microbial biomass and activity measurement methods in metal-contaminated soils. Bioresour Technol 96:1405–1414
Bernal MP, Sánchez-Monedero MA, Paredes C, Roig A (1998a) Carbon mineralisation from organic wastes at different composting stages during their incubation with soil. Agric Ecosyst Environ 69:175–189
Bernal MP, Navarro AF, Sánchez-Monedero MA, Roig A, Cegarra J (1998b) Influence of sewage sludge compost stability and maturity on carbon and nitrogen mineralisation in soil. Soil Biol Biochem 30:305–313
Bremner JM, Keeney DR (1965) Steam distillation methods for determination of ammonium, nitrate and nitrite. Anal Chim Acta 32:485–495
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Burton CH (2007) The potential contribution of separation technologies to the management of livestock manure. Livest Sci 112:208–216
Bustamante MA, Alburquerque JA, Restrepo A, de la Fuente C, Paredes C, Moral R, Bernal MP (2012) Co-composting of the solid fraction of anaerobic digestates, to obtain added-value materials for use in agriculture. Biomass Bioenerg 43:26–35
Clemente R, de la Fuente C, Moral R, Bernal MP (2007) Changes in microbial biomass parameters of a heavy metal-contaminated calcareous soil during a field remediation experiment. J Environ Qual 36:1137–1144
Clemens J, Huschka A (2001) The effect of biological oxygen demand of cattle slurry and soil moisture on nitrous oxide emissions. Nutr Cycl Agroecosyst 59:193–198
Chroni C, Kyriacou A, Manios T, Lasaridi K (2009) Investigation of the microbial community structure and activity as indicators of compost stability and composting process evolution. Bioresour Technol 100:3745–3750
de la Fuente C, Clemente R, Martinez J, Bernal MP (2010) Optimization of pig slurry application to heavy metal polluted soils monitoring nitrification processes. Chemosphere 81:603–610
Dendooven L, Bonhomme E, Merckx R, Vlassak K (1998) N dynamics and sources of N2O production following pig slurry application to a loamy soil. Biol Fertil Soils 26:224–228
Fuchs JC, Berner A, Mayer J, Smidt E, Schleiss K (2008) Influence of compost and digestates on plant growth and health: potentials and limits. In: Fuch JS, Kupper T, Tamm L, Schenk K (Eds) Proceedings of the International Congress CODIS2008. 27–29 February 2008, Solothurn, Switzerland (available at: http://orgprints.org/13135/1/fuchs-etal-proceedings-codis-2008.pdf).
Holm-Nielsen JB, Al Seadi T, Oleskowicz-Popiel P (2009) The future of anaerobic digestion and biogas utilization. Bioresour Technol 100:5478–5484
Jørgensen K, Jensen LS (2009) Chemical and biochemical variation in animal manure solids separated using different commercial separation technologies. Bioresour Technol 100:3088–3096
Kempers AJ, Zweers A (1986) Ammonium determination in soil extracts by the salicylate method. Comm Soil Sci Plant Anal 17:715–723
Kirchmann H, Lundvall A (1993) Relationship between N immobilization and volatile fatty acids in soil after application of pig and cattle slurry. Biol Fertil Soils 15:161–164
McLachlan KL, Chong C, Vorony RP, Liu HW, Holbein BE (2004) Assessing the potential phytotoxicity of digestates during processing of municipal solid waste by anaerobic digestion: comparison to aerobic composts 638: 225-230
Moral R, Moreno-Caselles J, Perez-Murcia MD, Perez-Espinosa A, Rufete B, Paredes C (2005) Characterisation of the organic matter pool in manures. Bioresour Technol 96:153–158
Myrold DD, Posavatz NR (2007) Potential importance of bacteria and fungi in nitrate assimilation in soil. Soil Biol Biochem 39:1737–1743
Morris DR, Lathwell DJ (2004) Anaerobically digested dairy manure as fertilizer for maize in acid and alkaline soils. Comm Soil Sci Plant Anal 35:1757–1771
Möller HB, Sommer SG, Ahring BK (2002) Separation efficiency and particle size distribution in relation to manure type and storage conditions. Bioresour Technol 85:189–196
Nelson DW, Sommers LE (1982) Total carbon, organic carbon and organic matter. In: Page AL (Ed.) Methods of soil analysis. Part 2. Chemical and microbiological properties. American Society of Agronomy–Soil Science Society of America, Madison, WI, pp. 539–579.
Nieder R, Benbi DK, Scherer HW (2011) Fixation and defixation of ammonium in soils. Biol Fertil Soils 47:1–14
Nielsen LH, Hjort-Gregersen K, Thygesen P, Christensen J (2002) Samfundsøkonomiske analyser af biogas fællesanlæg. Rapport 136. Fødevareøkonomisk Institut, København.
Paredes C, Roig A, Bernal MP, Sánchez-Monedero MA, Cegarra J (2000) Evolution of organic matter and nitrogen during co-composting of olive mill wastewater with solid organic wastes. Biol Fertil Soils 32:222–227
Poggi-Varaldo HM, Trejo-Espino J, Fernandez-Villagomez G, Esparza-Garcia F, Caffarel-Mendez S, Rinderknecht-Seijas N (1999) Quality of anaerobic compost from paper mill and municipal solid wastes for soil amendment. Water Sci Technol 40:179–186
Preusch PL, Adler PR, Sikora LJ, Tworkoski TJ (2002) Nitrogen and phosphorous availability in composted and uncomposted poultry litter. J Environ Qual 31:2051–2057
Rehl T, Müller J (2011) Life cycle assessment of biogas digestate processing technologies. Resour Conserv Recycl 56:92–104
Saviozzi A, Levi-Minzi R, Riffaldi R (1993) Mineralisation parameters from organic materials added to soil as a function of their chemical composition. Bioresour Technol 45:131–135
Sinha MK, Sinha DP, Sinha H (1977) Organic matter transformations in soils. V. Kinetics of carbon and nitrogen mineralization in soil amended with different organic materials. Plant Soil 46:579–590
Smet E, Van-Langenhore H, De-Bo IZ (1998) The emission of volatile compounds during the aerobic and the combine anaerobic/aerobic composting of biowaste. Atmos Environ 33:1295–1303
Sommer SG, Olesen JE (1991) Effects of dry matter content and temperature on ammonia loss from surface-applied cattle slurry. J Environ Chem 20:679–683
Sørensen P (1998) Effects of storage time and straw content of cattle slurry on the mineralisation of nitrogen and carbon in soil. Biol Fertil Soils 27:85–91
Tchobanoglous G, Kreith F, Williams ME (2002) Introduction. In: Tchobanoglous G, Kreith F (eds) Handbook of solid waste management, 2nd edn. McGraw Hill, London
USEPA (2005) Method 1682: Salmonella in sewage sludge (biosolids) by modified semisolid Rappaport–Vassiliadis (MSRV) medium, U.S. EPA Office of Water, p. 41 EPA/821/R-04/028.
USEPA (2007) Method 9210A. Potentiometric determination of nitrate in aqueous samples with anion-selective electrode (available at: http://www. epa.gov/epawaste/hazard/testmethods/sw846/pdfs/9210a.pdf).
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:689–696
Watanabe FS, Olsen SR (1965) Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil. Soil Sci Soc Am Proc 29:677–678
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of soil microbial biomass by fumigation–extraction—an automated procedure. Soil Biol Biochem 22:1167–1169
Wu J, Huang M, Xiao H-A, Su Y-R, Tong C-L, Huang D-Y, Syers J (2007) Dynamics in microbial immobilization and transformations of phosphorus in highly weathered subtropical soil following organic amendments. Plant Soil 290:333–342
Acknowledgements
This work has been financed by the Ministry of Economy and Competitiveness of Spain (previous Science and Innovation) (Ref. PSS-120000-2008-58 and -65) and the EU through FEDER Funds (Fondo Europeo de Desarrollo Regional “una manera de hacer Europa”). Part of Dr. Clemente’s salary is paid by the European Social Funds of the European Union through “Ramón y Cajal” contract.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de la Fuente, C., Alburquerque, J.A., Clemente, R. et al. Soil C and N mineralisation and agricultural value of the products of an anaerobic digestion system. Biol Fertil Soils 49, 313–322 (2013). https://doi.org/10.1007/s00374-012-0719-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-012-0719-9