Abstract
Purpose
Organic waste (OW), such as anaerobic digestates and compost, can provide nitrogen (N) and valuable crop available phosphorous (P) and is a potential alternative to chemical P fertilizers, which is a non-renewable resource. However, little is known about the short- to medium-term P supply capacity of OW within the framework of optimal soil organic carbon management.
Methods
In this study, four products were investigated: a sewage sludge digestate (D1) and biowaste digestate (D2), in addition to their respective composts (C1 and C2). These products were compared at 170 kg N ha−1 along with a chemical fertilizer (Chem) and digestate from animal manure (DM) in two successive tests on potted ryegrass (84 + 84 days), without (N −) and with (N +) mineral N supply (170 kg N ha−1). Their agronomic performance and apparent bioavailable P (ABP mg pot−1) were assessed. In addition, the variation in the total organic carbon (TOC), labile carbon (CL), and carbon management index (CMI) was monitored in potting soil at the end of the two growth cycles.
Results
The pot test showed that after 84 days, Chem mostly exploited its ABP (95%), similar to DM, resulting in a low-P inheritance over 168 days, while the compared products (D1; C1; D2; C2) ensured long-lasting P release. CL was more sensitive than Corg to changes occurring during the test, thus proving that biowaste products, especially compost (C2), gained the best CMI.
Conclusions
This promising approach may provide comprehensive insight into rational P fertilization via OW soil distribution and can provide important information on its effect on overall soil carbon management, a currently notable issue, especially in the Mediterranean region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
It is widely recognized that soil organic carbon (SOC) plays a fundamental role in soil health conditions and also plays a crucial role in many soil functions and ecosystem services (Stolte et al. 2016; Stavi et al. 2016). In this context, SOC represents a key factor in the overall balance of life, with important effects on fundamental components such as biodiversity and carbon storage. However, in recent decades, modern agricultural practices have heavily reduced the SOC content. In this framework, continuous mineral fertilization is often coupled to heavy soil ploughing, deeply stressing soil organic matter (SOM) mineralization processes; therefore, it appears that approximately 50% of European soils suffer organic matter deficiency, which is currently a current issue in many other parts of the world (de Brogniez et al. 2015). Within this context, the reutilization of recycled organic matter (animal slurries and manures; compost; anaerobic digestates; sewage sludge) is receiving increasing attention, aimed at both crop nutrition and carbon conservation/storage in agricultural soils according to the Circular Economy framework promoted by the European Union (EU), in the vision of more sustainable development (European Commission 2020).
There have been several investigations about the nitrogen (N) fertilizing capacity of these types of organic products (Grigatti et al. 2011; Akbar et al. 2021), while fewer, although an increasing number, of studies have researched phosphorus (P), an emerging critical raw material (Grigatti et al. 2019; Stamm et al. 2021). Phosphorous fertilizers are primarily derived from mined phosphate rock. These economic reservoirs are found in only a few regions of the world, so phosphate rock mining is controlled by five countries, the main ones being Morocco, China, and the USA, representing a security problem (Schroder et al. 2010; Stamm et al. 2021). Therefore, in recent years, there has been a growing interest in the strategic recycling of P from OW for crop nutrition (Fuentes et al. 2006; Jakubus 2016), and investigations addressing this topic have often proven the reliable efficiency of recycled organic products as P sources (Bachmann et al. 2011; Grigatti et al. 2019; Majaule et al. 2022).
As previously stated, N is a primary element for plant, and crop fertilization plans are typically N based; however, the recycled OW to be used in agricultural soil can have a significant amount of P, thus resulting in a potential imbalance between applied P and plant P requirements (Fuentes et al. 2006; Rowe et al. 2016). According to the different feedstocks and processes, P can be found in different forms (free, labile, partially available, unavailable) in this type of product, making soil P management challenging (Fuentes et al. 2008; Mazzini et al. 2020; Grigatti et al. 2020). The different P forms in recycled OM sources can be successfully determined via different sequential chemical extraction (SCE) approaches. According to Dou et al. (2000), this approach can provide a reliable assessment of the free P forms (water-extractable) in addition to the easily and medium- to long-term plant available P extractable in bicarbonate and alkali. Finally, the sparingly soluble P determined in the acid extract (HCl-P) is generally considered unavailable for plants. Based on this method, simplified procedures are proposed in the literature, obtaining robust results, such as those reported by Turner and Leytem (2004), which adopt a sequence of NaHCO3 and NaOH + EDTA or NaHCO3, NaOH + EDTA, and HCl, as suggested by Mazzini et al. (2020). In this context, some studies have proven the relationship between the various P forms determined via SCE and plant P uptake (Grigatti et al. 2017; 2019), and this approach is helpful for predicting potentially plant available P in addition to the potential environmental risk (leaching and runoff) following the soil distribution of recycled OW.
OW production is continuously increasing; thus, the anaerobic digestion (AD) process is rapidly expanding as a technology successfully applied to reduce the amount of easily degradable OM in organic waste, representing a renewable energy source via biogas production (Lytras et al. 2021). Anaerobic digestion is often coupled with the composting process with the aim of producing biologically stable products to be safely utilized in agricultural soil. It is generally recognized that the distribution of biologically unstable OM to the soil can cause high CO2 emissions in addition to undesired N2O and possible NH3 emissions (Verdi et al. 2019; Grigatti et al. 2011; 2020). Among OW types, sewage sludge and biowaste represent an important, and ever increasing, fraction of the waste stream in developed countries (Di Capua et al. 2020; Lytras et al. 2021). Proper management of these products can represent a cost and management problem, being, on the other hand, an opportunity for possible nutrient recovery aimed at plant nutrition, soil carbon conservation, and potential storage (Regelink et al. 2021; Slepetiene et al. 2022). However, few studies have addressed P plant availability from this type of product, especially in the context of the best soil carbon management practices (Slepetiene et al. 2020). As previously stated, SOC plays a fundamental role in ecosystems, and increasing interest is being paid to its conservation and possible increase. At the same time, it is recognized that the total amount of organic carbon in soil is not a robust indicator of the soil health status, and labile carbon (CL), determined via weak oxidation with potassium permanganate (KMnO4), is a more suitable indicator to describe soil functionality. The use of the carbon management index (CMI), proposed by Blair et al. in 1995, can be helpful to this aim. This index is based on the distribution of SOC in labile and nonlabile fractions, producing a lability index (LI), as well as the total carbon-related index, the carbon pool index (CPI), to determine the relative sustainability of systems under different management options (i.e., cropping; fertilization) compared to a reference system. Later, many authors adopted the CMI as an indicator of SOC quality changes resulting from different management practices (Gong et al. 2009; De Bona et al. 2008; Verma et al. 2013; Li et al. 2018). This approach was adopted to assess the variation occurring in SOC following long-term, simple mineral/organic or combined fertilization (Lou et al. 2011; Li et al. 2018). As previously stated, the reutilization of organic waste for agricultural purposes is generally N based, while the potential and actual plant P availability following the soil application of these products are poorly investigated. In addition, the soil functionality variation following the utilization of different recycled OW is poorly addressed in the literature, which is especially true for anaerobic digestates. While compost is generally considered a soil amendment with important organic carbon restoring capacity, anaerobic digestates are more often used in light of their fertilizing capacity (mainly N), and their effect on soil carbon and overall carbon management appears to be scarcely addressed in the literature (Slepetiene et al. 2022).
As mentioned above, these products can be used alternating with chemical fertilization; as fertilization plans are generally N based, there is a lack of relatively complete knowledge about the short- and medium-term plant P availability following the agricultural use of processed (via AD and/or composting) sewage sludge and biowastes, especially in the context of enhanced soil carbon management.
In this regard, the aim of this experiment was to (i) evaluate the current and residual potential plant P availability following the distribution of high- and low-P products from raw and composted anaerobic digestates from either sewage sludge or biowaste in a simulated sequence of organic and mineral fertilization on potted ryegrass. In addition (ii), this experiment explored the effect of the different organic fertilizers on the variation in the TOC and CMI in comparison with those of commonly applied chemical fertilizers at the end of two growth cycles.
2 Materials and Methods
2.1 Organic Products
Four organic products from either sewage sludge or biowaste were selected for this work. The solid fraction (press-filtered) of an anaerobic digestate was collected after 20 days of thermophilic continuous wet digestion of a municipal sewage sludge (D1); a compost (C1) was obtained after 30 days of composting the filter-pressed D1 along with green waste (30/70; V/V). Another anaerobic digestate was collected after 20 days of dry-continuous anaerobic digestion of biowaste (D2); the respective compost (C2) was obtained after 20 days of composting with green waste (50/50; V/V). In addition, a dry-batch anaerobic digestate from cow manure (DM) was added as an organic reference in the pot test. For the fresh products, we measured total solids (TS) and volatile solids (VS), NH+4-N, and the oxygen uptake rate (OUR, Grigatti et al., 2007). The tested products were then freeze-dried and ball milled for further analytical determinations. Total organic carbon (TOC) and total nitrogen (TN) were determined via an elemental analyzer (EA 1110, Thermo Electron, Germany). The total nutrients and heavy metals were determined using inductively coupled plasma–optical emission spectrometry (ICP–OES; Spectro Arcos, Ametek, Berwin, PA, USA) on ≈ 300-mg samples after microwave-assisted digestion (37% HCl and 65% HNO3). The main product characteristics and heavy metal contents are summarized in Table 1 and Table 2.
2.2 Phosphorous Fractionation
The organic products were subjected to P fractionation via sequential chemical extraction (SCE) according to the method of Mazzini et al. (2020). Briefly, freeze-dried and ball-milled products were extracted for 24 h with 0.5 M NaHCO3 (pH 8.5) in an end-over-end shaker and then centrifuged. The supernatants were filtered (Whatman #42), and the recovered pellets were extracted with NaOH + EDTA (0.25 N + 0.05 M) for 24 h. The same procedure was repeated with 1 N HCl. Inorganic P (Pi) in the extracts was determined via the molybdenum blue method (Murphy and Riley, 1962); total P in the extracts was determined via ICP. Organic P (Po) was calculated as the difference between total P and Pi in the extracts. The P recovered (%) in each fraction [bicarbonate extractable P (NaHCO3-P), alkali extractable P (NaOH + EDTA-P), acid extractable P (HCl-P), residual-P (H2SO4-P)] was calculated as follows:
where Pfraction x is the inorganic/organic P determined in each fraction (NaHCO3, NaOH + EDTA, HCl, residual), and Ptot OW is the total P determined in the different organic waste via ICP following microwave-assisted acid digestion. The total recovery was calculated as the sum of all fractions (NaHCO3-P + NaOH + EDTA-P + HCl-P + residual-P) by using the following equation:
where \({\sum }_{NaHCO3}^{Residual}{P}_{i;o fraction \, x}\) represents the sum of the single P recovery values (Pi; Po) in each fraction (NaHCO3-P + NaOH + EDTA-P + HCl-P + residual-P) and Ptot OW is the total P determined in the different products via ICP after microwave-assisted acid digestion.
2.3 Pot Test
The soil utilized in the pot experiments was taken from the upper layer (0–20 cm) of a field in the Po Valley (Bologna, Italy) as reported in Grigatti et al. (2015). This soil showed the following characteristics: pH (H2O 1:2.5), 7.90; particle-size distribution, sand (184 g kg−1), silt (425 g kg−1), and clay (391 mg kg−1); total CaCO3, 85 g kg−1; total organic carbon (TOC), 10.2 g kg−1; total Kjeldahl nitrogen (TKN), 1.60 g kg−1; C:N, 8.3; exchangeable K, 330 mg kg−1 (as K2O); and CEC, 27.2 meq. 100 g−1. The total (extractable in aqua regia + HF) Al, Fe, and P levels were 35,661, 22,224, and 808 mg kg−1, respectively. The NH4-oxalate (pH 3)-extractable Al and Fe levels were 764 and 2158 mg kg−1, respectively, while the Na dithionite-citrate-extractable Al and Fe levels were 281 and 2462 mg kg−1, respectively. The potted assay involved two subsequent growing cycles, each lasting 84 days (total 168 days). During the first growing cycle, the organic product (D1; D2; C1; C2) and the organic reference (DM) were applied to the soil at the target rate of 170 kg N ha−1. Then, amended soil (1 kg on a TS basis) was added into 2 L pots (ø 16 cm), previously half-filled (1 L) with (washed) sand. A complete randomized design with three replicates was adopted, including one chemical reference (Chem, N-P-K: 170–60-100 kg ha−1) through the addition of a solution containing NH4NO3 + Ca(H2PO4)2 + K2SO4 in addition to unamended soil (Ctrl). Seeds of ryegrass (Lolium multiflorum subsp. italicum) cv. were sown (0.8 g pot−1). Pots were set in a growing chamber with a 14-h photoperiod and temperatures of 23/13 °C day/night. Lighting was ensured by Master Tld 58 W-840 tubes (Philips, Amsterdam, The Netherlands). The pots were watered on a regular basis with tap water to maintain 60% water-holding capacity. At the end of the first cycle, ryegrass plants were harvested by cutting 2 cm above the soil and air-dried in a forced air oven (60 °C). Roots were carefully separated by soil by water washing and then air-dried. Tissue and roots were then ball-milled and analyzed for P content via ICP after microwave-assisted acid digestion. The soil recovered from the first cycle (from each treatment) was utilized in the second cycle of cultivation. The pots were filled with soil and seeded with ryegrass. To ensure a nonlimiting nutrient environment, a solution providing N was distributed to all the treatments (Table 1S) and managed as previously described. At the end of the second growth cycle, tissue and roots were collected and treated as previously described. The ryegrass P recovery (mg pot−1) was calculated by multiplying the dry biomass by the total P content. The Olsen-P in the pot soil was determined at the beginning and at the end of the first and second growth cycles (days 84 and 168). The apparent bioavailability of phosphorus (ABP) was calculated similarly to that described by Jimenez et al. (1993), as follows:
where Ptreatment (tn) is the P uptake (mg pot−1) at time t (day 84; 168) of plant tissue and roots of treated pots (digestates and compost; Chem), Pctrl (tn) is the P uptake (mg pot−1) (days 84, 168) of plant tissue and roots of control pots, Olsen-Ptreatment (tn) is the Olsen-P content (mg pot−1) of soil in treated pots (digestate, compost or Chem) at time n (days 84, 168), and Olsen-Pctrl (tn) is the Olsen-P content (mg pot−1) of soil in control pots (days 84, 168).
2.4 Soil Organic Carbon and KMnO 4 Oxidizable C
At the end of the first (day 84) and second (day 168) growth cycles, the organic carbon content was determined in pot soil via an elemental analyzer (EA 1110, Thermo Electron, Germany). In addition, the KMnO4 oxidizable C (CL) content was determined (in duplicate) on 2.5 g of pot soil according to Weil et al. (2003), assuming that 1.0 mmol L−1 MnO4 was consumed (Mn7+ → Mn2+) in the oxidation of 0.75 mmol L−1 (9.0 mg) carbon. The carbon management index (CMI) was obtained according to the method of Blair et al. (1995). CMI was calculated as follows:
CMI (%) = carbon pool index (CPI) × lability index (LI) × 100.where CPI was calculated according to the following equation:
where C-treated soil is the organic carbon (g kg−1) from soil treated with the organic products (D1, D2, C1, C2) and C reference soil is the organic carbon (g kg−1), in soil from Chem.
and LI was calculated according to the following equation
where C lability treated soil sample is the carbon lability from soil treated with the organic products (D1, D2, C1, C2), and C lability reference soil is the carbon lability in soil from Chem. The C lability is expressed as the ratio of labile C (CL) to nonlabile C (CNL). Nonlabile C was determined as the difference between the total C content and CL content of the soil.
2.5 Statistical Analysis
One-way ANOVA was conducted for the fertilizer treatments, addressing the shoot parameters (DW; P uptake) and soil parameters (TOC; CL; CNL) at the end of each growing cycle (days 84 and 168). Tukey’s HSD test at p < 0.05 was used to separate levels in significant ANOVA sources. The Statistica 7® package (Statsoft, Tulsa, OK, USA) was used to perform ANOVA and the Tukey test.
3 Results
3.1 Organic Products
The selected organic products for this work had different characteristics depending on their feedstock and processes. As displayed in Table 1, the initial TS level in the fresh anaerobic digestates (D1 and D2) was very low in comparison to that of the respective composts C1 and C2 (210 vs. 560 mg g−1), with the reference Ad (DM) being in the low range. Table 1 also shows the VS level, which was higher in anaerobic digestates than in respective compost (510 vs. 460 mg g−1 on average). Accordingly, the TOC of the tested Ads (D1 and D2) was higher than that of their respective compost (C1 and C2), thus averaging 269 and 240 mg g−1, respectively. Furthermore, the TN level showed notable variation ranging between 41 and 26 mg g−1 in sewage sludge-derived products (D1 and C1), being detected at a significantly lower level in D2 and C2 (17 and 15 mg g−1) and giving a wide C:N ratio ranging between ≈ 7 and 16 in digestates (D1 and D2) and between 10 and 15 in compost (C1 and C2). The NH+4-N level was approximately 10 mg g−1 in anaerobic digestates (D1 and D2) and was below 1 mg g−1 in the respective compost samples (C1 and C2). In this context, the organic reference (DM) exhibited extremely high VS (67%) and TOC (364 mg g−1) levels and intermediate TN and NH+4-N levels (27 and 6.3 mg g−1), thus achieving an intermediate C:N ratio of 14. In addition to these characteristics, the stability of the compared products showed important variation; the OUR (mmol O2 kg−1 VS h−1) ranged between 96 and 46 (D1 and D2) and between 8 and 11 (C1 and C2). The OUR of the reference material (DM) reached 11 mmol O2 kg−1 VS h−1. Table 1 also reports the total P content of the tested samples; this content was the highest in the sewage sludge-derived products (D1 and C1), which attained total P contents of ≈ 16 and ≈ 20 mg g−1, respectively, 4–fivefold higher than those exhibited by the biowaste-derived products (D2 and C2), which were both at ≈ 4 mg g−1. Among the other elements, total Ca content was the highest in D1 (69 mg g−1), while the content was lower (≈ 40 mg g−1) in the other tested products (C1; D2; C2). Moreover, the manure-Ad (DM) sample showed a notably low Ca content (≈ 20 mg g−1). Accordingly, the Ca:P ratio was lowest in D1 and C1 (Ca:P; 3, on average), very close to that in DM, and this ratio was approximately 9 in D2 and C2 (Table 1). Finally, the Fe content was highest in D1 and C1 (≈ 40 mg g−1) and that in the organic reference (DM) was lowest (≈ 2 mg g−1), with the Fe content in D2 and C2 being intermediate (≈ 7 mg g−1). The Fe:P ratio was at a similar level in both digested and composted products (D1; D2; C1; C2) at ≈ 1.5, on average, which was higher than that of DM (Fe:P, 0.3). As reported in Table 2, the compared products showed very different heavy metal contents. The sewage sludge-based products (D1 and C1) showed the highest Cd, Cu, Pb, and Zn contents. Last, the heavy metal content in the organic reference (DM) was the lowest.
3.2 Phosphorus Fractionation
The organic products compared in this work showed very different phosphorus fractionation (Table 3). The products from sewage sludge (D1 and C1) showed NaHCO3-Pi values ranging between 2.8 and 1.7 mg g−1, which were slightly higher than those of D2 and C2 (1.8 and 1.2 mg g−1), thus attaining very different recoveries in the two groups (≈ 10 vs. 35%), and DM exhibited an intermediate value (1.5 mg g−1; 28%). Conversely, the NaOH + EDTA-Pi level showed wide variation, being ≈ 6.5 mg g−1 in D1 and C1 and 1.7 mg g−1 in D2 and C2, which reflected the close recoveries of these products: 33–40% (D1-C1) and 40–41% (D2-C2). The NaOH + EDTA-Pi level of DM was in the lower range (1.4 mg g−1; 26%). The acid extractable P (HCl-P) level was 1.3 and ≈ 0.5 mg g−1 in sewage sludge (D1 and C1) and biowaste products (D2 and C2), thus attaining ≈ 7 and ≈ 12% recovery, respectively. The HCl-P level of DM was relatively low (0.2 mg g−1; 3%). The total recovery of Pi showed very notable variation, being approximately 55% in D1 and C1 and notably higher in D2 and C2 (≈ 90%) and that in DM was lower (58%). Contextually, the Po level was ≈ 10 mg g−1 in D1 and C1 (recovery ≈ 50%) and was detected at notably lower levels in D2 and C2 (≈ 1 mg g−1; recovery ≈ 20%). The reference digestate (DM) showed an intermediate Po level (1.5 mg g−1; 28%). Organic P was mostly detectable in the NaOH + EDTA extracts (Table 3), while residual-P was undetectable in all the tested samples. Overall, the P fractionation (%) was in the order of NaOH + EDTA (86) > NaHCO3 (14) > HCl (8) for D1, NaOH + EDTA (93) > NaHCO3 (12) > HCl (8) for C1, NaOH + EDTA (56) > NaHCO3 (47) > HCl (10) for D2, NaOH + EDTA (64) > NaHCO3 (31) > HCl (5) for C2, and NaOH + EDTA (54) > NaHCO3 (34) > HCl (5) for DM.
3.3 Plant Test
The ryegrass pot test showed that plant tissue dry biomass was affected by the different treatments. As shown in Table 4, the cumulative DW of tissue obtained during the first cycle of cultivation (day 84) ranged between 1.53 and 2.09 g pot−1 for the Ctrl and Chem; that of D1 and C1 was relatively high (1.91 g pot−1, on average). D2 and more C2 showed relatively low DW (1.79 and 1.61 g pot−1), and the reference Ad (DM) was intermediate (1.67 g pot−1). Root DW assessed at the end of the first growth cycle was not significantly different between the treatments; however, in the Ctrl and Chem groups, this value ranged between 1.18 and 1.56 g pot−1. D1 performed better than the associated compost C1 in terms of root DW (1.91 vs. 1.32 g pot−1). The opposite trend in root DW was observed for D2 and C2 (1.55 vs. 1.87 g pot−1), and DM exhibited an intermediate root DW (1.51 g pot−1). At the end of the second growth cycle (day 168), the tissue DW in Ctrl and Chem ranged between 2.66 and 2.80 g pot−1, without significant differences. The organic products (D1, D2, C1, C2) similarly performed without significant differences in tissue DW in comparison with that of the organic reference DM, averaging 2.58 g pot−1. Root dry biomass varied between 0.74 and 0.79 g pot−1 in Ctrl and Chem and was not different from that in the organic treatment groups (D1, D2, C1, C2) and the organic reference (DM), exhibiting an average value of 0.81 g pot−1. Overall, at the end of the first cycle, the best DW (g pot−1) was observed in Chem (4.89), followed by D1 (4.57), C1 (4.41), DM (4.39), D2 (4.30), Ctrl (4.19), and C2 (4.11). Although not significantly different, the biomass sum (g pot−1) over the two growth cycles was as follows: Chem, 7.24; C1, 7.15; D2, 7.07; DM, 6.72; D1, 6.72; C2, 6.31; and Ctrl, 6.11.
The ryegrass tissue P uptake at the end of the first growth cycle (day 84) was recorded at 4.03 mg pot−1 in Ctrl, which was the lowest value. D1 and D2 performed the best in terms of P uptake (5.09 mg pot−1), whereas the composted products (C1 and C2) achieved a P uptake value of 4.77 mg pot−1 (on average), which was similar to that of DM and Chem (4.69 and 4.67 mg pot−1). At the same sampling time, the root P uptake was 2.43 and 2.61 mg pot−1 in Ctrl and Chem, respectively, which were similar to those in the other treatments (D1, D2, C1, C2) and DM (2.78 mg pot−1, on average). At the end of the second cycle of cultivation (day 168), the ryegrass tissue P uptake ranged between 6.03 and 5.81 mg pot−1 in Ctrl and Chem, without significant differences, averaging 5.50 mg pot−1 in the other treatments (D1, D2, C1, C2), which was slightly lower than that in DM (5.98 mg pot−1). The root P uptake was also unaffected by treatments, with a value of 1.34 and 1.38 mg pot−1 in Ctrl and Chem, respectively, and an average of 1.47 mg pot−1 in the other treatments (D1, D2, C1, C2) and the organic reference (DM). Although the P uptake (mg pot−1) was not significantly different between treatments, the cumulative P uptake after the first growth cycle was in the order of D2 (10.99), D1 (10.90), DM (10.67), Chem (10.47), C2 (10.38), Ctrl (10.05), and C1 (9.46). The whole P uptake (mg pot−1) over the two growth cycles was the best in D2 (15.77), followed by D1 (15.71), DM (14.63), Chem (14.47), C2 (14.13), C1 (13.42), and Ctrl (13.82).
3.4 Olsen-P and Apparent Plant Bioavailable P (ABP)
As reported in Fig. 1, at the beginning of the experiment, the Olsen-P in the pot soil ranged between 13 and 33 mg kg−1 in Ctrl and Chem. Among the soil treated with the organic products, the Olsen-P of D1 and C1 was 23 mg kg−1 (on average), which was very close to that of DM (21 mg kg−1). The Olsen-P of D2 and C2 was lower at 18 mg kg−1 (on average). Throughout the pot experiment, the control soil (Ctrl) always had the lowest Olsen-P, which reached 9 and 6 mg kg−1 at the end of the first and second growth cycles (days 84 and 168), respectively. In this timeframe, the Olsen-P in the chemical reference (Chem) decreased linearly to 21 and 7 mg kg−1 on days 84 and 168, respectively. The other products (D1, D2, C1, C2) achieved intermediate Olsen-P on day 84 (15 mg kg−1) that were slightly lower than that of DM on the same sampling day (18 mg kg−1). In the following growth cycle (day 168), D1 and C1 showed further decreases in Olsen-P to 10 mg kg−1, which was slightly higher than that of D2 and C2 (8 mg kg−1), and that of DM which reached the background level (6 mg kg−1). Figure 2 shows the apparent bioavailable P (ABP) level at the end of the first growth cycle (day 84), which was highest in Chem (12.8 mg pot−1). At the same sampling time (day 84), among the organic products, the ABP level of D1 and C1 ranged between 9.2 and 8.4 mg pot−1, which was higher than that of D2 and C2 (8.0 and 6.4 mg pot−1), and the APB level of all these treatments were lower than that of the organic reference (DM: 10.1 mg pot−1). At the end of the second growth cycle (day 168), the APB level (mg pot−1) dramatically decreased in Chem (0.9), and that of D1 and C1 was highest (6.0 and 5.4), surpassing that of D2 and C2 (4.2 and 2.0) and of the organic reference DM (1.3). The cumulative ABP level over 168 days (mg pot−1) was the highest in D1 (15.2), followed by C1 (13.8), Chem (13.7), D2 (12.1), DM (11.4), and C2 (8.4).
Olsen-P determined in pot soil at the beginning of the experiment and after the first (day 84), and the second growth cycle (day 168) in the compared treatments. Ctrl: unamended control soil; D1: anaerobic digestate from wastewater sewage sludge; C1: compost from D1; D2: anaerobic digestate from biowaste; C2 compost from D2; DM digestate from animal manure. Chem: chemical reference (NH4NO3 + Ca(H2PO4)2 + K2SO4). Error bars = standard error (n. 3)
Apparent bioavailable phosphorous (ABP) determined in the compared treatments at the end of the first (day 84) and the second growth cycle (day 168). D1: anaerobic digestate from wastewater sewage sludge; C1: compost from D1; D2: anaerobic digestate from biowaste; C2 compost from D2; DM digestate from animal manure. Chem: chemical reference (NH4NO3 + Ca(H2PO4)2 + K2SO4). Different letter intervals indicate statistically different mean data according to the Tukey test (P < 0.05)
3.5 Total and KMnO 4 Oxidizable Carbon (Labile Carbon)
As shown in Fig. 3 a, the SOC level at the end of the first growth cycle (at day 84) showed no significant difference between the compared treatments; however, the SOC levels were similar in Ctrl and Chem (8.75 and 8.58 g kg−1). At the same sampling time (day 84) among the organic products, D1 showed a slightly higher Corg than C1 (9.28 vs. 9.01 g kg−1), which was similar to, although to a lesser extent, the Corg pattern in D2 and C2 (8.86 vs. 8.77 mg kg−1). The organic reference (DM) exhibited an intermediate Corg value (9.11 g kg−1). At the end of the second growth cycle (at day 168, Fig. 3b), a significant Corg decrease to 7.67 g kg−1 was detectable in pot soil, averaging − 16% with respect to day 84. Ctrl and Chem showed an important reduction in Corg in comparison to the first cycle, achieving values of 7.26 and 7.35 g kg−1 at this stage, respectively, which were similar to that of DM (7.23 g kg−1). D1 and C1 also showed important C mineralization down to 8.03 and 7.00 g kg−1, similar to what was shown by D2 and C2, although to a different extent (8.56 and 7.87 g kg−1).
Total organic carbon (TOC), nonlabile carbon (CNL), labile carbon (CL), and carbon lability in pot soil in the different treatments at the end of the two growth cycles (day 84 (a); day168 (b)). Ctrl: unamended soil; D1: anaerobic digestate from wastewater sewage sludge; C1: compost from D1; D2: anaerobic digestate from biowaste; C2 compost from D2; DM digestate from animal manure. Chem: chemical reference (NH4NO3 + Ca(H2PO4)2 + K2SO4). Different letter intervals indicate statistically different mean data according to the Tukey test (P < 0.05). ns, not significant
Labile carbon (CL) represented the minor C fraction showing any significant difference among the compared treatments at the end of the first growth cycle (at day 84, Fig. 3a). However, the CL value in Ctrl and Chem ranged between 0.35 and 0.26 g kg−1. At the same sampling time, among the organic treatments, the CL values in D1 and C1 were 0.29 and 0.38 g kg−1, respectively, while those in D2 and C2 were much higher (0.46 and 0.49 g kg−1, respectively), and that in DM was intermediate (0.42 g kg−1). At the end of the second growth cycle (at day 168, Fig. 3b), the CL value showed a significant reduction in comparison to the previous sampling time (at day 84); the CL value in Ctrl and Chem ranged between 0.09 and 0.15 g kg−1. Labile C in the soil treated with D1 and C1 varied between 0.19 and 0.17 g kg−1, which was lower than that in D2 and C2 (0.24 and 0.35 g kg−1), with that in DM being intermediate (0.22 g kg−1). Figure 3 a also reports the carbon lability, which, at the end of the first cultivation cycle (day 84), ranged between 4.2 and 3.1% in Ctrl and Chem. In D1 and C1, this value varied between 3.2 and 4.5%, while that in D2 and C2 ranged between 5.4 and 6.0% and that in DM was intermediate at 4.8%. Sampling at day 168 (Fig. 3b) showed that the second growth cycle produced an important reduction in carbon lability in comparison to the previous sampling time at day 84 (Fig. 3a). In this context, the C lability in Ctrl and Chem varied between 1.2 and 2.1%. Among the organic treatments, this value in D1 and C1 was similar (2.5%), while that in D2 and C2 was higher (2.9 and 4.6%) and that in DM was intermediate at 3.8%.
3.6 Carbon Pool Index, Lability Index, and Carbon Management Index
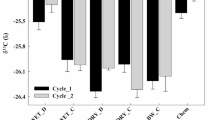
In this study, the carbon pool index (CPI) and the lability index (LI) were calculated for the different treatments vs. Chem. As reported in Fig. 4 a, at the end of the first cultivation cycle (day 84), the CPI in D1 and C1 ranged between 1.08 and 1.03; the CPI in D2 and C2 (1.05 and 1.02) was lower but similar to that in D1 and C1 and that in DM was intermediate (1.06). At the second sampling time at the end of the second growing cycle (at day 168, Fig. 4 b), the CPI in D1 and C1 was 1.09 and 0.95, respectively, and was slightly higher in D2 and C2 (1.16 and 1.07, respectively), while that in DM was intermediate (0.98).
Carbon pool index (CPI) and lability index (LI) calculated in the compared organic treatments (vs. Chem) at the end the two growth cycles (day 84 (a); day168 (b)). D1: anaerobic digestate from wastewater sewage sludge; C1: compost from D1; D2: anaerobic digestate from biowaste; C2 compost from D2; DM digestate from animal manure
The lability index (LI) at the end of the first growth cycle (day 84) shown in Fig. 4a ranged between 1.03 and 1.45 in D1 and C1, being notably higher in D2 and C2 (1.75 and 1.93) and exhibiting an intermediate level in DM (1.54). At the following sampling date (at day 168, Fig. 4b), the LI was similar in D1 and C1 (1.17, on average), while that in D2 and C2 showed notable differences (1.36 vs. 2.15); in this growth cycle, DM also showed an intermediate LI (1.79). The carbon management index (CMI) calculated at the end of the two growth cycles vs. Chem is reported in Fig. 5. At the first sampling date (at day 84), the organic treatments showed very different CMI values: C2 (197) ≥ D2 (184) > DM (167) > C1 (149) > D1 (112). At the second sampling date (at Day 168), the CMI values were slightly different: C2 (231) > DM (176) ≥ D2 (159) > C1 (127) ≥ D1 (109).
Carbon management index (CMI) calculated in the compared organic treatments (vs. Chem) at the end the two growth cycles. D1: anaerobic digestate from wastewater sewage sludge; C1: compost from D1; D2: anaerobic digestate from biowaste; C2 compost from D2; DM digestate from animal manure. Error bars = standard error (n. 3)
4 Discussion
The products tested in this work displayed remarkable differences according to the different feedstocks and processes, which was evident from the Cd, Cu, Pb, and Zn contents being the highest in products derived from sewage sludge; this result represents a characteristic trait of sewage sludge even if the reported values met the Italian legal constraints (Cristina et al. 2019). However, both sewage sludge and biowaste digestates showed little difference in VS and TOC values, being in the normal range for these types of products (Cristina et al. 2019; Reuland et al. 2022). In addition, their different TN values resulted in different C:N ratios in the two products: 7 vs. 16 (D1 vs. D2). Moreover, the C:N ratio of the composted products remained fairly unchanged compared to that of the input digestates, which was related to the addition of a bulking agent. However, according to the thermophilic stabilization process (composting), a steep reduction in ammonium nitrogen was recorded, which was in agreement with what was reported in the literature for similar products (Wang and Zeng 2018). In addition, poor biological stability of both digestates was registered, ranging between ≈ 100 and ≈ 50 mmol O2 kg−1 VS h−1, and these values are in agreement with those reported in the literature for similar products (Grigatti et al. 2020). It is generally recognized that stable products exhibit limited carbon mineralization, thus saving CO2 emissions in addition to N2O and NH3 emissions (Alburquerque et al. 2012; Iocoli et al. 2019; Grigatti et al. 2020). In this framework, composting deeply increased the biological stability, thus placing both composts within the safe limits suggested by the EU Fertilizer Regulation (EU, 2019; < 25 mmol O2 kg−1 VS h−1). Therefore, the composting of digestates can be a useful strategy for improved soil carbon conservation. Moreover, the tested products showed very different phosphorus contents, which could be strategically applied for agricultural purposes. As previously mentioned according to their distribution rate (170 kg N ha−1), D1 and C1 provided the highest estimated P content (80–100 kg P ha−1), which was twice that in D2 and C2 (40–50 kg P ha−1); however, as previously mentioned in the introduction, the potential plant P availability plays a major role in sustainable plant nutrition, and this trait was assessed via SCE. In this frame, the products compared in this work showed very different P fractionation, and the sewage sludge-derived products showed the lowest inorganic labile-P content. This outcome was in agreement with wastewater processing, generally following the utilization of chemicals (i.e., iron salts), therefore converting P into less available forms (Fe–P compounds), which are mostly detectable in alkali extracts (Regelink et al. 2021). This result was observed also for organic P, which had the highest content in sewage sludge-derived products. This fraction (Po), possibly becoming available over intermediate and long time spans as a result of possible mineralization interacts with Pi, thus acting as a medium- and long-term P source (Park et al. 2022). These SCE outcomes showed good relationships with the pot test results. In this study, analysis of the ABP level confirmed that the chemical P treatment was a fast P releaser, yielding the most of its potential over the first cultivation cycle (95%) with minimal residual-P availability. In contrast, sewage sludge and biowaste-based products released about 60–70% of their P potential in the first growth phase, ensuring a significant P contribution during the second growing cycle. Few data are available in the literature about this topic for these types of products. Some information is available about soil residual-P from manure and/or compost (Sharpley, 1996; Eghball et al. 2004), which shows that the N-based fertilization plan produced important soil P release, similar to the results determined in our work.
As mentioned above the use of recycled OW for agricultural purposes is generally N based, depending on crop requirements. However, the recycled OW to be used as fertilizer can have varying TOC contents, with very different inherent properties (biological stability and recalcitrance), thus giving very different organic carbon management outputs in soil in the short to medium term. To gain deeper insight into the application rate and different C:N ratios, sewage sludge and biowaste-based products provided different estimated amounts of TOC to the soil (≈ 1 vs. ≈ 2.5 Mg ha−1). After one growth cycle, the soil treated with recycled OW generally had a higher TOC content than references according to the organic carbon application. However, to a deeper insight, no appreciable difference was recorded between CL values from the different organic treatments at the end of the first growth cycle, while following the external mineral N addition, the unamended control attained the strongest CL depletion, while Chem and DM exhibited a more limited CL decline. In this framework, the organic treatments generally showed higher CL values than both the unamended control soil and the chemical reference, confirming the general capacity of these organic fertilizers to preserve the active fraction of the SOM. CL refers to the highly active fraction being sensitive to plant and microbial activities, which are very susceptible to oxidation and decomposition (Chen et al. 2010). Thus, an increasing amount of scientific literature suggests CL as a useful indicator that can describe the impacts of agricultural practices (including fertilization), becoming a representative index for estimating the quality and productivity of the soil (Yang et al. 2017). The soil receiving the sewage sludge-based digestate showed a lower CL than the soil receiving the biowaste digestate, suggesting a key role of their origin on the total organic carbon dynamics in soil and possibly lower long-term soil quality following the utilization. Unfortunately, not even composting positively affected this characteristic in sewage sludge, additionally, the LI generally confirmed the CL output, thus showing that the sewage sludge-derived products had a lower active carbon content than the biowaste-derived products. In the literature, the KMnO4 oxidation procedure has been proven to oxidize lignin from analyzed samples, and cellulose is not oxidized (Tirol-Padre and Ladha, 2004). The same authors reported that KMnO4 oxidizable C (CL) was directly related to the lignin content of manure and plant residues. Therefore, the chemical composition of raw materials (sewage sludge/biowaste) played a key role in this parameter. The lower C:N ratio of the sewage sludge digestate in comparison to the biowaste digestate (7 vs. 16) can support this hypothesis, with the C:N ratio in the reference digestate (DM) being consistent. Moreover, composting affected this parameter, thus showing that the biowaste digestate compost exhibited a generally higher LI than the raw digestates, which also performed best over 168 days. Accordingly, the CMI in soil treated with the sewage sludge-derived products was notably lower than that in soil treated with the biowaste-derived products and the organic reference (DM) intermediate. Few studies have reported data about the soil carbon management outputs following the soil distribution of raw and composted digestates from sewage sludge and biowaste. Some information is available about the CMI following the soil utilization of raw sewage sludge (Kalisz et al. 2012), reporting a CMI in the same range we observed for both sewage sludge-based products. Kalisz et al. (2017) reported the CMI variation following a field comparison between sewage sludge and selected municipal solid waste compost (MSWC) application, thus showing similar average output values between the treatments. Biowaste and its anaerobically digested/composted products are relatively new products in comparison to MSWC; moreover, there is little information on their utilization in soils. Biowaste originates from food waste selection, which generally produces less inert materials than MSWC thus showing higher OM, on average (Grigatti et al. 2020). Furthermore, biowaste is generally formed by waste rich in fiber components with low nitrogen and high carbon contents, and these products are very different from the sewage sludge waste products. The different feedstocks used to produce the tested products played a key role in their short- to medium-term organic carbon management in soil. This issue is widely discussed in a review by Karimi et al. (2022), who underlined the poor information about the soil quality parameters following the distribution of anaerobic digestates from various raw materials. In this context, the heavy metal content of the biowaste products was generally lower than that of sewage sludge products. Although we did not investigate this specific trait, we cannot exclude some inhibitory effect of this component on the overall soil health (functionality), as proven in the literature following the utilization of similar products (Banerjee et al. 1997).
5 Conclusion
The current management of raw organic waste mainly uses anaerobic digestion and composting; thus, the biologically treated products are commonly used in agriculture for nitrogen-based fertilization plans. However, due to their very different phosphorus contents and solubilities, these materials exhibit different apparent plant available phosphorus. The pot test showed that all compared organic products satisfied the phosphorus plant request during a simulated growing season. In addition, the study revealed that alternating organic and chemical fertilization with nitrogen can maximize the plant phosphorus utilization efficiency from the applied recycled organic wastes, thus possibly saving phosphatic fertilizers application as suggested by European Union guidelines. The study also showed that the use of the carbon management index in the assessment of soil carbon quality following the application of recycled organic waste to the soil proved to be robust and strategic. In this context, sewage sludge-derived products had little effect on soil carbon management, while biowaste-derived products performed better, suggesting that the latter products are best suited to improve organic carbon management in carbon-poor soils in the frame of an integrated fertilization approach.
References
Akbar S, Ahmed S, Khan S, Badshah M (2021). Anaerobic Digestate: a sustainable source of bio-fertilizer. In sustainable intensification for agroecosystem services and management ( 493–542). Springer, Singapore. https://doi.org/10.1007/978-981-16-3207-5
Alburquerque JA, De la Fuente C, Campoy M, Carrasco L, Nájera I, Baixauli C, Bernal MP (2012) Agricultural use of digestate for horticultural crop production and improvement of soil properties. Eur J Agron 43:119–128. https://doi.org/10.1016/j.eja.2012.06.001
Bachmann S, Wentzel S, Eichler-Löbermann B (2011) Codigested dairy slurry as a phosphorus and nitrogen source for Zea mays L. and Amaranthus cruentus L. J Plant Nutr Soil Sci 174:908–915. https://doi.org/10.1002/jpln.201000383
Banerjee MR, Burton DL, Depoe S (1997) Impact of sewage sludge application on soil biological characteristics. Agric Ecosys Environ 66:241–249. https://doi.org/10.1016/S0167-8809(97)00129-1
Blair GJ, Lefroy RD, Lisle L (1995) Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust J Agr Res 46:1459–1466. https://doi.org/10.1071/AR9951459
Chen HL, Zhou JM, Xiao BH (2010) Characterization of dissolved organic matter derived from rice straw at different stages of decay. J Soils Sediments 10:915–922. https://doi.org/10.1007/s11368-010-0210-x
Cristina G, Camelin E, Pugliese M, Tommasi T, Fino D (2019) Evaluation of anaerobic digestates from sewage sludge as a potential solution for improvement of soil fertility. Waste Manage 99:122–134. https://doi.org/10.1016/j.wasman.2019.08.018
De Bona FD, Bayer C, Dieckow J, Bergamaschi H (2008) Soil quality assessed by carbon management index in a subtropical Acrisol subjected to tillage systems and irrigation. Soil Res 46:469–475. https://doi.org/10.1071/SR08018
de Brogniez D, Ballabio C, Stevens A, Jones RJA, Montanarella L, van Wesemael B (2015) A map of the topsoil organic carbon content of Europe generated by a generalized additive model. Eur J Soil Sci 66:121–134. https://doi.org/10.1111/ejss.12193
Di Capua F, Spasiano D, Giordano A, Adani F, Fratino U, Pirozzi F, Esposito G (2020) High-solid anaerobic digestion of sewage sludge: challenges and opportunities. Appl Energ 278:115608. https://doi.org/10.1016/j.apenergy.2020.115608
Dou Z, Toth JD, Galligan DT, Ramberg CF Jr, Ferguson JD (2000) Laboratory procedures for characterizing manure phosphorus. J Environ Qual 29:508–514. https://doi.org/10.2134/jeq2000.00472425002900020019x
Eghball B, Ginting D, Gilley JE (2004) Residual effects of manure and compost applications on corn production and soil properties. Agron J 96:442–447. https://doi.org/10.2134/agronj2004.4420
European Commission, Directorate-General for Communication, Circular economy action plan: for a cleaner and more competitive Europe, Publications Office, 2020, https://doi.org/10.2779/717149
Fuentes B, Bolan N, Naidu R, Mora MDLL (2006) Phosphorus in organic waste-soil systems. J Soil Sci Plant Nutr 6:64–83. https://doi.org/10.4067/S0718-27912006000200006
Fuentes B, de la Luz MM, Bolan NS, Naidu R (2008) Assessment of phosphorus bioavailability from organic wastes in soil. Dev Soil Sci 32:363–411. https://doi.org/10.1016/S0166-2481(07)32016-3
Gong W, Yan XY, Wang JY, Hu TX, Gong YB (2009) Long-term manuring and fertilization effects on soil organic carbon pools under a wheat–maize cropping system in North China Plain. Plant Soil 314:67–76. https://doi.org/10.1007/s11104-008-9705-2
Grigatti M, Pérez MD, Blok WJ, Ciavatta C, Veeken A (2007). A standardized method for the determination of the intrinsic carbon and nitrogen mineralization capacity of natural organic matter sources. Soil Biol Biochem 39: 1493–1503. https://doi.org/10.1016/j.soilbio.2006.12.035
Grigatti M, Di Girolamo G, Chincarini R, Ciavatta C, Barbanti L (2011) Potential nitrogen mineralization, plant utilization efficiency and soil CO2 emissions following the addition of anaerobic digested slurries. Biomass Bioenerg 35:4619–4629. https://doi.org/10.1016/j.biombioe.2011.09.007
Grigatti M, Boanini E, Cavani L, Ciavatta C, Marzadori C (2015) Phosphorus in digestate-based compost: chemical speciation and plant-availability. Waste Biomass Valor 6:481–493. https://doi.org/10.1007/s12649-015-9383-2
Grigatti M, Boanini E, Mancarella S, Simoni A, Centemero M, Veeken AH (2017) Phosphorous extractability and ryegrass availability from bio-waste composts in a calcareous soil. Chemosphere 174:722–731. https://doi.org/10.1016/j.chemosphere.2017.02.039
Grigatti M, Boanini E, Bolzonella D, Sciubba L, Mancarella S, Ciavatta C, Marzadori C (2019) Organic wastes as alternative sources of phosphorus for plant nutrition in a calcareous soil. Waste Manage 93:34–46. https://doi.org/10.1016/j.wasman.2019.05.028
Grigatti M, Barbanti L, Hassan MU, Ciavatta C (2020) Fertilizing potential and CO2 emissions following the utilization of fresh and composted food-waste anaerobic digestates. Sci Total Environ 698:134198. https://doi.org/10.1016/j.scitotenv.2019.134198
Iocoli GA, Zabaloy MC, Pasdevicelli G, Gómez MA (2019) Use of biogas digestates obtained by anaerobic digestion and co-digestion as fertilizers: characterization, soil biological activity and growth dynamic of Lactuca sativa L. Sci Total Environ 647:11–19. https://doi.org/10.1016/j.scitotenv.2018.07.444
Jakubus M (2016) Estimation of phosphorus bioavailability from composted organic wastes. Chem Spec Bioavailab 28:189–198. https://doi.org/10.1080/09542299.2016.1227687
Jimenez EI, Garcia VP, Espino M, Moreno JM (1993) City refuse compost as a phosphorus source to overcome the P-fixation capacity of sesquioxide-rich soils. Plant Soil 148:115–127. https://doi.org/10.1007/BF02185391
Kalisz B, Lachacz A, Glazewski R, Klasa A (2012) Effect of municipal sewage sludge under Salix plantations on dissolved soil organic carbon pools. Arch Environ Prot 38:87–97. https://doi.org/10.2478/v10265-012-0030-8
Kalisz B, Łachacz A, Głażewski R, Grabowski K (2017) Labile organic carbon fractions after amendment of sandy soil with municipal sewage sludge and compost. J Elem 22:785–797. https://doi.org/10.5601/jelem.2017.22.1.1264
Karimi B, Sadet-Bourgeteau S, Cannavacciuolo M, Chauvin C, Flamin C, Haumont A, Ranjard L (2022). Impact of biogas digestates on soil microbiota in agriculture: a review. Environ Chem Letters, 1-24. https://doi.org/10.1007/s10311-022-01451-8
Li J, Wen Y, Li X, Li Y, Yang X, Lin Z, Zhao B (2018) Soil labile organic carbon fractions and soil organic carbon stocks as affected by long-term organic and mineral fertilization regimes in the North China Plain. Soil till Res 175:281–290. https://doi.org/10.1016/j.still.2017.08.008
Lou Y, Wang J, Liang W (2011) Impacts of 22-year organic and inorganic N managements on soil organic C fractions in a maize field, northeast China. CATENA 87:386–390. https://doi.org/10.1016/j.catena.2011.07.006
Lytras G, Lytras C, Mathioudakis D, Papadopoulou K, Lyberatos G (2021) Food waste valorization based on anaerobic digestion. Waste Biomass Valor 12:1677–1697. https://doi.org/10.1007/s12649-020-01108-z
Majaule U, Dikinya O, Glaser B (2022). Biochar and sewage sludge phosphorus fertilizer effects on phosphorus bioavailability and spinach (Spinacia oleracea L.) yields under no-till system in semi-arid soils. Int J Recycl Org Waste Agric. https://doi.org/10.30486/ijrowa.2022.1927076.1231
Mazzini S, Borgonovo G, Scaglioni L, Bedussi F, D’Imporzano G, Tambone F, Adani F (2020) Phosphorus speciation during anaerobic digestion and subsequent solid/liquid separation. Sci Tot Environ 734:139284. https://doi.org/10.1016/j.scitotenv.2020.139284
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Park Y, Solhtalab M, Thongsomboon W, Aristilde L (2022) Strategies of organic phosphorus recycling by soil bacteria: acquisition, metabolism, and regulation. Env Microbiol Rep 14:3–24. https://doi.org/10.1111/1758-2229.13040
Regelink IC, Egene CE, Tack FM, Meers E (2021) Speciation of P in solid organic fertilisers from digestate and biowaste. Agronomy 11:2233. https://doi.org/10.3390/agronomy11112233
Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019. Official Journal of the European Union, http://data.europa.eu/eli/reg/2019/1009/oj
Reuland G, Sigurnjak I, Dekker H, Sleutel S, Meers E (2022) Assessment of the carbon and nitrogen mineralisation of digestates elaborated from distinct feedstock profiles. Agronomy 12:456. https://doi.org/10.3390/agronomy12020456
Rowe H, Withers PJ, Baas P, Chan NI, Doody D, Holiman J, Weintraub MN (2016) Integrating legacy soil phosphorus into sustainable nutrient management strategies for future food, bioenergy and water security. Nutr Cycl Agroecosys 104:393–412. https://doi.org/10.1007/s10705-015-9726-1
Schroder JJ, Cordell D, Smit AL, Rosemarin A (2010). Sustainable use of phosphorus: EU tender ENV.B1/ETU/2009/0025. Report/Plant Research International; No. 357. https://edepot.wur.nl/163942
Sharpley AN (1996) Availability of residual phosphorus in manured soils. Soil Sci Soc Am J 60:1459–1466. https://doi.org/10.2136/sssaj1996.03615995006000050025x
Slepetiene A, Volungevicius J, Jurgutis L, Liaudanskiene I, Amaleviciute-Volunge K, Slepetys J, Ceseviciene J (2020) The potential of digestate as a biofertilizer in eroded soils of Lithuania. Waste Manage 102:441–451. https://doi.org/10.1016/j.wasman.2019.11.008
Slepetiene A, Kochiieru M, Jurgutis L, Mankeviciene A, Skersiene A, Belova O (2022) The effect of anaerobic digestate on the soil organic carbon and humified carbon fractions in different land-use systems in lithuania. Land 11:133. https://doi.org/10.3390/land11010133
Stamm C, Binder CR, Frossard E, Haygarth PM, Oberson A, Richardson AE, Schaum C, Schoumans O, Udert KM (2021) Towards circular phosphorus: the need of inter- and transdisciplinary research to close the broken cycle. Ambio. https://doi.org/10.1007/s13280-021-01562-6
Stavi I, Bel G, Zaady E (2016) Soil functions and ecosystem services in conventional, conservation, and integrated agricultural systems. A Review Agron Sustain Dev 36:1–12. https://doi.org/10.1007/s13593-016-0368-8
Stolte J, Tesfai M, Øygarden L, Kværnø S, Keizer J, Verheijen F, Panagos P, Ballabio C, Hessel R. Soil threats in Europe. EUR 27607. Luxembourg (Luxembourg): publications Office of the European Union; 2016. JRC98673. https://doi.org/10.2788/828742
Tirol-Padre A, Ladha JK (2004) Assessing the reliability of permanganate-oxidizable carbon as an index of soil labile carbon. Soil Sci Soc Am J 68:969–978. https://doi.org/10.2136/sssaj2004.9690
Turner BL, Leytem AB (2004) Phosphorus compounds in sequential extracts of animal manures: chemical speciation and a novel fractionation procedure. Environ Sci Tech 38:6101–6108. https://doi.org/10.1021/es0493042
Verdi L, Kuikman PJ, Orlandini S, Mancini M, Napoli M, Dalla Marta A (2019) Does the use of digestate to replace mineral fertilizers have less emissions of N2O and NH3? Agr Forest Meteorol 269:112–118. https://doi.org/10.1016/j.agrformet.2019.02.004
Verma BC, Datta SP, Rattan RK, Singh AK (2013). Labile and stabilised fractions of soil organic carbon in some intensively cultivated alluvial soils. J Environ Biol 34: 1069–1075. SSN: 0254- 8704
Wang S, Zeng Y (2018) Ammonia emission mitigation in food waste composting: a review. Bioresource Technol 248:13–19. https://doi.org/10.1016/j.biortech.2017.07.050
Weil RR, Islam KR, Stine MA, Gruver JB, Samson-Liebig SE (2003) Estimating active carbon for soil quality assessment: a simplified method for laboratory and field use. Am J Alternative Agr 18:3–17. https://doi.org/10.1079/AJAA200228
Yang X, Meng J, Lan Y, Chen W, Yang T, Yuan J, Han J (2017) Effects of maize stover and its biochar on soil CO2 emissions and labile organic carbon fractions in Northeast China. Agric Ecosys Environ 240:24–31. https://doi.org/10.1016/j.agee.2017.02.001
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Grigatti, M. Effect of Raw and Composted Anaerobic Digestates from Sewage Sludge and Biowaste on Ryegrass Phosphorous Availability and Soil Carbon Management. J Soil Sci Plant Nutr 23, 854–866 (2023). https://doi.org/10.1007/s42729-022-01088-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-022-01088-0