Abstract
Pinocembrin (PB; 5,7-dihydroxyflavanone) is found in propolis and exhibits antioxidant activity in several experimental models. The antioxidant capacity of PB is associated with the activation of the nuclear factor erythroid 2-related factor 2/antioxidant response element (Nrf2/ARE) signaling pathway. The Nrf2/ARE axis mediates the expression of antioxidant and detoxifying enzymes, such as glutathione peroxidase (GPx), glutathione reductase (GR), heme oxygenase-1 (HO-1), and the catalytic (GCLC) and regulatory (GCLM) subunits of the rate-limiting enzyme in the synthesis of glutathione (GSH), γ-glutamate-cysteine ligase (γ-GCL). Nonetheless, it is not clear how PB exerts mitochondrial protection in mammalian cells. Human neuroblastoma SH-SY5Y cells were pretreated (4 h) with PB (0–25 µM) and then exposed to methylglyoxal (MG; 500 µM) for further 24 h. Mitochondria were isolated by differential centrifugation. PB (25 µM) provided mitochondrial protection (decreased lipid peroxidation, protein carbonylation, and protein nitration in mitochondrial membranes; decreased mitochondrial free radical production; enhanced the content of GSH in mitochondria; rescued mitochondrial membrane potential—MMP) and blocked MG-triggered cell death by a mechanism dependent on the activation of the extracellular-related kinase (Erk1/2) and consequent upregulation of Nrf2. PB increased the levels of GPx, GR, HO-1, and mitochondrial GSH. The PB-induced effects were suppressed by silencing of Nrf2 with siRNA. Therefore, PB activated the Erk1/2–Nrf2 signaling pathway resulting in mitochondrial protection in SH-SY5Y cells exposed to MG. Our work shows that PB is a strong candidate to figure among mitochondria-focusing agents with pharmacological potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pinocembrin (PB; 5,7-dihydroxyflavanone) is major component of propolis and exhibits antioxidant, anti-inflammatory, and antimicrobial activities in several experimental models [1–4]. Furthermore, PB exerts neuroprotection in animal experimental models of cerebral ischemic injury [5–9] and in amyloid-β-induced neurodegeneration [10, 11]. PB also counteracted cell death triggered by 1-methyl-4-phenylpyridinium (MPP+) [12] and glutamate [13] in human neuroblastoma SH-SY5Y cells. Jin et al. [14] published that PB induced neuroprotection by the activation of the nuclear factor erythroid 2-related factor 2/antioxidant response element (Nrf2/ARE) axis in SH-SY5Y cells exposed to 6-hydroxydopamine (6-OHDA). Recently, Zhou et al. [3] also reported that PB abrogated lipopolysaccharide (LPS)-elicited inflammatory response in BV2 microglial cells by a mechanism involving the inhibition of the phosphoinositide 3-kinase/Akt/nuclear factor-κB (PI3K/Akt/NF-κB) signaling pathway.
Redox impairment takes a central role in several human pathologies, including neurodegeneration, cancer, and cardiovascular diseases [15–24], as well as in the aging process [25, 26]. Increased generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) may lead to cellular stress by widespread damage to biomolecules, such as proteins, lipids, DNA, and RNA [27]. ROS and RNS may also inactivate enzymes, causing inhibition of detoxification reactions and bioenergetics deficits in mammalian cells [28–30]. Moreover, intermediates of lipid peroxidation, for example, have been viewed as toxic agents involved in the amplification of the pro-oxidant insult, leading to a vicious cycle that culminates in cell death [31–33]. On the other hand, ROS and RNS exert physiological roles mediating cellular signaling and the defenses against microorganisms [26, 27]. The mitochondria are the major source of free radicals in mammalian cells due to the activity of the oxidative phosphorylation (OXPHOS) system [34–36]. In this context, disruption of the mitochondrial function favors increased generation of reactive species and the triggering of cell death due to the release of pro-apoptotic factors, such as cytochrome c and the apoptosis-inducing factor (AIF), to the cytosol and consequent activation of the apoptosome [37]. Actually, mitochondrial dysfunction has been associated with neurodegeneration in in vitro [38–40] and in in vivo [41–44] experimental models, as well as in patients suffering from Alzheimer’s disease (AD) [45, 46], Parkinson’s disease (PD) [47, 48], and Huntington’s disease (HD) [16, 49, 50], and diabetes mellitus (DM) [51–53]. Furthermore, exposure to chemical stressors impairs mitochondrial function and enhances production of ROS and RNS by the organelle [54–59]. A growing body of evidence points to mitochondrial protection by natural compounds as an interesting pharmacological strategy in order to alleviate neurodegeneration [60–66]. In spite of this, the complete mechanism underlying the mitochondrial benefits elicited by natural compounds remains to be fully understood.
Therefore, we aimed to investigate here whether and how PB would protect mitochondria of SH-SY5Y cells exposed to methylglyoxal (MG), which is derived from glycolysis and induces redox impairment and cell death in mammalian cells through autoxidation [67] and by causing mitochondrial dysfunction [68–70]. Additionally, advanced glycation end products (AGEs) derived from MG have been detected in samples obtained from the brain and cerebrospinal fluid of AD patients [71–73]. Hence, the experimental model using MG as a stressor is excellent in linking redox impairment, mitochondrial dysfunction, metabolic disturbances, and neurodegeneration.
Experimental Procedures
Materials
We obtained plastic materials used in cell culture from Corning, Inc (NY, USA) and Beckton Dickson (NJ, USA). Culture analytical grade reagents and MG were purchased from Sigma–Aldrich (MO, USA). Erk1/2 inhibitor (PD98059) was achieved from Santa Cruz (Dallas, TX, USA). All other chemicals and assay kits we utilized here have been acquired as described below in details.
Cell Culture and Treatment
The human neuroblastoma SH-SY5Y cells were acquired from the American Type Culture Collection (ATCC; Manassas, VA, USA) and further cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F-12 HAM nutrient medium (1:1 mixture) supplemented with 10% fetal bovine serum (FBS) and 2 mM l-glutamine in a 5% CO2 humidified incubator at 37 °C. The SH-SY5Y cell line was plated at an appropriate density according to the different experimental protocols utilized in the herein presented work. The results were achieved by performing three or five independent experiments each done in triplicate. In order to induce cellular impairment and mitochondrial dysfunction, we utilized methylglyoxal (MG) at 500 µM, i.e. a concentration that causes a 50%-decrease in cell viability, as previously reported by our research group [74] and others [75]. The cells were treated with PB (dissolved in DMSO) at varying concentrations (1–25 µM) for 4 h before administration of MG for further 24 h, in agreement with the protocol of each experiment. More detailed information, i.e. concentrations and incubation time of treatments, are described in the figure legends.
Analyses of Cellular Viability, Cytotoxicity, and Apoptosis-Related Parameters
We examined cell viability by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, as previously described [76]. Cytotoxicity was evaluated by utilizing the lactate dehydrogenase (LDH) leakage assay according to the manufacturer instructions (CytoTox 96-NonRadioactive Cytotoxicity Assay, Promega). Quantification of caspase-3 and caspase-9 enzyme activities were measured by utilizing commercial kits following the instructions of the manufacturer (Abcam, MA, USA; Ex/Em = 400/505 nm). DNA fragmentation was assessed by using a commercial ELISA kit in which 5′-bromo-2′-deoxy-uridine (BrdU) is used to label nuclear DNA (Roche, Germany). We measured the levels of cytoplasmic BrdU-labeled DNA fragments according to the instructions of the manufacturer, because it is a marker of apoptosis. After specific treatments, the samples were read (450 nm) in a plate reader (Molecular Devices, CA, USA). The immunocontents of Bcl-2, Bax, and cytochrome c (mitochondrial and cytosolic) were measured by using ELISA assay kits following the instructions of the manufacturer (Abcam, MA, USA).
Mitochondrial Isolation
In order to obtain viable mitochondria, the cells were washed and re-suspended, after each treatment, in a buffer with 250 mM sucrose, 20 mM HEPES - pH 7.4–10 mM KCl, 1 mM EGTA, 1 mM EDTA, 1 mM MgCl2, 1 mM dithiothreitol, 1 mM phenylmethylsulphonyl floride, 1 mM benzamidine, 1 mM pepstatin A, 10 mg/mL leupeptin, and 2 mg/mL aprotonin [77]. Then, the cells were homogenized and nuclei, unbroken cells, as well as cell debris were achieved after centrifugation at 1000 × g for 10 min at 4 °C. The supernatant obtained in the previous centrifugation was centrifuged once again (13,000 × g, 20 min, 4 °C) in order to isolate cytosolic fraction. The final supernatant was utilized as the cytosolic fraction and the final pellet contained the mitochondria.
Quantification of Reduced Glutathione (GSH)
The quantification of GSH in mitochondria was performed according to the protocol of a commercial kit using the Thiol Green Indicator (Abcam, MA, USA) in a fluorescence plate reader (Molecular Devices, USA; Ex/Em 490/520 nm). We utilized this same assay kit to quantify the protein thiol content in mitochondrial membranes obtained from SH-SY5Y cells after extraction of submitochondrial membranes, as described below.
Quantification of Mitochondrial Membrane Potential (MMP)
Mitochondrial membrane potential (MMP) was measured by using a commercial kit that utilizes tetraethylbenzimidazolylcarbocyanide iodine (JC-1), a lipophilic cationic dye that accumulates in functional mitochondria according to its membrane potential (Abcam, MA, USA). JC-1 is predominantly a monomer that yields green fluorescence at low ΔΨm (emission of 530 ± 15 nm). On the other hand, JC-1 aggregates at high ΔΨm rendering a red to orange fluorescence (emission of 590 ± 17.5 nm). The cells (1.5 × 104) were stained with 20 µM JC-1 in dilution buffer for 10 min at 37 °C. Then, SH-SY5Y cells were washed twice with dilution buffer. The treatments were applied and incubated for specific periods. The samples were read (excitation at 485 nm, emission at 40 and 590 nm, and cut-off at 530 nm) in a fluorescence plate reader (MolecularDevices, USA) [74, 78, 79]. FCCP was used as positive control in order to elicit loss of ΔΨm (data not shown).
Extraction of Submitochondrial Particles (SMP)
After cell culture reaching 80%-confluence, the medium was removed and the treatments were added. After each specific period of incubation, we homogenized the cells in specific buffer (230 mM mannitol, 70 mM sucrose, 10 mM Tris–HCl and 1 mM EDTA—pH 7.4) to isolate SMP. Mitochondria were freeze and thaw (three times) to generate superoxide dismutase-free SMP, that were washed (twice) with another buffer (140 mM KCl, 20 mM Tris–HCl—pH 7.4) resulting in Mn-SOD release from the organelles. This protocol was used to measure O2 −⋅ generation and to examine the consequences of the treatments on the contents of malondialdehyde (MDA), protein carbonylation, protein thiol content, and 3-nitrotyrosine in the membranes of the organelles.
Quantification of Superoxide Anion Radical (O2 −⋅) Production in SMP
Mitochondrial O2 −⋅ production was measured in SMP isolated from the SH-SY5Y cells in a reaction medium with 230 mM mannitol, 70 mM sucrose, 10 mM HEPES-KOH (pH 7.4), 4.2 mM succinate, 0.5 mM KH2PO4, 0.1 µM catalase, and 1 mM epinephrine, and the increase in the absorbance (that represents the autoxidation of adrenaline to adrenochrome) was read in a plate reader (Molecular Devices, CA, USA; 480 nm at 32 °C), as described [80–82].
Analyses of the Levels of ATP
In this work, we quantified the levels of ATP by using a commercial kit (Abcam, MA, USA). Briefly, SH-SY5Y cells (1 × 106 cells) were re-suspended in specific ATP assay buffer and homogenized. Then, the cells were centrifuged (13,000 × g for 2 min at 4 °C) and the supernatants were collected and transferred to another tube. These samples were deproteinizated, centrifuged again (13,000 × g for 2 min at 4 °C), and the supernatants utilized to measure ATP levels. After the reaction of the samples with ATP probe, the samples were read in a fluorescence plate reader (Molecular Devices, USA) as indicated: excitation at 535 nm and emission at 590 nm [78, 79, 83].
Measurement of Lipid Peroxidation and Protein Carbonylation in Mitochondrial Membranes
We quantified lipid peroxidation (using malodialdehyde—MDA—as an index of oxidative damage in lipids) and protein carbonylation in the samples by using commercial kits according to the instructions of the manufacturer (Abcam, MA, USA). After specific reactions, MDA and DNP hydrazones were read in a plate reader (Molecular Devices, CA, USA) at 532 and 375 nm, respectively.
Measurement of 3-Nitrotyrosine, GCLM (Modifier Subunit), GCLC (Catalytic Subunit), GPx, GR, and HO-1 by Enzyme-Linked Immunosorbent Assay (ELISA)
We utilized an indirect ELISA assay in order to quantify such parameters, as previously described [74, 78, 79, 83, 84]. Briefly, the levels of 3-nitrotyrosine in SH-SY5Y cells were examined by using a polyclonal antibody to 3-nitrotyrosine [diluted 1:2000 in phosphate-buffered saline (PBS) containing 5% albumin, pH 7.4] (Calbiochem, Germany). The polyclonal antibodies (Abcam, MA, USA) used to detect GCLM, GCLC, GPx, GR, and HO-1 were diluted 1:1000 in PBS with 5% albumin. Microtiter plates (96-well flat-bottom) were coated for 24 h with the samples (30 µg protein). Plates were then washed (four times) with wash buffer (PBS containing 0.05% Tween-20), and each specific antibody was added to the plates for 2 h (room temperature). After washing (four times), the samples were incubated with anti-rabbit antibody peroxidase conjugated (diluted 1:1000) during 1 h at room temperature. Then, after adding the substrates (hydrogen peroxide and 3, 3′, 5, 5′-tetramethylbenzidine 1:1 v:v), the samples were read at 450 nm in a plate reader (Molecular Devices, CA, USA).
Isolation of Cell Nucleus
Nuclei were isolated with a Nuclear Extraction Kit, which was acquired from Cayman Chemical (MI, USA). Briefly, 1 × 107 cells (after reaching 80–90% confluence) were suspended in phosphate buffered saline (ice-cold). After centrifuging the samples at 300 × g for 5 min at 4 °C, the cells were pelleted and then resuspended in ice-cold hypotonic buffer (responsible for inducing swelling of the cells). Then, 10% Nonidet P-40 was added in order to dissolve the cell membranes in order to access the cytoplasmic fraction while conserving nuclear membrane. Another centrifugation (13,000 × g for 30 s at 4 °C) was done, resulting in isolate nuclei. The pelleted nuclei were lysed in ice-cold extraction buffer. A final centrifugation (14,000 × g for 10 min at 4 °C) was performed generating nuclear extracts, which were utilized in the measurements of Nrf2 translocation to the cell nucleus.
Quantification of Nrf2 Immunocontent in Nuclear Samples
Quantification of the nuclear immunocontent of Nrf2 was performed after nuclear extraction by using an ELISA assay kit following the instructions of the manufacturer (Active Motif, CA, USA). The samples (30 µg protein) were added into the wells, which contain an Nrf2 specific-monoclonal capture antibody. Then, a detection antibody specific for Nrf2 was added to each well. A horseradish peroxidase labeled anti-rabbit IgG was pipetted into the wells after washing. Finally, a substrate solution (TMB) was added, leading to color generation, which was read in a microplate reader (Molecular Devices, CA, USA) at 450 nm.
Nrf2 Silencing by Transfection with siRNA
Silencing of Nrf2 was obtained by the utilization of siRNA targeting Nrf2, as previously described [74, 78, 79, 83].
Statistical Analyses
Statistical analyses were performed by using the GraphPad 5.0 software. Data are presented as the mean ± standard error of the mean (SEM) of three or five independent experiments each done in triplicate; p values were considered significant when p < 0.05. Differences in experimental groups were determined by one-way ANOVA followed by the post hoc Tukey’s test.
Results
PB Affords Cytoprotection in SH-SY5Y Cells Exposed to MG
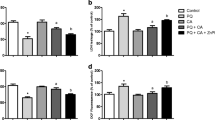
We have previously reported that 500 µM MG caused a 50% loss of cell viability and induced cytotoxicity in SH-SY5Y cells [74]. Therefore, we examined here the concentration of PB that efficiently counteracted the MG-induced cytotoxic and pro-apoptotic effects. A treatment with PB at 1–25 µM for 4 h prior exposure to MG for additional 24 h significantly prevented loss of cell viability (Fig. S1A) and cytotoxicity (Fig. S1B) in SH-SY5Y cells. We next tested whether PB would be able to suppress MG-triggered cell death regarding the mitochondria-related parameters in this experimental model. Pretreatment with PB at 25 µM abrogated the mitochondria-related pro-apoptotic effects triggered by MG (Fig. 1). MG decreased the levels of Bcl-2 protein (Fig. 1a) and increased the Bax protein contents (Fig. 1b), leading to an increase levels of cytochrome c in the cytosol (Fig. 1c) and a decrease in the mitochondrial content of this protein (Fig. 1d). Consequently, we observed that MG treatment enhanced the activities of caspase-9 (Fig. 1e) and caspase-3 (Fig. 1f). In this context, exposure to MG caused an increase in DNA fragmentation (Fig. 2), a hallmark of apoptosis [37]. A pretreatment with PB at 25 µM for 4 h abrogated these pro-apoptotic alterations associated with mitochondria (Fig. 1a–f), alleviating MG-induced DNA fragmentation (Fig. 2).
The effects of a treatment with pinocembrin (PB; 1–25 μM for 4 h) prior exposure to Methylglyoxal (MG; 500 µM for additional 24 h) on the contents of a Bcl-2, b Bax, c cytosolic cytochrome c (cyt c), d mitochondrial cyt c, and on the activities of e caspase-9 and f caspase-3 in human neuroblastoma SH-SY5Y cells. Data are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from control cells, #p < 0.05 different from MG-treated cells
The effects of a treatment with pinocembrin (PB; 1–25 μM for 4 h) prior exposure to methylglyoxal (MG; 500 µM for additional 24 h) on the levels of DNA fragmentation in human neuroblastoma SH-SY5Y cells. Data are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from control cells, #p < 0.05 different from MG-treated cells
PB Attenuated the MG-Induced Mitochondrial Dysfunction and Redox Impairment
We next investigated whether PB would prevent mitochondrial dysfunction elicited by MG in SH-SY5Y cells. Therefore, we quantified MMP in SH-SY5Y cells exposed or not to PB and/or MG. PB at 25 µM blocked the MG-dependent loss of MMP in this experimental model (Fig. 3a). Additionally, PB alleviated the effects of MG on O2 −⋅ production, as assessed in SMP obtained from SH-SY5Y cells (Fig. 3b). PB also significantly abrogated the decrease in ATP levels elicited by MG (Fig. 3c).
The effects of a treatment with pinocembrin (PB; 1–25 μM for 4 h) prior exposure to methylglyoxal (MG; 500 µM for additional 24 h) on a MMP, b superoxide anion radical (O2 −⋅) generation, and c ATP levels in human neuroblastoma SH-SY5Y cells. Data are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from control cells, #p < 0.05 different from MG-treated cells
PB pretreatment efficiently prevented lipid peroxidation (Fig. 4a), protein carbonylation (Fig. 4b), formation of 3-nitrotyrosine (Fig. 4c), and oxidation of protein thiol groups (Fig. 4e) in the membranes of mitochondria obtained from MG-treated SH-SY5Y cells. PB also enhanced the levels of GSH in the mitochondria and prevented loss of GSH induced by MG in this experimental model (Fig. 4e).
The effects of a treatment with pinocembrin (PB; 1–25 μM for 4 h) prior exposure to methylglyoxal (MG; 500 µM for additional 24 h) on the levels of a lipid peroxidation, b protein carbonylation, c protein nitration, d protein thiol groups, and e intramitochondrial GSH in human neuroblastoma SH-SY5Y cells. Data are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from control cells, #p < 0.05 different from MG-treated cells
PB Upregulated Antioxidant Enzymes
In order to verify whether the PB-induced increase in the mitochondrial levels of GSH were associated with upregulation of enzymes involved in GSG synthesis, we examined the changes in GCLM and GCLC subunits of the γ-GCL enzyme, the rate-limiting step in the synthesis of GSH [85]. We found that PB at 25 µM induced a time-dependent (0–24 h) increase in the contents of both GCLM (Fig. S3A) and GCLC (Fig. S3B). Furthermore, PB elicited a similar effect on the levels of GPx (Fig. S3C) and GR (Fig. S3D). Thus, it is very likely that the increase in the mitochondrial levels of GSH is related to a PB-induced upregulation in the levels of the subunits of γ-GCL enzyme, as well as in the contents of GR, which is responsible for regenerates GSH from GS-SG (oxidized glutathione) by consuming NADPH [86]. In addition, PB caused an increase in the levels of HO-1 enzyme (Fig. S4), which has been viewed as an important antioxidant and anti-inflammatory agent in several experimental models involving disturbances in the redox biology of mammalian cells [87].
PB Treatment Activates the Nrf2 Transcription Factor Through an Erk1/2-Dependent Mechanism
Nrf2 is the master regulator of the expression of antioxidant enzymes, such as GPx, GR, γ-GCL, and HO-1 [26, 27, 85]. Nrf2 activation involves the interaction of electrophiles agents with the complex KEAP1-Nrf2 in the cytosol and consequent release of Nrf2 to the cell nucleus [88]. Moreover, phosphorylation by several protein kinases leads to activation and translocation of the Nrf2 transcription factor to the cell nucleus [89]. As depicted in Fig. S5A, exposure to PB caused a dose-dependent increase in the nuclear contents of Nrf2. PB at 25 µM triggered a time-dependent activation of Nrf2, as assessed through the quantification of Nrf2 in the nucleus of SH-SY5Y cells (Fig. S5B). Thus, we investigated whether the activation of Nrf2 would be associated with protein kinases that are well-known regulators of this transcription factor [89]. SH-SY5Y cells were pretreated with protein kinase inhibitors for 1 h before exposure to PB at 25 µM for additional 12 h (in which we found a peak of Nrf2 in the cell nucleus). In this context, we found that inhibition of the Erk1/2, but not of other protein kinases, suppressed the translocation of Nrf2 to the cell nucleus (Fig. 5). Hence, it is very likely that Erk1/2 protein kinase mediated the Nrf2 activation and consequent translocation to the cell nucleus.
The effects of protein kinases inhibitors on the levels of the transcription factor Nrf2 in the nucleus of human neuroblastoma SH-SY5Y cells exposed to pinocembrin (PB) at 25 µM for 12 h. The cells were exposed to each protein kinase inhibitor for 1 h prior administration of PB. We used each inhibitor at the concentrations as follows: 20 µM PD98059 (Erk1/2 inhibitor), 10 µM SP600125 [c-jun N-terminal kinase (JNK) inhibitor], 10 µM SB203580 (p38 inhibitor), 10 µM LY294002 (PI3K/Akt inhibitor). Data are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, a p < 0.01 vs the control group, b p < 0.01 vs the cells that received only PB
PB Upregulates Antioxidant Enzymes Levels and Mitochondrial GSH Content by an Erk1/2-Dependent Mechanism
We then measured the levels of antioxidant enzymes in PB-treated SH-SY5Y cells exposed to protein kinases inhibitors (Fig. 6). We observed that only the inhibition of Erk1/2 protein kinase caused a blockade in the PB-dependent increase in the contents of GCLM (Fig. 6a), GCLC (Fig. 6b), GPx (Fig. 6c), and GR (Fig. 6d). Additionally, Erk1/2 protein kinase inhibition also blocked the PB-induced increase in HO-1 levels in SH-SY5Y cells (Fig. 7). Erk1/2 protein kinase activation also mediated the effects of PB on the mitochondrial content of GSH, as depicted in Fig. 8.
The effects of protein kinases inhibitors on the levels of a glutamate-cysteine ligase modifier subunit (GCLM), b glutamate-cysteine ligase catalytic subunit (GCLC), c glutathione peroxidase (GPx), and d glutathione reductase (GR) in human neuroblastoma SH-SY5Y cells exposed to pinocembrin (PB) at 25 µM for 24 h. The cells were exposed to each protein kinase inhibitor for 1 h prior administration of PB. We used each inhibitor at the concentrations as follows: 20 µM PD98059 (Erk1/2 inhibitor), 10 µM SP600125 [c-jun N-terminal kinase (JNK) inhibitor], 10 µM SB203580 (p38 inhibitor), 10 µM LY294002 (PI3K/Akt inhibitor). Data are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, a p < 0.01 vs the control group, b p < 0.01 vs the cells that received only PB
The effects of protein kinases inhibitors on the levels of heme oxygenase-1 (HO-1) in human neuroblastoma SH-SY5Y cells exposed to pinocembrin (PB) at 25 µM for 24 h. The cells were exposed to each protein kinase inhibitor for 1 h prior administration of PB. We used each inhibitor at the concentrations as follows: 20 µM PD98059 (Erk1/2 inhibitor), 10 µM SP600125 [c-jun N-terminal kinase (JNK) inhibitor], 10 µM SB203580 (p38 inhibitor), 10 µM LY294002 (PI3K/Akt inhibitor). Data are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, a p < 0.01 vs the control group, b p < 0.01 vs the cells that received only PB
The effects of protein kinases inhibitors on the levels of reduced glutathione (GSH) in human neuroblastoma SH-SY5Y cells exposed to pinocembrin (PB) at 25 µM for 24 h. The cells were exposed to each protein kinase inhibitor for 1 h prior administration of PB. We used each inhibitor at the concentrations as follows: 20 µM PD98059 (Erk1/2 inhibitor), 10 µM SP600125 [c-jun N-terminal kinase (JNK) inhibitor], 10 µM SB203580 (p38 inhibitor), 10 µM LY294002 (PI3K/Akt inhibitor). Data are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, a p < 0.01 vs the control group, b p < 0.01 vs the cells that received only PB
PB Exerted Antioxidant Effects on Mitochondria by an Erk1/2-Dependent Manner
Erk1/2 protein kinase inhibition suppressed the antioxidant effects elicited by PB, in mitochondria of cells exposed to MG, regarding lipid peroxidation (Fig. 9a), protein carbonylation (Fig. 9b) in mitochondrial membranes, and production of O2 −⋅ by the organelles (Fig. 9c).
The effect of Erk1/2 protein kinase inhibition on the levels of a lipid peroxidation and b protein carbonylation in mitochondrial membranes, and on c the production of O2 −⋅ by submitochondrial particles obtained from human neuroblastoma SH-SY5Y cells exposed to PB. The cells were pretreated with the Erk1/2 protein kinase inhibitor PD98059 at 20 µM during 1 h before exposure of the cells to 25 µM pinocembrin (PB) for additional 4 h. Methylglyoxal (MG) was administrated at 100 µM for additional 24 h. Data are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, a p < 0.05 vs the control group, b p < 0.05 vs the cells exposed to MG alone, c p < 0.05 vs the PB + MG-treated cells
PB Counteracted the Effects of MG on Mitochondrial Function and Cell Viability by an Erk1/2-Dependent Mechanism
We next examined whether Erk1/2 would participate in the preventive effects elicited by PB regarding mitochondrial function and cell viability (Fig. 10). We found that Erk1/2 protein kinase inhibition blocked the protective effects resulting from exposure to PB prior administration of MG in SH-SY5Y cells in relation to MMP (Fig. 10a) and cell viability (Fig. 10b).
The effect of Erk1/2 protein kinase inhibition on a mitochondrial membrane potential (MMP) and b cell viability of human neuroblastoma SH-SY5Y cells exposed to PB. The cells were pretreated with the Erk1/2 protein kinase inhibitor PD98059 at 20 µM during 1 h before exposure of the cells to 25 µM pinocembrin (PB) for additional 4 h. Methylglyoxal (MG) was administrated at 100 µM for additional 24 h. Data are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, a p < 0.05 vs the control group, b p < 0.05 vs the cells exposed to MG alone, c p < 0.05 vs the PB + MG-treated cells
PB Upregulated Antioxidant Enzymes Levels and Mitochondrial GSH Content by an Nrf2-Dependent Manner
In order to confirm the role of Nrf2 in mediating the upregulation of antioxidant enzymes in PB-treated cells, we utilized siRNA targeting Nrf2 in SH-SY5Y cells exposed to PB. Nrf2 knockdown abolished the increase in GCLM (Fig. 11a), GCLC (Fig. 11b), GPx (Fig. 11c), and GR (Fig. 11d) elicited by PB. Nrf2 silencing also abrogated the PB-induced effects on HO-1 enzyme, as demonstrated in Fig. 12. The levels of GSH in the mitochondria were increased by PB by an Nrf2-dependent fashion, since knocking down of this transcription factor reduced the levels of GSH in PB-treated cells (Fig. 13).
The effects of silencing Nrf2 with siRNA (for 48 h) on the levels of a glutamate-cysteine ligase modifier subunit (GCLM), b glutamate-cysteine ligase catalytic subunit (GCLC), c glutathione peroxidase (GPx), and d glutathione reductase (GR) in human neuroblastoma SH-SY5Y cells exposed to pinocembrin (PB) at 25 µM for 24 h. Data are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, a p < 0.05 vs the control group, b p < 0.05 vs PB-treated cells transfected with negative control (NC) siRNA
The effects of silencing Nrf2 with siRNA (for 48 h) on the levels of heme oxygenase-1 (HO-1) in human neuroblastoma SH-SY5Y cells exposed to pinocembrin (PB) at 25 µM for 24 h. Data are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, a p < 0.05 vs the control group, b p < 0.05 vs PB-treated cells transfected with negative control (NC) siRNA
The effects of silencing Nrf2 with siRNA (for 48 h) on the levels of reduced glutathione (GSH) in the mitochondria of human neuroblastoma SH-SY5Y cells exposed to pinocembrin (PB) at 25 µM for 24 h. Data are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, a p < 0.05 vs the control group, b p < 0.05 vs PB-treated cells transfected with negative control (NC) siRNA
PB Rescued Mitochondrial Function and Cell Viability by an Nrf-2-Dependent Mechanism
We tested the efficiency of siRNA against Nrf2 regarding the translocation of this transcription factor to the cell nucleus. Nrf2 silencing abrogated the PB-induced translocation of Nrf2 to the nucleus of SH-SY5Y cells, as demonstrated in Fig. S2. We next verified whether the PB-induced Nrf2 activation would really affect mitochondrial function in MG-treated SH-SY5Y cells. We found that Nrf2 silencing blocked the preventive effects elicited by PB regarding mitochondrial function (Fig. 14). Furthermore, PB exerted anti-apoptotic effects by activating Nrf2, since the knockdown of Nrf2 abolished the protective role of PB regarding DNA fragmentation, a hallmark of apoptosis, in SH-SY5Y cells exposed to MG (Fig. 15a). Consequently, silencing of Nrf2 also suppressed the cytoprotective effects elicited by PB in this experimental model (Fig. 15b).
The consequence of Nrf2 silencing with siRNA (for 48 h) on the mitochondrial membrane potential (MMP) of methylglyoxal (MG) and/or pinocembrin (PB)-treated human neuroblastoma SH-SY5Y cells. Data are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 vs the PB + MG-treated cells transfected with negative control (NC) siRNA
The consequence of Nrf2 silencing with siRNA (for 48 h) on a the levels of DNA fragmentation and b cell viability of human neuroblastoma SH-SY5Y cells exposed to methylglyoxal (MG) and/or pinocembrin (PB) for 24 h. Data are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 vs the PB + MG-treated cells transfected with negative control (NC) siRNA
Discussion
In the herein presented work, we found that PB, a major flavonoid found in propolis, activated the Erk1/2–Nrf2 signaling pathway causing mitochondrial and cellular protection in cells exposed to MG, a toxic derivative from the glycolysis [67–69]. Flavonoids exert antioxidant and cytoprotective effects by the activation of several signaling pathways [90]. Actually, Wang et al. [91] first demonstrated that PB activates Erk1/2 protein kinase in SH-SY5Y cells. Also, Jin et al. [14] reported that PB activated Nrf2 in the same cell line. However, it was not previously studied whether Erk1/2 activation would be involved in the upregulation of Nrf2 mediated by PB. Moreover, it was not examined whether the activation of the Erk1/2–Nrf2 axis by PB would induce mitochondrial and cellular protection against chemical stressors. Therefore, we found here a causative link between Erk1/2 activation and the posterior Nrf2 translocation to the cell nucleus, consequently triggering the expression of antioxidant enzymes responsible for the amelioration observed in redox parameters associated with mitochondria, including prevention of oxidative and nitrosative damage in mitochondrial membranes and upregulation of the GSH levels in the organelles.
MG is a derivative from the glycolysis and a potent inducer of protein carbonylation [67, 68]. This reactive aldehyde may play a crucial role in the pathogenesis of diabetes mellitus and AD [71–73]. Moreover, MG is a mitochondrial toxicant, since impairs mitochondrial function causing bioenergetics impairments in several mammalian cells [68–70]. In this regard, MG activates the intrinsic apoptotic pathway, which is closely related to mitochondrial function and dynamics, enhancing cell death and disrupting tissue function [72, 73]. Therefore, investigating strategies that would prevent the MG-elicited effects and the mechanisms underlying the cytoprotective action of natural compounds is interesting in order to deal with the consequences of exposure to MG.
The inhibition of the MG-induced lipid peroxidation by PB may mediate the anti-apoptotic effect exerted by this flavonoid in this experimental model, since oxidation of mitochondrial lipids favor the release of cytochrome c from the organelles [27, 37]. Moreover, downregulation of Bax by PB is very likely to decrease the formation of the mitochondrial permeability transition pore (MPTP), which participates in the release of cytochrome c to the cytosol [37]. In the other hand, the inhibitory action of PB on the oxidation of components of the mitochondrial membranes may be important to prevent loss of MMP, which has been used as an index of mitochondrial function (as a result of the formation and maintenance of an electrochemical gradient in the intermembrane space) and of apoptosis, depending on the extent of mitochondrial impairment [37]. In this context, mitochondrial protection as seen here takes a crucial role in both regulation of cell fate and maintenance of bioenergetics reactions in mammalian cells. Importantly, the PB-induced increment in the levels of GSH in the mitochondria is a strong candidate to be central in mitochondrial protection against MG, since GSH is the main non-enzymatic antioxidant agent in mammalian cells [27, 85]. At the best of our knowledge, this is the first work demonstrating the ability of PB in upregulating mitochondrial GSH and the mechanism underlying this effect. GSH is utilized by GPx in the conversion oh H2O2 to water in both cytosol and mitochondria [85]. The consumption of GSH in that reaction leads to the formation of GS-SG, which is recycled to GSH by GR in an NADPH-dependent manner [85, 86]. NADPH is obtained mainly from the pentose phosphate pathway, which consumes glucose-6-phosphate that is also used in the glycolysis [92]. Hence, the chemically-induced increase in the mitochondrial GSH is crucial in the detoxification of H2O2, which is diffusible and may induce widespread redox disturbances by easily crossing biological membranes [26, 27].
We also observed here that PB upregulated the antioxidant enzyme HO-1. This protein converts heme to biliverdin, iron, and carbon monoxide (CO) [93]. Biliverdin exhibits antioxidant capacity in several cell types [94]. CO is also considered an antioxidant agent in mammalian cells [95]. Interestingly, it was demonstrated that HO-1 may upregulate both SOD and CAT in an experimental model of diabetes [96]. HO-1 downregulates NADPH oxidase activity, causing a decrease in the generation of O2 −⋅ by the enzyme [97]. Additionally, HO-1 enhances the levels of GSH experimentally [98]. In this regard, HO-1 upregulation may play a role in the cytoprotective effects seen here. Nonetheless, it remains to be fully understood whether HO-1 would exert a role in the mitochondria-related antioxidant actions observed in the present work in PB-treated SH-SY5Y cells.
In conclusion, PB activated the Erk1/2–Nrf2–GSH axis alleviating MG-induced cytotoxicity and mitochondrial impairment in SH-SY5Y cells. Further research would be needed in order to investigate whether PB would afford mitochondrial protection in experimental models of diabetes mellitus and neurodegenerative disorders in which MG plays a role.
References
Rasul A, Millimouno FM, Ali Eltayb W, Ali M, Li J, Li X (2013) Pinocembrin: a novel natural compound with versatile pharmacological and biological activities. Biomed Res Int 2013:379850. doi:10.1155/2013/379850
Nina N, Quispe C, Jiménez-Aspee F, Theoduloz C, Feresín GE, Lima B, Leiva E, Schmeda-Hirschmann G (2015) Antibacterial activity, antioxidant effect and chemical composition of propolis from the Región del Maule, Central Chile. Molecules 20:18144–18167. doi:10.3390/molecules201018144
Zhou LT, Wang KJ, Li L, Li H, Geng M (2015) Pinocembrin inhibits lipopolysaccharide-induced inflammatory mediators production in BV2 microglial cells through suppression of PI3K/Akt/NF-κB pathway. Eur J Pharmacol 761:211–216. doi:10.1016/j.ejphar.2015.06.003
Promsan S, Jaikumkao K, Pongchaidecha A, Chattipakorn N, Chatsudthipong V, Arjinajarn P, Pompimon W, Lungkaphin A (2016) Pinocembrin attenuates gentamicin-induced nephrotoxicity in rats. Can J Physiol Pharmacol 94:808–818. doi:10.1139/cjpp-2015-0468
Liu R, Gao M, Yang ZH, Du GH (2008) Pinocembrin protects rat brain against oxidation and apoptosis induced by ischemia–reperfusion both in vivo and in vitro. Brain Res 1216:104–115. doi:10.1016/j.brainres.2008.03.049
Meng F, Liu R, Gao M, Wang Y, Yu X, Xuan Z, Sun J, Yang F, Wu C, Du G (2011) Pinocembrin attenuates blood–brain barrier injury induced by global cerebral ischemia–reperfusion in rats. Brain Res 1391:93–101. doi:10.1016/j.brainres.2011.03.010
Shi LL, Chen BN, Gao M, Zhang HA, Li YJ, Wang L, Du GH (2011) The characteristics of therapeutic effect of pinocembrin in transient global brain ischemia/reperfusion rats. Life Sci 88:521–528. doi:10.1016/j.lfs.2011.01.011
Wu CX, Liu R, Gao M, Zhao G, Wu S, Wu CF, Du GH (2013) Pinocembrin protects brain against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress induced apoptosis. Neurosci Lett 546:57–62. doi:10.1016/j.neulet.2013.04.060
Zhao G, Zhang W, Li L, Wu S, Du G (2014) Pinocembrin protects the brain against ischemia–reperfusion injury and reverses the autophagy dysfunction in the penumbra area. Molecules 19:15786–15798. doi:10.3390/molecules191015786
Liu R, Wu CX, Zhou D, Yang F, Tian S, Zhang L, Zhang TT, Du GH (2012) Pinocembrin protects against β-amyloid-induced toxicity in neurons through inhibiting receptor for advanced glycation end products (RAGE)-independent signaling pathways and regulating mitochondrion-mediated apoptosis. BMC Med 10:105. doi:10.1186/1741-7015-10-105
Liu R, Li JZ, Song JK, Sun JL, Li YJ, Zhou SB, Zhang TT, Du GH (2014) Pinocembrin protects human brain microvascular endothelial cells against fibrillar amyloid-β(1–40) injury by suppressing the MAPK/NF-κB inflammatory pathways. Biomed Res Int 2014:470393. doi:10.1155/2014/470393
Wang Y, Gao J, Miao Y, Cui Q, Zhao W, Zhang J, Wang H (2014) Pinocembrin protects SH-SY5Y cells against MPP+-induced neurotoxicity through the mitochondrial apoptotic pathway. J Mol Neurosci 53:537–545. doi:10.1007/s12031-013-0219-x
Gao M, Zhang WC, Liu QS, Hu JJ, Liu GT, Du GH (2008) Pinocembrin prevents glutamate-induced apoptosis in SH-SY5Y neuronal cells via decrease of bax/bcl-2 ratio. Eur J Pharmacol 591:73–79. doi:10.1016/j.ejphar.2008.06.071
Jin X, Liu Q, Jia L, Li M, Wang X (2015) Pinocembrin attenuates 6-OHDA-induced neuronal cell death through Nrf2/ARE pathway in SH-SY5Y cells. Cell Mol Neurobiol 35:323–333. doi:10.1007/s10571-014-0128-8
Juurlink BH (1999) Management of oxidative stress in the CNS: the many roles of glutathione. Neurotox Res 1:119–140
Sorolla MA, Rodríguez-Colman MJ, Vall-llaura N, Tamarit J, Ros J, Cabiscol E (2012) Protein oxidation in Huntington disease. Biofactors 38:173–185
Sharma S, Moon CS, Khogali A, Haidous A, Chabenne A, Ojo C, Jelebinkov M, Kurdi Y, Ebadi M (2013) Biomarkers in Parkinson’s disease (recent update). Neurochem Int 63:201–229. doi:10.1016/j.neuint.2013.06.005
Taylor JM, Main BS, Crack PJ (2013) Neuroinflammation and oxidative stress: co-conspirators in the pathology of Parkinson’s disease. Neurochem Int 62:803–819. doi:10.1016/j.neuint.2012.12.016
Okon IS, Zou MH (2015) Mitochondrial ROS and cancer drug resistance: Implications for therapy. Pharmacol Res 100:170–174. doi:10.1016/j.phrs.2015.06.013
Ali Sheikh MS, Salma U, Zhang B, Chen J, Zhuang J, Ping Z (2016) Diagnostic, prognostic, and therapeutic value of circulating miRNAs in heart failure patients associated with oxidative stress. Oxid Med Cell Longev 2016:5893064. doi:10.1155/2016/5893064
Bu J, Dou Y, Tian X, Wang Z, Chen G (2016) The role of omega-3 polyunsaturated fatty acids in stroke. Oxid Med Cell Longev 2016:6906712. doi:10.1155/2016/6906712
Kurian GA, Rajagopal R, Vedantham S, Rajesh M (2016) The Role of oxidative stress in myocardial ischemia and reperfusion injury and remodeling: revisited. Oxid Med Cell Longev 2016:1656450. doi:10.1155/2016/1656450
Lipchick BC, Fink EE, Nikiforov MA (2016) Oxidative stress and proteasome inhibitors in multiple myeloma. Pharmacol Res 105:210–215. doi:10.1016/j.phrs.2016.01.029
Vyas S, Zaganjor E, Haigis MC (2016) Mitochondria and cancer. Cell 166:555–566. doi:10.1016/j.cell.2016.07.002
Urano S, Sato Y, Otonari T, Makabe S, Suzuki S, Ogata M, Endo T (1998) Aging and oxidative stress in neurodegeneration. Biofactors 7:103–112
Forman HJ (2016) Redox signaling: an evolution from free radicals to aging. Free Radic Biol Med 97:398–407. doi:10.1016/j.freeradbiomed.2016.07.003
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658
Lynch RE, Fridovich I (1979) Autoinactivation of xanthine oxidase: the role of superoxide radical and hydrogen peroxide. Biochim Biophys Acta 571:195–200
Kono Y, Fridovich I (1982) Superoxide radical inhibits catalase. J Biol Chem 257:5751–5754
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552:335–344
Grimsrud PA, Xie H, Griffin TJ, Bernlohr DA (2008) Oxidative stress and covalent modification of protein with bioactive aldehydes. J Biol Chem 283:21837–21841. doi:10.1074/jbc.R700019200
Fritz KS, Petersen DR (2011) Exploring the biology of lipid peroxidation-derived protein carbonylation. Chem Res Toxicol 24:1411–1419. doi:10.1021/tx200169n
Guo J, Prokai-Tatrai K, Nguyen V, Rauniyar N, Ughy B, Prokai L (2011) Protein targets for carbonylation by 4-hydroxy-2-nonenal in rat liver mitochondria. J Proteom 74:2370–2379. doi:10.1016/j.jprot.2011.07.009
Petrosillo G, Matera M, Casanova G, Ruggiero FM, Paradies G (2008) Mitochondrial dysfunction in rat brain with aging Involvement of complex I, reactive oxygen species and cardiolipin. Neurochem Int 53:126–131. doi:10.1016/j.neuint.2008.07.001
Abeti R, Abramov AY (2015) Mitochondrial Ca(2+) in neurodegenerative disorders. Pharmacol Res 99:377–381. doi:10.1016/j.phrs.2015.05.007
Angelova PR, Abramov AY (2016) Functional role of mitochondrial reactive oxygen species in physiology. Free Radic Biol Med. doi:10.1016/j.freeradbiomed.2016.06.005. (in press).
Green DR, Galluzzi L, Kroemer G (2014) Metabolic control of cell death. Science 345:1250256. doi:10.1126/science.1250256
Liu Y, Song XD, Liu W, Zhang TY, Zuo J (2003) Glucose deprivation induces mitochondrial dysfunction and oxidative stress in PC12 cell line. J Cell Mol Med 7:49–56
Yi F, He X, Wang D (2013) Lycopene protects against MPP(+)-induced cytotoxicity by maintaining mitochondrial function in SH-SY5Y cells. Neurochem Res 38:1747–1757. doi:10.1007/s11064-013-1079-z
Ye X, Han Y, Zhang L, Liu W, Zuo J (2015) MTERF4 regulates the mitochondrial dysfunction induced by MPP(+) in SH-SY5Y cells. Biochem Biophys Res Commun 464:214–220. doi:10.1016/j.bbrc.2015.06.119
Avetisyan AV, Samokhin AN, Alexandrova IY, Zinovkin RA, Simonyan RA, Bobkova NV (2016) Mitochondrial dysfunction in neocortex and hippocampus of olfactory bulbectomized mice, a model of Alzheimer’s disease. BioChemistry 81:615–623. doi:10.1134/S0006297916060080
Demarest TG, Schuh RA, Waddell J, McKenna MC, Fiskum G (2016) Sex-dependent mitochondrial respiratory impairment and oxidative stress in a rat model of neonatal hypoxic-ischemic encephalopathy. J Neurochem 137:714–729. doi:10.1111/jnc.13590
Santa-Cruz LD, Guerrero-Castillo S, Uribe-Carvajal S, Tapia R (2016) Mitochondrial dysfunction during the early stages of excitotoxic spinal motor neuron degeneration in vivo. ACS Chem Neurosci 7:886–896. doi:10.1021/acschemneuro.6b00032
Tatarkova Z, Kovalska M, Timkova V, Racay P, Lehotsky J, Kaplan P (2016) The effect of aging on mitochondrial complex I and the extent of oxidative stress in the rat brain cortex. Neurochem Res 41:2160–2172. doi:10.1007/s11064-016-1931-z
Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS et al (2001) Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci 21:3017–3023
Sun AY, Draczynska-Lusiak B, Sun GY (2001) Oxidized lipoproteins, beta amyloid peptides and Alzheimer’s disease. Neurotox Res 3: 167–178
Calì T, Ottolini D, Brini M (2011) Mitochondria, calcium, and endoplasmic reticulum stress in Parkinson’s disease. Biofactors 37:228–240
Keane PC, Kurzawa M, Blain PG, Morris CM (2011) Mitochondrial dysfunction in Parkinson’s disease. Parkinsons Dis 2011: 716871. doi:10.4061/2011/716871
Guedes-Dias P, Pinho BR, Soares TR, de Proença J, Duchen MR, Oliveira JM (2016) Mitochondrial dynamics and quality control in Huntington’s disease. Neurobiol Dis 90:51–57. doi:10.1016/j.nbd.2015.09.008
Mejia EM, Chau S, Sparagna GC, Sipione S, Hatch GM (2016) Reduced mitochondrial function in human Huntington disease lymphoblasts is not due to alterations in cardiolipin metabolism or mitochondrial supercomplex assembly. Lipids 51:561–569. doi:10.1007/s11745-015-4110-0
Rochette L, Zeller M, Cottin Y, Vergely C (2014) Diabetes, oxidative stress and therapeutic strategies. Biochim Biophys Acta 1840:2709–2729. doi:10.1016/j.bbagen.2014.05.017
Antoun G, McMurray F, Thrush AB, Patten DA, Peixoto AC, Slack RS, McPherson R, Dent R, Harper ME (2015) Impaired mitochondrial oxidative phosphorylation and supercomplex assembly in rectus abdominis muscle of diabetic obese individuals. Diabetologia 58:2861–2866. doi:10.1007/s00125-015-3772-8
Wu F, Liu Y, Luo L, Lu Y, Yew DT, Xu J, Guo K (2015) Platelet mitochondrial dysfunction of DM rats and DM patients. Int J Clin Exp Med 8:6937–6946
Finsterer J, Ohnsorge P (2013) Influence of mitochondrion-toxic agents on the cardiovascular system. Regul Toxicol Pharmacol 67:434–445. doi:10.1016/j.yrtph.2013.09.002
Jia G, Aroor AR, Martinez-Lemus LA, Sowers JR (2015) Mitochondrial functional impairment in response to environmental toxins in the cardiorenal metabolic syndrome. Arch Toxicol 89:147–153. doi:10.1007/s00204-014-1431-3
de Oliveira MR (2015) Vitamin A and retinoids as mitochondrial toxicants. Oxid Med Cell Longev 2015:140267. doi:10.1155/2015/140267
Oliveira MR (2015) The neurotoxic effects of vitamin A and retinoids. Acad Bras Cienc 87:1361–1373. doi:10.1590/0001-3765201520140677
de Oliveira MR (2016) Fluoxetine and the mitochondria: a review of the toxicological aspects. Toxicol Lett 258:185–191. doi:10.1016/j.toxlet.2016.07.001
de Oliveira MR, Jardim FR (2016) Cocaine and mitochondria-related signaling in the brain: a mechanistic view and future directions. Neurochem Int 92:58–66. doi:10.1016/j.neuint.2015.12.006
Gruber J, Fong S, Chen CB, Yoong S, Pastorin G, Schaffer S, Cheah I, Halliwell B (2013) Mitochondria-targeted antioxidants and metabolic modulators as pharmacological interventions to slow ageing. Biotechnol Adv 31:563–592. doi:10.1016/j.biotechadv.2012.09.005
Gibellini L, Bianchini E, De Biasi S, Nasi M, Cossarizza A, Pinti M (2015) Natural compounds modulating mitochondrial functions. Evid Based Complement Altern Med 2015:527209. doi:10.1155/2015/527209
de Oliveira MR (2016) Evidence for genistein as a mitochondriotropic molecule. Mitochondrion 29:35–44. doi:10.1016/j.mito.2016.05.005
de Oliveira MR, Nabavi SF, Manayi A, Daglia M, Hajheydari Z, Nabavi SM (2016) Resveratrol and the mitochondria: from triggering the intrinsic apoptotic pathway to inducing mitochondrial biogenesis, a mechanistic view. Biochim Biophys Acta 1860:727–745. doi:10.1016/j.bbagen.2016.01.017
de Oliveira MR, Nabavi SM, Braidy N, Setzer WN, Ahmed T, Nabavi SF (2016) Quercetin and the mitochondria: a mechanistic view. Biotechnol Adv 34:532–549. doi:10.1016/j.biotechadv.2015.12.014
de Oliveira MR, Jardim FR, Setzer WN, Nabavi SM, Nabavi SF (2016) Curcumin, mitochondrial biogenesis, and mitophagy: exploring recent data and indicating future needs. Biotechnol Adv 34:813–826. doi:10.1016/j.biotechadv.2016.04.004
Oliveira MR, Nabavi SF, Daglia M, Rastrelli L, Nabavi SM (2016) Epigallocatechin gallate and mitochondria—a story of life and death. Pharmacol Res 104:70–85. doi:10.1016/j.phrs.2015.12.027
Thornalley PJ, Wolff SP, Crabbe MJ, Stern A (1984) The oxidation of oxyhaemoglobin by glyceraldehyde and other simple monosaccharides. Biochem J 217:615–622
Roy SS, Biswas S, Ray M, Ray S (2003) Protective effect of creatine against inhibition by methylglyoxal of mitochondrial respiration of cardiac cells. Biochem J 372:661–669
Cardoso S, Carvalho C, Marinho R, Simões A, Sena CM, Matafome P, Santos MS, Seiça RM, Moreira PI (2014) Effects of methylglyoxal and pyridoxamine in rat brain mitochondria bioenergetics and oxidative status. J Bioenerg Biomembr 46:347–355. doi:10.1007/s10863-014-9551-2
Seo K, Seo S, Han JY, Ki SH, Shin SM (2014) Resveratrol attenuates methylglyoxal-induced mitochondrial dysfunction and apoptosis by Sestrin2 induction. Toxicol Appl Pharmacol 280:314–322. doi:10.1016/j.taap.2014.08.011
Smith MA, Taneda S, Richey PL, Miyata S, Yan SD, Stern D, Sayre LM, Monnier VM, Perry G (1994) Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc Natl Acad Sci USA 91:5710–5714
Harrington CR, Colaco CA (1994) Alzheimer’s disease. A glycation connection. Nature 370:247–248
Reddy VP, Obrenovich ME, Atwood CS, Perry G, Smith MA (2002) Involvement of Maillard reactions in Alzheimer disease. Neurotox Res. 4:191–209
de Oliveira MR, Ferreira GC, Schuck PF, Dal Bosco SM (2015) Role for the PI3K/Akt/Nrf2 signaling pathway in the protective effects of carnosic acid against methylglyoxal-induced neurotoxicity in SH-SY5Y neuroblastoma cells. Chem Biol Interact 242:396–406. doi:10.1016/j.cbi.2015.11.003
Angeloni C, Malaguti M, Rizzo B, Barbalace MC, Fabbri D, Hrelia S (2015) Neuroprotective effect of sulforaphane against methylglyoxal cytotoxicity. Chem Res Toxicol 28:1234–1245. doi:10.1021/acs.chemrestox.5b00067
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Wang K, Zhu L, Zhu X, Zhang K, Huang B, Zhang J, Zhang Y, Zhu L, Zhou B, Zhou F (2014) Protective effect of paeoniflorin on Aβ25-35-induced SH-SY5Y cell injury by preventing mitochondrial dysfunction. Cell Mol Neurobiol 34:227–234. doi:10.1007/s10571-013-0006-9
de Oliveira MR, Ferreira GC, Schuck PF (2016) Protective effect of carnosic acid against paraquat-induced redox impairment and mitochondrial dysfunction in SH-SY5Y cells: Role for PI3K/Akt/Nrf2 pathway. Toxicol In Vitro 32:41–54. doi:10.1016/j.tiv.2015.12.005
de Oliveira MR, Schuck PF, Bosco SM (2016) Tanshinone I induces mitochondrial protection through an Nrf2-dependent mechanism in paraquat-treated human neuroblastoma SH-SY5Y cells. Mol Neurobiol. doi:10.1007/s12035-016-0009-x (in press)
Poderoso JJ, Carreras MC, Lisdero C, Riobó N, Schöpfer F, Boveris A (1996) Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch Biochem Biophys 328:85–92
de Oliveira MR, Moreira JC (2007) Acute and chronic vitamin A supplementation at therapeutic doses induces oxidative stress in submitochondrial particles isolated from cerebral cortex and cerebellum of adult rats. Toxicol Lett 173:145–150
de Oliveira MR, Oliveira MW, Lorenzi R, Fagundes da Rocha R, Fonseca Moreira JC (2009) Short-term vitamin A supplementation at therapeutic doses induces a pro-oxidative state in the hepatic environment and facilitates calcium-ion-induced oxidative stress in rat liver mitochondria independently from permeability transition pore formation : detrimental effects of vitamin A supplementation on rat liver redox and bioenergetic states homeostasis. Cell Biol Toxicol 25:545–560. doi:10.1007/s10565-008-9111-9
de Oliveira MR, Peres A, Ferreira GC, Schuck PF, Bosco SM (2016) Carnosic acid affords mitochondrial protection in chlorpyrifos-treated Sh-Sy5y Cells. Neurotox Res. doi:10.1007/s12640-016-9620-x (in press)
de Oliveira MR, Lorenzi R, Schnorr CE, Morrone M, Moreira JC (2011) Increased 3-nitrotyrosine levels in mitochondrial membranes and impaired respiratory chain activity in brain regions of adult female rats submitted to daily vitamin A supplementation for 2 months. Brain Res Bull 86:246–253. doi:10.1016/j.brainresbull.2011.08.006
Lu SC (2013) Glutathione synthesis. Biochim Biophys Acta 1830:3143–3153. doi:10.1016/j.bbagen.2012.09.008
Couto N, Wood J, Barber J (2016) The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic Biol Med 95:27–42. doi:10.1016/j.freeradbiomed.2016.02.028
Otterbein LE, Foresti R, Motterlini R (2016) Heme oxygenase-1 and carbon monoxide in the heart: the balancing act between danger signaling and pro-survival. Circ Res 118:1940–1959. doi:10.1161/CIRCRESAHA.116.306588
Satoh T, McKercher SR, Lipton SA (2013) Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radic Biol Med 65:645–657. doi:10.1016/j.freeradbiomed.2013.07.022
Sandberg M, Patil J, D’Angelo B, Weber SG, Mallard C (2014) NRF2-regulation in brain health and disease: implication of cerebral inflammation. Neuropharmacology 79:298–306. doi:10.1016/j.neuropharm.2013.11.004
Matias I, Buosi AS, Gomes FC (2016) Functions of flavonoids in the central nervous system: astrocytes as targets for natural compounds. Neurochem Int 95:85–91. doi:10.1016/j.neuint.2016.01.009
Wang H, Wang Y, Zhao L, Cui Q, Wang Y, Du G (2016) Pinocembrin attenuates MPP(+)-induced neurotoxicity by the induction of heme oxygenase-1 through ERK1/2 pathway. Neurosci Lett 612:104–109. doi:10.1016/j.neulet.2015.11.048
Brekke E, Morken TS, Sonnewald U (2015) Glucose metabolism and astrocyte-neuron interactions in the neonatal brain. Neurochem Int 82:33–41. doi:10.1016/j.neuint.2015.02.002
Foresti R, Bains SK, Pitchumony TS, de Castro Brás LE, Drago F, Dubois-Randé JL, Bucolo C, Motterlini R (2013) Small molecule activators of the Nrf2-HO-1 antioxidant axis modulate heme metabolism and inflammation in BV2 microglia cells. Pharmacol Res 76:132–148. doi:10.1016/j.phrs.2013.07.010
Jansen T, Daiber A (2012) Direct antioxidant properties of bilirubin and biliverdin. Is there a role for biliverdin reductase? Front Pharmacol 3:30. doi:10.3389/fphar.2012.00030
Parfenova H, Leffler CW, Basuroy S, Liu J, Fedinec AL (2012) Antioxidant roles of heme oxygenase, carbon monoxide, and bilirubin in cerebral circulation during seizures. J Cereb Blood Flow Metab 32:1024–1034. doi:10.1038/jcbfm.2012.13
Turkseven S, Kruger A, Mingone CJ, Kaminski P, Inaba M, Rodella LF, Ikehara S, Wolin MS, Abraham NG (2005) Antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes. Am J Physiol Heart Circ Physiol 289:H701–H707
Taillé C, El-Benna J, Lanone S, Dang MC, Ogier-Denis E, Aubier M, Boczkowski J (2004) Induction of heme oxygenase-1 inhibits NAD(P)H oxidase activity by down-regulating cytochrome b558 expression via the reduction of heme availability. J Biol Chem 279:28681–28688
Stocker R, McDonagh AF, Glazer AN, Ames BN (1990) Antioxidant activities of bile pigments: biliverdin and bilirubin. Methods Enzymol 186:301–309
Acknowledgements
This work was supported by the Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq). GCF receives a “Bolsa Produtividade em Pesquisa”. GCF is supported by Edital APQ1/FAPERJ.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11064_2016_2140_MOESM1_ESM.pdf
Supplementary material 1 Figure S1. A pretreatment with pinocembrin (PB) at 1–25 μM for 4 h ameliorates (a) cell viability and suppressed (b) cytotoxicity in human neuroblastoma SH-SY5Y cells exposed to methylglyoxal (MG) for additional 24 h. The results are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, #p < 0.05 vs the control group, *p < 0.05 different from MG-treated cells, **p < 0.01 different from MG-treated cells (PDF 92 KB)
11064_2016_2140_MOESM2_ESM.pdf
Supplementary material 2 Figure S2. Silencing of Nrf2 by using siRNA targeting Nrf2 in human neuroblastoma SH-SY5Y cells. Cells were further exposed to pinocembrin (PB) at 25 µM for 12 h. Data are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, a p < 0.05 vs the control group, b p < 0.05 vs PB-treated cells transfected with negative control (NC) siRNA (PDF 102 KB)
11064_2016_2140_MOESM3_ESM.pdf
Supplementary material 3 Figure S3. The effects of a treatment with pinocembrin (25 µM) for different periods (0–24 h) on the cellular contents of a glutamate-cysteine ligase modifier subunit (GCLM), b glutamate-cysteine ligase catalytic subunit (GCLC), c glutathione peroxidase (GPx), and d glutathione reductase (GR) in human neuroblastoma SH-SY5Y cells. Data are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 vs the control group, ** p < 0.01 vs the control group (PDF 85 KB)
11064_2016_2140_MOESM4_ESM.pdf
Supplementary material 4 Figure S4. The effects of a treatment with pinocembrin (25 µM) for different periods (0–24 h) on the cellular contents of heme oxygenase-1 (HO-1) in human neuroblastoma SH-SY5Y cells. Data are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 vs the control group, **p < 0.01 vs the control group (PDF 99 KB)
11064_2016_2140_MOESM5_ESM.pdf
Supplementary material 5 Figure S5. The effects of a treatment with pinocembrin (PB) at varying concentrations (0–25 µM) for 12 h on the nuclear Nrf2 levels in human neuroblastoma SH-SY5Y cells (a). The time-dependent (0–12 h) effects of pinocembrin at 25 µM on the nuclear Nrf2 content (b). Data are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 vs the control group, **p < 0.01 vs the control group (PDF 103 KB)
Rights and permissions
About this article
Cite this article
de Oliveira, M.R., Peres, A. & Ferreira, G.C. Pinocembrin Attenuates Mitochondrial Dysfunction in Human Neuroblastoma SH-SY5Y Cells Exposed to Methylglyoxal: Role for the Erk1/2–Nrf2 Signaling Pathway. Neurochem Res 42, 1057–1072 (2017). https://doi.org/10.1007/s11064-016-2140-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-2140-5