Abstract
Isoflurane, a commonly used inhalation anesthetic, may induce neurocognitive deficits, especially in elderly patients after surgery. Recent study demonstrated that isoflurane caused endoplasmic reticulum (ER) stress and subsequent neuronal apoptosis in the brain, contributing to cognitive deficits. Taurine, a major intracellular free amino acid, has been shown to inhibit ER stress and neuronal apoptosis in several neurological disorders. Here, we examined whether taurine can prevent isoflurane-induced ER stress and cognitive impairment in aged rats. Thirty minutes prior to a 4-h 1.3 % isoflurane exposure, aged rats were treated with vehicle or taurine at low, middle and high doses. Aged rats without any treatment served as control. The brains were harvested 6 h after isoflurane exposure for molecular measurements, and behavioral study was performed 2 weeks later. Compared with control, isoflurane increased expression of hippocampal ER stress biomarkers including glucose-regulated protein 78, phosphorylated (P-) inositol-requiring enzyme 1, P-eukaryotic initiation factor 2-α (EIF2α), activating transcription factor 4 (ATF-4), cleaved ATF-6 and C/EBP homologous protein, along with activation of apoptosis pathways as indicated by decreased B cell lymphoma 2 (BCL-2)/BCL2-associated X protein, increased expressions of cytochrome-c and cleaved caspase-3. Taurine pretreatment dose-dependently inhibited isoflurane-induced increase in expression of ER stress biomarkers except for P-EIF2α and ATF-4, and reversed isoflurane-induced changes in apoptosis-related proteins. Moreover, isoflurane caused spatial working memory deficits in aged rats, which were prevented by taurine pretreatment. The results indicate that taurine pretreatment prevents anesthetic isoflurane-induced cognitive impairment by inhibiting ER stress-mediated activation of apoptosis pathways in the hippocampus in aged rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postoperative cognitive dysfunction (POCD) is generally described as a decline in cognitive function that occurs in patients after surgery and has been recognized as an important clinical complication of major surgery [1, 2]. POCD is a transient disturbance that can affect patients of any age, but elderly patients are particularly vulnerable to memory disturbances and other types of cognitive impairment after surgical operations due to physiologic, pharmacokinetic, and pharmacodynamic changes that are associated with aging. These changes may cause increased sensitivity and susceptibility to the insult from the surgical experience and anesthetic agents that are known to have negative cognitive outcomes [1, 3–5]. A recent study has showed that approximately 12 % of patients over age 60 have POCD 3 months after surgery [2]. This has become a critical issue in perioperative care as the incidence of surgery is high in elderly patients.
Isoflurane is a commonly used inhalation anesthetic in current clinical practice. Accumulating evidence from experimental and clinical studies has shown that isoflurane can induce neuroapoptosis and cognitive impairment, especially in elderly animals and humans [6–10]. Thus, it is important to understand the mechanisms underlying isoflurane-induced neurotoxicity and develop strategies to avoid or ameliorate potential brain injury in the elderly population.
Endoplasmic reticulum (ER) stress has been considered as an initial or early response of cells under stress and is associated with neuronal death in various neurodegenerative diseases [7, 11]. Recent studies have demonstrated that isoflurane induces ER stress and subsequent caspase-3 activation in the brain, contributing to neuroapoptosis and cognitive impairment in aged rats [7, 12].
Taurine, one of the most abundant amino acids in the brain, has been proposed as a neurotransmitter, neuromodulator and membrane stabilizer [13]. Taurine serves a wide variety of functions in the brain. It has been reported that taurine treatment protects against neuronal damage and epilepsy in several experimental studies [13–15]. In addition, taurine inhibited ER stress and caspase-3 activation, and thereby prevented P2X7 receptor-mediated apoptosis in neuronal cells [16] and reduced neurological deficits in animal stroke models of cerebral artery occlusion [17, 18]. However, no study to date has examined the effects of taurine on isoflurane-induced cognitive impairment. In the present study, we tested the hypothesis that taurine pretreatment would suppress isoflurane-induced ER stress and inhibit activation of apoptosis pathways in the hippocampus, and prevent cognitive impairment in aged rats.
Methods
Animals
All animal experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) at Shandong University. The research protocols and procedures were approved by IACUC of Shandong University. All efforts were made to minimize animal suffering and the number of animals used. Twenty-month old male Sprague-Dawley rats weighing 350 ± 400 g were purchased from Beijing Laboratory Animal Research Center (Beijing, China) and were housed in a temperature-controlled (23–25 °C) room under a 12-h light/dark cycle; standard rat chow and water were provided ad libitum.
Isoflurane Exposure and Taurine Treatment
The rats were randomly divided into five groups (n = 12 for each group): control (CON); isoflurane (Baxter, Deerfield, IL, USA) plus vehicle (saline, ISO+VEH); isoflurane plus low-dose (0.16 mmol/kg) taurine (ISO+T-L); isoflurane plus middle-dose (0.32 mmol/kg) taurine (ISO+T-M); and isoflurane plus high-dose (0.48 mmol/kg) taurine (ISO+T-H). Taurine (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 1 ml 0.09 % saline and intraperitoneally injected 30 min prior to isoflurane exposure. Isoflurane exposure was performed as previously described [7, 8]. Briefly, rats were placed in a temperature controlled chamber with two interfaces that were connected to an anesthesia machine and a multi-gas monitor, respectively. For rats assigned to isoflurane exposure groups, 1.3 % isoflurane was provided by a humidified 30 % O2 carrier gas from a calibrated vaporizer for 4 h. Rats assigned to control groups were also placed in the same chamber except no isoflurane was provided.
The concentrations of isoflurane, O2 and CO2 in the chamber were continuously monitored with a gas analyzer (Datex Ohmeda, Mississauga, ON, Canada). The rectal temperature was maintained at 37 °C during the experiment. Blood pressure (BP) and heart rate were measured hourly using a CODA Monitor (Kent Scientific Corp., Torrington, CT, USA). Arterial blood gases and blood glucose were measured in some rats (n = 6 for each group) immediately after isoflurane exposure. Six hours after isoflurane exposure, these rats were sacrificed and brains were quickly removed for molecular studies. The rest of rats from each group (n = 6 for each group) were used to assess cognitive function after 2 weeks.
Western Blot Analysis
The hippocampus was dissociated from brain and homogenized in a mammalian tissue lysis buffer with protease inhibitor (Sigma-Aldrich, St. Louis, MO, USA). The western blot was performed as previously described [17], using the following primary antibodies: anti- glucose-regulated protein 78 (GRP78), anti-phosphorylated (P-) inositol-requiring enzyme 1 (P-IRE-1), anti-activating transcription factor 4 (ATF-4) (Abcam, Cambridge, MA, USA), anti-B cell lymphoma 2 (BCL-2), anti-BCL2-associated X protein (BAX), anti-caspase-3, anti-cytochrome-c, anti-C/EBP homologous protein (CHOP) (Cell Signaling Technology, Danvers, MA, USA), anti-activating transcription factor 6 (ATF-6, imgenex, San Diego, CA, USA), anti-P-eukaryotic initiation factor 2-α (EIF2α) and anti-β-actin (Santa Cruz Biotechnology, Dallas, USA). After incubation with horseradish peroxidase-conjugated anti-rabbit or anti-mouse second antibodies (Abcam, Cambridge, MA, USA), the signal was visualized with the enhanced chemiluminescence (ECL) detection reagents (Amersham, UK), and the densities of the immunobands were analyzed by NIH ImageJ software (Bethesda, MD, USA). Each sample was measured in duplicate and the average of duplicate measurements was used for analysis. All data were corrected by β-actin.
Open-Field Test
Open-field test was carried out to evaluate the emotional responses and exploration activity to a novel environment [7, 19]. Fifteen days after isoflurane exposure and taurine treatment, each rat was released in the center of the arena and the activity was measured as the total distance (meters) traveled by rats in 10 min time period. After every test, the arena was carefully cleaned with 75 % alcohol and rinsed with water to avoid olfactory cues.
Morris Water Maze
Morris water maze (MWM) was performed to evaluate the learning and memory functions of rats as previously described [7, 20]. Briefly, a circular tank (diameter, 150 cm; depth, 50 cm) was filled with opaque water (25 ± 1 °C) and the escape platform in the third quadrant was submerged 1.5 cm below the water level. The rats swim patterns were recorded by a video tracking system, and the data were analyzed with motion-detection software for the MWM (Actimetrics Software, Evanston, IL, USA).
Eighteen days after isoflurane exposure and taurine treatment, the place trials were carried out for 4 days to determine the rats’ ability to obtain spatial information. The pool was surrounded by a dark black curtain to prevent confounding visual cues. Each rats received four trials per day in each of the four quadrants of the swimming pool. On each trial, rats were released into the water facing the pool wall, and allowed to search for the platform for a maximum of 120 s and sit on the platform for 20 s before being removed from the pool. A rat that failed to find the platform within 120 s was guided to the platform and remained there for 20 s. For all training trials, the time required for a rat to reach the hidden platform (escape latency), swimming speed and swimming path were automatically digitized and recorded. The average of four trials per day was calculated as the escape latency.
The day after the completion of the hidden platform training, probe trials were performed to determine whether the rats had developed a spatial bias for the former platform quadrant. The platform in the third quadrant was removed from the pool and rats were allowed to swim for 120 s in any of the four quadrants of the swimming pool. The number of crossings over the original position of the platform and time spent in each quadrant were recorded.
Statistical Analysis
All data are presented as mean ± SE. The normality of the data was checked by the Shapiro–Wilk statistics (W test) prior to further analysis. Hemodynamic, physiologic and some behavioral data (place trials) were analyzed by a two-way repeated measures ANOVA (treatment as between-groups and time as repeated measures factors) followed by Bonferroni multiple comparison testing. Comparisons of other data among multiple groups were analyzed using one-way ANOVA followed by Tukey’s multiple comparison tests. P < 0.05 was considered statistically significant in all experiments. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS 15.0, SPSS Inc., Chicago, IL, USA).
Results
Taurine Treatment Prevents Isoflurane-Induced ER Stress in the Hippocampus
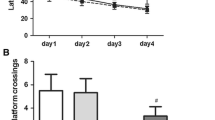
As shown in Fig. 1, compared with control, isoflurane exposure induced ER stress in the hippocampus of rats as evidenced by increased expression of GRP-78, P-IRE-1, cleaved ATF-6, P-EIF2α, ATF-4 and CHOP protein. Pretreatment with taurine dose-dependently attenuated isoflurane-induced increase in expression of GRP-78, P-IRE-1, cleaved ATF-6 and CHOP protein without altering expression of P-EIF2α and ATF-4 protein. Notably, pretreatment with a middle-dose of taurine (0.32 mmol/kg) completely normalized expressions of these ER stress biomarkers except for P-EIF2α and ATF-4. Pretreatment with a high-dose of taurine (0.48 mmol/kg) had no further effects on expression of ER stress biomarkers.
Effects of taurine on isoflurane-induced ER stress in the hippocampus of aged rats. Taurine pretreatment inhibited isoflurane-induced increase in expression of ER stress biomarkers GRP-78 (a), P-IRE-1 (b), cleaved ATF-6 (c) and CHOP (f) but not P-EIF2α (d) and ATF-4 (e). Representative western blots from each group (g). Western blot data were corrected by β-actin. Data are presented as mean ± SE (n = 6 for each group). Statistical differences were analyzed by one-way ANOVA followed by Tukey’s multiple comparison tests. *P < 0.05 vs CON; †P < 0.05 vs ISO+VEH. CON control, ISO isoflurane, T-L low dose (0.16 mmol/kg) of taurine, T-M middle dose (0.32 mmol/kg) of taurine, T-H high dose (0.48 mmol/kg) of taurine
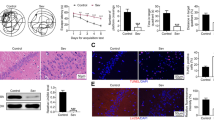
Taurine Treatment Inhibits Isoflurane-Induced Activation of Apoptosis Pathways in the Hippocampus
ER stress, especially increased transcription factor CHOP, has been demonstrated to play a key role in the regulation of apoptosis by altering the balance of pro- and anti-apoptotic proteins. We next examined whether attenuation of ER stress by taurine would inhibit isoflurane-induced activation of apoptosis pathways in the hippocampus of rats. Our results showed that isoflurane exposure induced activation of apoptosis pathways as indicated by decreased ratio of BCL-2 protein (an inhibitor of apoptosis) to BAX protein (an inducer of apoptosis), increased expressions of cytochrome-c and cleaved caspase-3 protein, compared with control (Fig. 2). Importantly, pretreatment with taurine before exposure to isoflurane significantly increased the ratio of BCL-2 protein to BAX protein, decreased expressions of cytochrome-c and cleaved caspase-3 protein in a dose-dependent manner. Of note, pretreatment with a middle-dose of taurine completely reversed isoflurane-induced changes in apoptosis-related proteins. Pretreatment with a high-dose of taurine did not exhibit further beneficial effects on expression of apoptosis-related proteins.
Effects of taurine on isoflurane-induced activation of apoptosis pathways in the hippocampus of aged rats. Taurine pretreatment reversed isoflurane-induced changes in apoptosis-related proteins, including the ratio of BCL-2 to BAX (a), expressions of cytochrome-c (CYT, b) and cleaved caspase-3 (CAS3, c). Representative western blots from each group (d). Western blot data were corrected by β-actin. Data are presented as mean ± SE (n = 6 for each group). Statistical differences were analyzed by one-way ANOVA followed by Tukey’s multiple comparison tests. *P < 0.05 vs CON; †P < 0.05 vs ISO+VEH. CON control, ISO isoflurane, T-L low dose of taurine, T-M middle dose of taurine, T-H high dose of taurine
Taurine Treatment Protects Against Cognitive Impairment Induced by Isoflurane
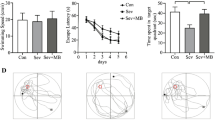
The protocol of behavior study was illustrated in Fig. 3a. Results of the Open-field test revealed that there were no significant differences in travel distances among five experimental groups (Fig. 3b). In the place trial, no differences in swimming speeds were observed among five experimental groups (Fig. 3c). However, rats exposed to isoflurane spent significantly more time to find the submerged platform at trial day 2 and trial day 3 when compared with control group (Fig. 3d). While the time spent to find the submerged platform in rats pretreated with a middle-dose or a high-dose of taurine before exposure to isoflurane was similar to that in control group.
a Experimental protocol of behavior study; b emotional responses and exploration activity to a novel environment was assessed by open-field test; c, d the ability of spatial information acquisition was assessed by place trial that measured the swimming speed and the latency for the rats to reach at the platform. Data are presented as mean ± SE (n = 6 for each group). Statistical differences were analyzed by one-way ANOVA followed by Tukey’s multiple comparison tests (b) or two-way repeated measures ANOVA followed by Bonferroni multiple comparison testing (c, d). *P < 0.05, ISO+VEH vs CON, ISO+T-M or ISO+T-H. CON control, ISO isoflurane, T-L low dose of taurine, T-M middle dose of taurine, T-H high dose of taurine
In the probe trial, rats exposed to isoflurane had a decreased number of crossings over the former platform location (Fig. 4a) and spent significantly less time in the third quadrant where the platform was located (Fig. 4b), compared with control group. Pretreatment with taurine before exposure to isoflurane in rats significantly improved the performance. Rats pretreated with a middle-dose or a high-dose of taurine before exposure to isoflurane performed similar with the control group. The representative swimming paths of each group were showed in Fig. 4c.
a, b The memory retention capabilities were assessed by Probe trial that measured the number of original platform (the third quadrant) crossings and the time spent in each quadrant. The representative swimming paths of each group (c). Data are presented as mean ± SE (n = 6 for each group). Statistical differences were analyzed by one-way ANOVA followed by Tukey’s multiple comparison tests. *P < 0.05 vs CON; †P < 0.05 vs ISO+VEH. CON control, ISO isoflurane, T-L low dose of taurine, T-M middle dose of taurine, T-H high dose of taurine
Taurine Treatment has no Effects on Hemodynamic and Physiologic Parameters
Hemodynamic and physiologic parameters before (0 h) and 4 h after isoflurane exposure were shown in Table 1. Compared with control, isoflurane exposure slightly decreased mean BP and heart rate but the differences did not reach statistical significance. Pretreatment with taurine at any dose had no effects on mean BP and heart rate. Four hours after isoflurane exposure, there were no differences in blood gas values and blood glucose levels across five experimental groups. These results excluded the possibility that molecular and behavioral changes observed in the study were due to hemodynamic or physiologic side effects of isoflurane or taurine.
Discussion
The present study examined the effects of taurine on anesthetic isoflurane-induced cognitive deficits and underlying mechanisms in aged rats. The novel finding of this study is that pretreatment with taurine before isoflurane exposure inhibits isoflurane-induced ER stress and activation of apoptosis pathways in the hippocampus, thereby preventing the cognitive impairment in aged rats. This finding demonstrate a protective role of taurine in isoflurane-induced cognitive deficits in aged rats and these beneficial effects are mediated by inhibition of ER stress-associated apoptosis in the hippocampus, a brain region that plays a key role in learning and memory.
The ER is an important organelle responsible for the synthesis and folding of proteins that traffic through the secretory pathway [21–23]. Multiple disturbances can induce accumulation of unfolded proteins in the ER, a cellular condition termed ER stress [22, 23]. There are three ER transmembrane receptors: pancreatic ER kinase (PKR)-like ER kinase (PERK), ATF6 and IRE1 [22, 23]. On accumulation of unfolded proteins, ER chaperone GRP78 dissociates from the three receptors, which leads to their activation and triggers an evolutionarily conserved response termed the unfolded protein response (UPR) [21–23]. The initial intent of the UPR serves as a pro-survival response to reduce the accumulation of unfolded proteins and restore normal ER functioning [24]. However, if protein aggregation is persistent and ER homeostasis cannot be recovered, signaling switches from pro-survival to pro-apoptotic by activating apoptosis pathways [24]. The BCL-2 protein family plays an essential role in the control of apoptosis under ER stress conditions [21, 24]. Activated PERK blocks general protein synthesis by phosphorylating eukaryotic initiation factor 2α (eIF2α), which increases the specific translation of ATF4 [21, 24]. Following dissociation of GRP78, ATF6 moves to the Golgi apparatus where it is cleaved into its active form [21, 24]. Both ATF4 and cleaved ATF6 can lead to the induction of transcription factor CHOP in the nucleus [21, 24]. Chop is known to regulate the mitochondria-mediated apoptosis by downregulation of anti-apoptotic protein BCL-2 and upregulation of pro-apoptotic protein BAX, which lead to the release of cytochrome c and activation of downstream effector caspases (caspase-3 in particular), resulting in apoptosis [21, 24]. In the third ER stress pathway, activation of IRE1 induces activation of apoptosis signal-regulating kinase 1 (ASK1) and c-Jun N-terminal kinase (JNK), which targets BCL2 proteins and allow the activation of BAX and BAK, leading to the execution of apoptosis [24]. It has been widely accepted that the neuronal apoptosis in the hippocampus disturbs learning ability and memory function, causing cognitive deficits [25, 26]. In the present study, we found that isoflurane exposure induced significantly increase in expression of GRP-78, P-IRE-1, cleaved ATF-6, P-EIF2α, ATF-4 and CHOP protein in the hippocampus of aged rats, which was associated with decreased ratio of BCL-2 to BAX protein and increased expressions of cytochrome-c and cleaved caspase-3 protein. The Morris water maze test revealed that aged rats exposed to isoflurane had longer escape latency to reach the platform, less number of original platform crossing and less time spent in the target quadrant. These results confirm previous in vitro and in vivo studies indicating that isoflurane exposure causes ER stress, which leads to activation of apoptosis pathways in the hippocampus, contributing to cognitive dysfunction [7, 12]. Importantly, our results demonstrated that pretreatment with taurine inhibited isoflurane-induced ER stress and activation of apoptosis pathways in the hippocampus, preventing the cognitive dysfunction in aged rats.
Taurine as a semi-essential amino acid has recently been shown to exert neuroprotective effects in several neurological disorders such as stroke, traumatic brain injury, hypoxia and epilepsy [13, 16, 17, 27, 28]. Taurine may confer neuroprotection by preventing mitochondrial dysfunction and ER stress [18, 29, 30] and by decreasing ER stress-induced apoptosis in the brain [16, 17, 27]. As a neuroprotective agent, taurine needs to cross the blood–brain barrier and enter into the brain under neuropathological conditions. Previous studies have demonstrated that taurine crosses the blood–brain barrier [31, 32] using a specific beta amino acid taurine transporter (TAUT) [31], which is abundantly expressed in hippocampal neurons [33]. In the present study, pretreatment with taurine dose-dependently attenuated isoflurane-induced increase in expression of ER stress biomarkers GRP-78, P-IRE-1, cleaved ATF-6 and CHOP, which was associated with reduction in activation of apoptosis pathways in the hippocampus. Pretreatment with a middle-dose or a high-dose of taurine completely reversed expressions of above ER stress biomarkers, resulting in inhibition of apoptosis pathways and normalization of cognitive dysfunction in aged rats. These data clearly demonstrated that taurine protects against cognitive deficits induced by isoflurane via inhibition of ER stress-mediated apoptosis in the hippocampus. Of note, isoflurane-induced increase in expression of ER stress biomarkers P-EIF2α and ATF-4 was not altered by pretreatment with taurine. These results are consistent with recent in vitro and in vivo studies suggesting that taurine prevents the ER stress-induced apoptosis by mediating ATF6 and the IRE1 pathways, but not the PERK pathway [17, 18, 27].
It is worth noting that GRP78 is primarily located in the ER lumen with a subfraction detected as a transmembrane protein in non-stressed cells. Under ER stress conditions, the induction of GRP78 not only leads to an increase in GRP78 in the ER compartment, but also promotes GRP78 relocalization from the ER to the cell surface, mitochondria, cytoplasm or nucleus where it acts in multifaceted cellular activities [34]. For example, ER stress induces promotion of cell surface expression of GRP78, which has been considered as an important receptor in cell signaling, viability and therapeutic targeting [34]. The cytoplasmic GRP78 isoform induced by ER stress is a newly identified regulator of the ER stress signaling pathway, in addition to the function of canonical GRP78 in the cytoplasm [34]. Beyond the ER, the mitochondrial, nuclear and secreted forms of GRP78 have been associated with cellular homeostasis and therapeutic resistance [34]. Therefore, taurine might exert beneficial effects on isoflurane-induced cognitive impairment in part by regulating GRP78 expression in the cell surface, mitochondria, cytoplasm or nucleus.
The present study focused on the effects of taurine on isoflurane-induced ER stress pathways. However, other mechanisms may also be involved in neuroprotective actions of taurine in isoflurane-induced cognitive impairment in aged rats. For example, intracellular calcium overload has been demonstrated to induce ER stress and mitochondrial membrane potential collapse, eventually resulting in cell apoptosis [35]. Isoflurane can induce neuronal apoptosis in developing rat brain by intracellular calcium overload [36]. Taurine has been shown to prevent intracellular calcium overload in primary neurons by inhibiting the reverse mode of Na+/Ca2+ exchanger, voltage-gated Ca2+ channels and NMDA receptors [37, 38]. In addition, taurine can regulate intracellular calcium homeostasis by reducing the release of calcium from intracellular storage pools and increasing the capacity of mitochondria to sequester calcium [18]. Thus, the possibility could not be excluded that taurine prevents isoflurane-induced ER stress and neuronal apoptosis in the hippocampus by inhibiting intracellular calcium overload in our study. Moreover, aging is associated with reduced hippocampal neurogenesis, alteration of the GABAergic system and impaired long-term potentiation of synaptic transmission in the hippocampus, all of which contribute to cognitive impairment [39–41]. Taurine has been shown to ameliorate cognitive impairment by increasing hippocampal neurogenesis and improving the function of the GABAergic system in aged mice [39, 40]. Taurine also induces long-term potentiation in the rat hippocampus [42]. Further studies are needed to determine whether reduced hippocampal neurogenesis, dysfunction of GABAergic system or impaired long-term potentiation in aged animals is exaggerated by isoflurane but improved by taurine.
In summary, the present study demonstrates that pretreatment with taurine prevents anesthetic isoflurane-induced cognitive impairment in aged rats and that these beneficial effects are mediated by inhibition of ER stress, which suppresses activation of apoptosis pathways in the hippocampus (Fig. 5). These findings may provide new insights into potential therapeutic use of taurine for preventing anesthetic isoflurane-induced cognitive impairment in elderly patients.
Schematic representation of taurine prevention of isoflurane-induced cognitive impairment in aged rats. Taurine pretreatment inhibits ER stress-induced apoptosis by mediating ATF6 and the IRE1 pathways but not the PERK pathway, leading to the prevention of isoflurane-induced cognitive impairment in aged rats
References
Shoair OA, Grasso Ii MP, Lahaye LA, Daniel R, Biddle CJ, Slattum PW (2015) Incidence and risk factors for postoperative cognitive dysfunction in older adults undergoing major noncardiac surgery: a prospective study. J Anaesthesiol Clin Pharmacol 31:30–36
Rundshagen I (2014) Postoperative cognitive dysfunction. Dtsch Arztebl Int 111:119–125
Mangoni AA, Jackson SH (2004) Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol 57:6–14
Lewis MC, Nevo I, Paniagua MA, Ben-Ari A, Pretto E, Eisdorfer S, Davidson E, Matot I, Eisdorfer C (2007) Uncomplicated general anesthesia in the elderly results in cognitive decline: does cognitive decline predict morbidity and mortality? Med Hypotheses 68:484–492
Pieperhoff P, Homke L, Schneider F, Habel U, Shah NJ, Zilles K, Amunts K (2008) Deformation field morphometry reveals age-related structural differences between the brains of adults up to 51 years. J Neurosci 28:828–842
Jevtovic-Todorovic V, Absalom AR, Blomgren K, Brambrink A, Crosby G, Culley DJ, Fiskum G, Giffard RG, Herold KF, Loepke AW, Ma D, Orser BA, Planel E, Slikker W Jr, Soriano SG, Stratmann G, Vutskits L, Xie Z, Hemmings HC Jr (2013) Anaesthetic neurotoxicity and neuroplasticity: an expert group report and statement based on the BJA Salzburg Seminar. Br J Anaesth 111:143–151
Ge HW, Hu WW, Ma LL, Kong FJ (2015) Endoplasmic reticulum stress pathway mediates isoflurane-induced neuroapoptosis and cognitive impairments in aged rats. Physiol Behav 151:16–23
Ni C, Li Z, Qian M, Zhou Y, Wang J, Guo X (2015) Isoflurane induced cognitive impairment in aged rats through hippocampal calcineurin/NFAT signaling. Biochem Biophys Res Commun 460:889–895
Kong FJ, Ma LL, Zhang HH, Zhou JQ (2015) Alpha 7 nicotinic acetylcholine receptor agonist GTS-21 mitigates isoflurane-induced cognitive impairment in aged rats. J Surg Res 194:255–261
Zhang B, Tian M, Zhen Y, Yue Y, Sherman J, Zheng H, Li S, Tanzi RE, Marcantonio ER, Xie Z (2012) The effects of isoflurane and desflurane on cognitive function in humans. Anesth Analg 114:410–415
Doyle KM, Kennedy D, Gorman AM, Gupta S, Healy SJ, Samali A (2011) Unfolded proteins and endoplasmic reticulum stress in neurodegenerative disorders. J Cell Mol Med 15:2025–2039
Wang H, Dong Y, Zhang J, Xu Z, Wang G, Swain CA, Zhang Y, Xie Z (2014) Isoflurane induces endoplasmic reticulum stress and caspase activation through ryanodine receptors. Br J Anaesth 113:695–707
Birdsall TC (1998) Therapeutic applications of taurine. Altern Med Rev 3:128–136
Louzada PR, Paula Lima AC, Mendonca-Silva DL, Noel F, De Mello FG, Ferreira ST (2004) Taurine prevents the neurotoxicity of beta-amyloid and glutamate receptor agonists: activation of GABA receptors and possible implications for Alzheimer’s disease and other neurological disorders. Fed Am Soc Exp Biol J 18:511–518
Sun M, Xu C (2008) Neuroprotective mechanism of taurine due to up-regulating calpastatin and down-regulating calpain and caspase-3 during focal cerebral ischemia. Cell Mol Neurobiol 28:593–611
Chao CC, Chan P, Kuo CS, Gong CL, Cheng TH, Liu ZM, Shen PC, Huang CC, Leung YM (2014) Protection of differentiated neuronal NG108-15 cells from P2X7 receptor-mediated toxicity by taurine. Pharmacol Rep 66:576–584
Gharibani P, Modi J, Menzie J, Alexandrescu A, Ma Z, Tao R, Prentice H, Wu JY (2015) Comparison between single and combined post-treatment with S-methyl-N, N-diethylthiolcarbamate sulfoxide and taurine following transient focal cerebral ischemia in rat brain. Neuroscience 300:460–473
Gharibani PM, Modi J, Pan C, Menzie J, Ma Z, Chen PC, Tao R, Prentice H, Wu JY (2013) The mechanism of taurine protection against endoplasmic reticulum stress in an animal stroke model of cerebral artery occlusion and stroke-related conditions in primary neuronal cell culture. Adv Exp Med Biol 776:241–258
Valentim AM, Di Giminiani P, Ribeiro PO, Rodrigues P, Olsson IA, Antunes LM (2010) Lower isoflurane concentration affects spatial learning and neurodegeneration in adult mice compared with higher concentrations. Anesthesiology 113:1099–1108
Wang WY, Luo Y, Jia LJ, Hu SF, Lou XK, Shen SL, Lu H, Zhang HH, Yang R, Wang H, Ma ZW, Xue QS, Yu BW (2014) Inhibition of aberrant cyclin-dependent kinase 5 activity attenuates isoflurane neurotoxicity in the developing brain. Neuropharmacology 77:90–99
Hetz C, Martinon F, Rodriguez D, Glimcher LH (2011) The unfolded protein response: integrating stress signals through the stress sensor IRE1alpha. Physiol Rev 91:1219–1243
Ellgaard L, Helenius A (2003) Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol 4:181–191
Kim I, Xu W, Reed JC (2008) Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 7:1013–1030
Szegezdi E, Logue SE, Gorman AM, Samali A (2006) Mediators of endoplasmic reticulum stress-induced apoptosis. Eur Mol Biol Organ Rep 7:880–885
Kuhn HG, Biebl M, Wilhelm D, Li M, Friedlander RM, Winkler J (2005) Increased generation of granule cells in adult Bcl-2-overexpressing mice: a role for cell death during continued hippocampal neurogenesis. Eur J Neurosci 22:1907–1915
Sun XQ, Xu ZP, Zhang S, Cao XS, Liu TS (2009) Simulated weightlessness aggravates hypergravity-induced impairment of learning and memory and neuronal apoptosis in rats. Behav Brain Res 199:197–202
Chen PC, Pan C, Gharibani PM, Prentice H, Wu JY (2013) Taurine exerts robust protection against hypoxia and oxygen/glucose deprivation in human neuroblastoma cell culture. Adv Exp Med Biol 775:167–175
Sun M, Gu Y, Zhao Y, Xu C (2011) Protective functions of taurine against experimental stroke through depressing mitochondria-mediated cell death in rats. Amino Acids 40:1419–1429
Pan C, Giraldo GS, Prentice H, Wu JY (2010) Taurine protection of PC12 cells against endoplasmic reticulum stress induced by oxidative stress. J Biomed Sci 17(Suppl 1):S17
Foos TM, Wu JY (2002) The role of taurine in the central nervous system and the modulation of intracellular calcium homeostasis. Neurochem Res 27:21–26
Benrabh H, Bourre JM, Lefauconnier JM (1995) Taurine transport at the blood–brain barrier: an in vivo brain perfusion study. Brain Res 692:57–65
Tamai I, Senmaru M, Terasaki T, Tsuji A (1995) Na(+)- and Cl(−)-dependent transport of taurine at the blood–brain barrier. Biochem Pharmacol 50:1783–1793
Pow DV, Sullivan R, Reye P, Hermanussen S (2002) Localization of taurine transporters, taurine, and (3)H taurine accumulation in the rat retina, pituitary, and brain. Glia 37:153–168
Ni M, Zhang Y, Lee AS (2011) Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem J 434:181–188
Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R (2008) Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 27:6407–6418
Liu H, Dai T, Guo W (2013) Isoflurane-induced neuronal apoptosis in developing hippocampal neurons. Neural Regen Res 8:825–832
Wu H, Jin Y, Wei J, Jin H, Sha D, Wu JY (2005) Mode of action of taurine as a neuroprotector. Brain Res 1038:123–131
Wu JY, Prentice H (2010) Role of taurine in the central nervous system. J Biomed Sci 17(Suppl 1):S1
Gebara E, Udry F, Sultan S, Toni N (2015) Taurine increases hippocampal neurogenesis in aging mice. Stem Cell Res 14:369–379
El Idrissi A (2008) Taurine improves learning and retention in aged mice. Neurosci Lett 436:19–22
Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER (1999) Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci USA 96:5280–5285
Dominy J Jr, Thinschmidt JS, Peris J, Dawson R Jr, Papke RL (2004) Taurine-induced long-lasting potentiation in the rat hippocampus shows a partial dissociation from total hippocampal taurine content and independence from activation of known taurine transporters. J Neurochem 89:1195–1205
Acknowledgments
This work was supported by Youth Science Foundation of Shandong Province, China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Rights and permissions
About this article
Cite this article
Zhang, Y., Li, D., Li, H. et al. Taurine Pretreatment Prevents Isoflurane-Induced Cognitive Impairment by Inhibiting ER Stress-Mediated Activation of Apoptosis Pathways in the Hippocampus in Aged Rats. Neurochem Res 41, 2517–2525 (2016). https://doi.org/10.1007/s11064-016-1963-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1963-4