Abstract
Aims Taurine as an endogenous substance possesses a number of cytoprotective properties. In the study, we have evaluated the neuroprotective effect of taurine and investigated whether taurine exerted neuroprotection through affecting calpain/calpastatin or caspase-3 actions during focal cerebral ischemia, since calpain and caspase-3 play central roles in ischemic neuronal death. Methods Male Sprague–Dawley rats were subjected to 2 h of middle cerebral artery occlusion (MCAo), and 22 h of reperfusion. Taurine was administrated intravenously 1 h after MCAo. The dose–responses of taurine to MCAo were determined. Next, the effects of taurine on the activities of calpain, calpastatin and caspase-3, the levels of calpastatin, microtubule-associated protein-2 (MAP-2) and αII-spectrin, and the apoptotic cell death in penumbra were evaluated. Results Taurine reduced neurological deficits and decreased the infarct volume 24 h after MCAo in a dose-dependent manner. Treatment with 50 mg/kg of taurine significantly increased the calpastatin protein levels and activities, and markedly reduced the m-calpain and caspase-3 activities in penumbra 24 h after MCAo, however, it had no significant effect on μ-calpain activity. Moreover, taurine significantly increased the MAP-2 and αII-spectrin protein levels, and markedly reduced the ischemia-induced TUNEL staining positive score within penumbra 24 h after MCAo. Conclusions Our data demonstrate the dose-dependent neuroprotection of taurine against transient focal cerebral ischemia, and suggest that one of protective mechanisms of taurine against ischemia may be blocking the m-calpain and caspase-3-mediated apoptotic cell death pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral ischemia initiates a cascade of detrimental events including release of glutamate, accumulation of intracellular calcium, formation of free radicals, and induction of inflammation, which lead to disruption of cellular homeostasis and structure damage of ischemic brain tissue (Lipton 1999). Many different approaches to influence the detrimental events after brain ischemia have been developed in animal models of stroke. Almost all of them result in dramatic neuroprotection in animal experiments, but all human studies, to date, have failed, and the possible reasons for the failure have been reviewed (Gladstone et al. 2002; Cheng et al. 2004). Recently, more attentions to the neuroprotection are focused on the endogenous neuroprotection (Dirnagl et al. 2003; Lizasoain et al. 2006), and it has been demonstrated that some endogenous substances, such as albumin, estrogens, erythropoietin, and growth factors, exhibit neuroprotection in focal cerebral ischemia (Labiche and Grotta 2004; Hoffman et al. 2006).
Taurine (2-aminoethanesulfonic acid) is the major intracellular free β-amino acid present in most mammalian tissues. It is not involved in primary metabolism, and neither is incorporated into proteins. Taurine possesses a number of cytoprotective properties through its actions as a neurotransmitter, neuromodulator, osmoregulator, modulator of intracellular calcium homeostasis, anti-oxidant, membrane stabilizer, and anti-inflammation factor (Huxtable 1992; Schuller-Levis and Park 2004). It has been reported to reduce glutamate-induced neurotoxicity (El Idrissi et al. 2003; Louzada et al. 2004), and improve the recovery of neuronal functions after cerebral hypoxia (Schurr et al. 1987). Moreover, taurine-containing neurons in hippocampus are more resistant to the damage induced by ischemia than other neurons (Wu et al. 1994). Under cell-damaging conditions, the release of taurine is increased, meanwhile, the uptake of taurine is inhibited. The increase in the extracellular levels of taurine in cell-damaging conditions may be an important endogenous protective mechanism (Saransaari and Oja 2000). These reports suggest that taurine may act as an endogenous neuroprotectant to block multiple targets of detrimental cascade after focal cerebral ischemia. For testing this hypothesis, we first evaluated the neuroprotective effect of taurine against focal cerebral ischemia in the present study.
If the neuroprotective effect of taurine on focal cerebral ischemia is positive, then we shall be interested to investigate what is its mechanism. In this study, we primarily investigated whether taurine exerted neuroprotection through blocking the actions of calpain/calpastatin and caspase-3 after focal cerebral ischemia, since calpain/calpastatin and caspase-3 play central roles in ischemic neuronal death (Lipton 1999).
Calpains, one family of cysteine proteases, are activated by calcium and autolytic processing. The two ubiquitous calpains, μ- and m-calpain are regulated reversibly by calcium and calpastatin, an endogenous inhibitor of calpain. They are proposed to participate in the turnover of cytoskeletal proteins and regulation of kinases, transcription factors, and receptors (Goll et al. 2003). Caspases, another family of cysteine protease with an unusual substrate specificity, have been identified as key executioners of apoptosis. Caspase-3, one member of caspase family, is believed as a final killer of apoptosis (Salvesen and Dixit 1997). During focal cerebral ischemia, calpains and caspase-3 are activated and involved in the apoptotic cell death, which have been demonstrated through investigating the protection of calpain and caspase-3 inhibitors and the proteolysis of substrates, such as microtubule-associated protein-2 (MAP-2), spectrin, and calpastatin (Fink et al. 1998; Davoli et al. 2002; Rami et al. 2003; Kambe et al. 2005; Han et al. 2006). Moreover, calpastatin has been shown to be induced by cerebral hypoxia-ischemia in immature rats (Blomgren et al. 1999), and involved in the neuroprotection against brain ischemia (Rami et al. 2003).
With this background, we further investigated whether taurine exerted neuroprotection through blocking the calpain- or caspase-3-mediated ischemic cell death pathway in penumbra after focal cerebral ischemia. Particularly, we paid more attentions on the effects of taurine on calpastatin actions after focal cerebral ischemia, since taurine (Schurr et al. 1987; El Idrissi et al. 2003; Louzada et al. 2004) and calpastatin (Rami et al. 2003; Han et al. 2006; Crocker et al. 2003), as two endogenous substances, are involved in the neuroprotection during central nervous system damage.
Materials and Methods
Rat Model of Focal Cerebral Ischemia
All animal protocols were approved by the national institutes of health guide for the care and the use of laboratory animals. Male Sprague–Dawley rats weighting from 315 to 330 g were anesthetized with chloral hydrate (400 mg/kg, i.p.) and the right middle cerebral artery occlusion (MCAo) was produced by inversion of a 4-0 nylon filament as described previously (Schmid-Elsaesser et al. 1998). The filament was withdrawn 2 h after onset of MCAo, and then reperfused. Rectal temperature was continuously monitored and maintained at 37 ± 0.5°C by a negative-feedback-controlled heating pad during the whole experiment.
Experimental Protocols
Dose–Response of Taurine to Focal Cerebral Ischemia
Rats were randomly assigned to 6 groups treated with taurine or vehicle: (1) taurine (Shanghai Chemical Reagents Company, dissolved in normal saline), 1 mg/kg; (2) taurine, 2.5 mg/kg; (3) taurine, 5 mg/kg; (4) taurine, 15 mg/kg; (5) taurine, 50 mg/kg, and (6) vehicle, a similar volume of normal saline (1 ml/kg). The respective agent was administrated intravenously 1 h after ischemia. Neurological deficits were evaluated 4 and 24 h after MCAo (n = 15 per group), and the infarct volumes were determined 24 h after MCAo (n = 14 per group).
Effects of Taurine on Activities of Calpastatin, Calpain and Caspase-3, and the Levels of Calpastatin, MAP-2 and αII-spectrin
Based on the dose–response study, 50 mg/kg of taurine was used in the study. Animals were randomly assigned to 3 groups treated with taurine or vehicle: (1) sham: normal saline (1 ml/kg); (2) vehicle: normal saline (1 ml/kg) and (3) taurine (50 mg/kg). The respective agent was administrated intravenously 1 h after ischemia. Rats were decapitated and the tissues of penumbras were dissected 24 h after MCAo in the vehicle- or taurine-treated rats, and the regions from the right hemispheres that corresponded to the ischemic penumbras were dissected 24 h after operation in sham-operated rats. The cytosolic fractions were prepared and used to determine the activities of calpastatin, calpain and caspase-3 (n = 12 per group), and the levels of calpastatin protein, MAP-2 and αII-spectrin (n = 8 per group).
Effect of Taurine on Apoptotic Cell Death
Animals were randomly assigned to 3 groups treated with taurine or vehicle: (1) sham: normal saline (1 ml/kg); (2) vehicle: normal saline (1 ml/kg), and (3) taurine (50 mg/kg). The respective agent was administrated intravenously 1 h after ischemia. Rats were decapitated and the brains were collected 24 h after operation in sham-operated rats, or 24 h after MCAo in vehicle- or taurine-treated rats. The brains were embedded in paraffin, and coronal slices (8 μm thick) were obtained. Terminal deoxynucleotidyl transferase-mediated biotin-dUTP nick-end labeling (TUNEL) staining was used to observe the apoptotic cell death (n = 9 per group).
Neurological Evaluation
Animals were examined for neurological deficits 4 and 24 h after MCAo by a blinder to the identity of the groups using a 6-point neurological function score that was described by Schmid-Elsaesser et al.: 0, no spontaneous motor activity; 1, spontaneous circling; 2, circling if pulled by tail; 3, lowered resistance to lateral push (and forelimb flexion) without circling; 4, contralateral forelimb flexion; 5, no observable neurological deficit (Schmid-Elsaesser et al. 1998).
Measurement of Infarct Volume
The rats were anesthetized with chloral hydrate (400 mg/kg, i.p.) and decapitated 24 h after MCAo. The brains were rapidly removed and sliced into 2-mm-thick slices. The brain slices were stained with 1% 2,3,5-triphenyltetrazolium chlorides (TTC, Sigma Co., St Louis, MO, USA) at 37°C for 15 min in the dark, and then fixed by 4% formaldehyde in phosphate buffered solution. The unstained area of the brain slice was defined as infarction. The infarct volume was measured using an image analysis program (Beijing Konghai Co., China). Since brain edema might significantly affect the accuracy of infarct estimation, the corrected infarct volume was calculated by the method described previously (Lin et al. 1993; Swanson et al. 1990).
Sample Collection and Preparation of Cytosolic Fraction
As has been previously described by Ashwal et al. (1998), the tissues of penumbras were dissected 24 h after MCAo in the vehicle- or taurine-treated rats, and the regions from the right hemispheres that corresponded to the ischemic penumbras were dissected 24 h after operation in sham-operated rats at 4°C, and homogenized in 7 volumes of homogenization buffer (25 mM HEPES, pH 7.4, 0.1% Triton X-100, 5 mM MgCl2, 2 mM DTT, 1.3 mM EDTA, 1 mM EGTA, 0.5 mM phenylmethanesulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml pepstatin A, 10 μg/ml leupeptin). The homogenates were centrifuged at 1,000 × g for 10 min at 4°C, and the supernatants were centrifuged at 17,000 × g for 90 min at 4°C again. The final supernatants (cytosolic fractions) were used to measure the activities of calpain, calpastatin, or caspase-3, and the levels of calpastatin, MAP-2, or αII-spectrin. The protein concentrations in supernatants were determined by the method of Bradford (Bradford 1976).
Calpastatin Activity Assay
The cytosolic fractions were boiled for 10 min and then centrifuged at 10,000 × g for 10 min at 4°C to remove denatured proteins, and the protein concentrations in supernatants were measured (Bradford 1976). The calpastatin activity was determined by the method described previously (Yoshida et al. 1993; Sorimachi et al. 1997). Briefly, the heat-treated sample (50 μg/ml protein) was incubated with purified m-calpain from rat (3 μg/ml, Calbiochem, San Diego, CA, USA) at 4°C for 5 min, and then was diluted in calpain assay mixture (20 mM Tris, pH 7.4, 4 mg/ml azocasein, 20 mM β-meraptoethanol, 1% Triton X-100) to a final volume of 1 ml. The mixture was incubated at 32 ± 1°C for 60 min, and calpain activity was determined as the release of trichloroacetic acid-soluble peptides from azocasein (Sigma Co., St Louis, MO, USA). Calpastatin activity (inhibition rate, %) was defined as m-calpain activity of purified m-calpain alone minus m-calpain activity of purified m-calpain with heat-treated samples/m-calpain activity of purified m-calpain alone.
Calpain Activity Assay
As has been previously described (Raser et al. 1995), 12% polyacrylamide gel contained 0.05% casein and 4% polyacrylamide gel were prepared and used as separating and stacking gel, respectively. The casein gel was pre-run at 50 V for 1 h at 4°C in a running buffer (25 mM Tris, 192 mM glycine, 1 mM EGTA, 1 mM DTT, pH 8.3). Proteins from each sample (45 μg) were loaded in each well, and then given electrophoresis at 50 V, 4°C. When bromophenol blue reached the base of the gel, the gel was removed and rinsed in incubation buffer (20 mM Tris, 10 mM DTT, 3 mM CaCl2, pH 7.6 for μ-calpain and pH 7.3 for m-calpain) twice, then incubated in the same incubation buffer at 32 ± 1°C for 24 h. The gel was stained in 0.2% Commassie blue R-250 for 2 h and incubated in a destaining solution overnight. The gel was analyzed by gel image analyzer (AlphaImager™ 2200, Aalpha Innotech Co., USA). Results were normalized with the integrated optical density (arbitrary densitometric units) of the lysed region (cleared band).
Caspase-3 Activity Assay
The caspase-3 activity was determined by use of ac-DEVD-AFC (Calbiochem, San Diego, CA, USA), a fluorescent substrate to be used as a susceptible fluorescent substrate for caspase-3 (Benchoua et al. 2001). Fifteen-microlitre sample was diluted in caspase-3 assay buffer (50 mM HEPES, pH 7.4, 100 mM NaCl, 1 mM EDTA, 10 mM DTT) to a final volume of 1,485 μl. The enzymatic reaction was started by addition of 15 μl of a 2 mM solution of ac-DEVD-AFC and incubated for 2 h at 37°C. Quantification of substrate cleavage leading to release of free AFC was determined with a spectrofluorometer set at 400 nm excitation wavelength and at 505 nm emission wavelength (Hitachi Co., Japan). Fluorescent arbitrary units were converted into micromoles of AFC released per hour and milligrams of protein using a standard curve of free AFC (Sigma Co., St Louis, MO, USA).
Western Blot Analysis
Calpastatin, MAP-2, and αII-spectrin were determined from cytosolic fraction separated by 10, 6, and 6% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), respectively. Twenty-microlitre sample was mixed with 5 μl of sample buffer, and then boiled for 5 min. Equal amount of proteins (40 μg) were separated by SDS-PAGE, and molecular weight markers (R&D Systems Inc., Minneapolis, MN, USA) were loaded on each gel for protein band identification. The proteins on the gel were subsequently transferred onto a nitrocellulose membrane using a semidry transfer apparatus. Blots were probed with either primary rabbit polyclonal antibody reactive with calpastatin (Santa Cruz Biotechnology, Santa Cruz, CA, USA, 1:100), or primary mouse monoclonal antibody reactive with MAP-2 (Sigma Co., St Louis, MO, USA, 1:800), or αII-spectrin (Santa Cruz Biotechnology, Santa Cruz, CA, USA, 1:100), and subsequently incubated with secondary anti-rabbit or mouse antibody conjugated with horseradish peroxidase (Chemicon International Inc., Temecula, CA, USA). Finally, the color reaction was observed by incubation of membrane with 3,3′-diaminobenzidine (Chemicon International Inc., Temecula, CA, USA), and the integrated optical densities of the protein bands detected by Western blot analysis were analyzed by gel image analyzer (AlphaImager™ 2200, Aalpha Innotech Co., USA).
TUNEL Staining for Apoptotic Cells
In situ detection of DNA fragmentation in brain cells after ischemia or after sham operation was performed using TUNEL as described by Gavrieli et al (1992). The 8-μm coronal slices fixed by 4% paraformaldehyde were deparaffized and rinsed in distilled water at room temperature. They were incubated with proteinase K (200 μg/ml) for 15 min at 37°C, and endogenous peroxidases were inactivated by covering the slices with 2% H2O2 for 10 min at room temperature. The sections were rinsed with 50 mM PBS, and immersed in TdT buffer (30 mM Tris, pH 7.2, 140 mM sodium acodylate, 1 mM cobalt chloride) for 30 min, followed by reaction with a TdT enzyme (Roche Diagnostics GmbH, Germany) and biotinylated 16-dUTP (Roche Diagnostics GmbH, Germany) at 37°C for 90 min. The slices were washed in reaction stopping buffer (150 mM sodium chloride, 15 mM sodium citrate, pH 7.4) for 15 min, followed by washing in PBS. The avidin–biotin technique was applied, and then the nuclei were counterstained with hematoxylin solution for 30 s. The apoptotic cells exhibiting DNA fragmentation were observed using a light microscope. Cells containing apoptotic bodies are referred to as apoptotic cells. In the current study, the following four scores were used to evaluate the degree of apoptotic dell death within frontoparietal cortex motor area (cortical penumbra) and medial part of striatum (striatal penumbra): 0, few positive staining cells; 1, positive staining cells were less than 30%; 2, positive staining cells were 30–60%, and 3, positive staining cells were >60%. The anatomic distribution of frontoparietal cortex motor area and medial part of striatum was shown in Fig. 6A (Memezawa et al. 1992).
Data Expression and Statistical Analysis
Data are presented as mean ± SEM. Comparisons among multiple groups were statistically evaluated by one-way ANOVA with a post hoc Fisher’s test and comparisons between two groups were evaluated by Student’s t-test. A probability of <0.05 was considered statistically significant.
Results
Dose–Responses of Taurine to Focal Cerebral Ischemia: Neurological Deficits and Infarct Volume
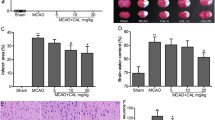
Before ischemia, neurological scores were normal (score = 5) in all animals. The vehicle-treated rats showed significant neurological deficits 4 and 24 h after MCAo. Treatment with 5, 15, or 50 mg/kg of taurine markedly reduced the neurological deficits 4 and 24 h after MCAo (4 h after MCAo: all P < 0.01 versus vehicle-treated rats at 5, 15, and 50 mg/kg of taurine; 24 h after MCAo: P < 0.05, 0.01 and 0.01 versus vehicle-treated rats at 5, 15, and 50 mg/kg of taurine, respectively). However, 1 or 2.5 mg/kg of taurine just had trend towards improving neurological function (see Fig. 1A).
Dose–response of taurine to focal cerebral ischemia. The rats received 2-h ischemia and then reperfused. Vehicle or various dose of taurine was injected intravenously at 1 h after ischemia. (A) Dose–response of taurine to neurological deficits (mean ± SEM, n = 15, *P < 0.05 and **P < 0.01 versus vehicle). (B) The infarct zone was displayed by TTC staining in vehicle- or various dose of taurine-treated rats. (C) The bar graph reflected the infarct volumes from TTC staining in various groups (mean ± SEM, n = 14, *P < 0.01 versus vehicle)
The dose–response of taurine to infarct volume is illustrated in Fig. 1B and C. A 2-h ischemia following 22-h reperfusion resulted in an infarct of 218 ± 11 mm3 in vehicle-treated rats. Taurine treatment at the dose of 5, 15, or 50 mg/kg decreased infarct volume significantly (all P < 0.01 versus vehicle-treated rats at 5, 15, or 50 mg/kg of taurine), however, taurine at the dose of 1 or 2.5 mg/kg had tendency to decrease the infarct volume.
Effects of Taurine on Calpastatin Protein Levels and Activities
The calpastatin protein levels in cytosolic fraction were determined by Western blot analysis. As illustrated in Fig. 2A and B, the calpastatin protein levels in penumbra 24 h after MCAo in vehicle-treated rats had no statistical significance compared with sham-operated rats, however, taurine treatment enhanced the calpastatin protein levels significantly (P < 0.05 versus vehicle-treated rats).
Effects of taurine on calpastatin protein levels and activities in cytosolic fractions of penumbras 24 h after MCAo in rats. The rats received 2-h ischemia and then reperfused. Taurine or saline was administrated intravenously 1 h after ischemia. (A) Western blot analysis using anti-calpastatin antibody. (B) The bar graph reflected the densitometric data from the experiment of calpastatin Western blot analysis (mean ± SEM, n = 8, *P < 0.05 versus vehicle). (C) The bar graph reflected the calpastatin activities through determining the inhibition of purified m-calpain by samples (mean ± SEM, n = 12, *P < 0.01 versus vehicle)
In order to determine whether the changes in calpastatin protein levels had functional significance, the calpastatin activities were determined through evaluating the inhibition of the purified m-calpain by the heat-treated sample. The calpastatin activities in penumbra 24 h after MCAo in vehicle-treated rats decreased compared with sham-operated rats, but it did not reach statistical significance. Taurine treatment increased the calpastatin activities significantly (P < 0.01 versus vehicle-treated rats, Fig. 2C).
Effects of Taurine on Calpain Activities
The μ- and m-calpain activities were determined by means of casein zymography. As shown in Fig. 3A and B, the μ- and m-calpain activities in penumbra 24 h after MCAo in vehicle-treated rats increased significantly (μ-calpain: P < 0.05 versus sham-operated rats. m-calpain: P < 0.01 versus sham-operated rats). Taurine treatment reduced m-calpain activities significantly (P < 0.05 versus vehicle-treated rats), however, it had no significant effect on μ-calpain activities.
Effects of taurine on μ- and m-calpain activities in cytosolic fractions of penumbras 24 h after MCAo in rats. The rats received 2-h ischemia and then reperfused. Taurine or saline was administrated intravenously 1 h after ischemia. (A) Casein zymography of μ- and m-calpain. (B) The bar graph reflected the densitometric data from the casein zymography (mean ± SEM, n = 12, μ-calpain: *P < 0.05 versus sham, m-calpain: *P < 0.01 versus sham, # P < 0.05 versus vehicle)
Effects of Taurine on Caspase-3 Activities
The caspase-3 activities in cytosolic fractions were measured through determining the cleavage of fluorescent substrate ac-DEVD-AFC. The caspase-3 activities in penumbra 24 h after MCAo in vehicle-treated rats increased significantly (P < 0.05 versus sham-operated rats), and taurine treatment reduced the caspase-3 activities significantly (P < 0.05 versus vehicle-treated rats, see Fig. 4).
Effects of taurine on caspase-3 activities in cytosolic fractions of penumbras 24 h after MCAo. The rats received 2-h ischemia and then reperfused. Taurine or saline was administrated intravenously 1 h after ischemia. Fluorescent arbitrary units were converted into picomoles of AFC released per hour and milligrams of protein (mean ± SEM, n = 8. *P < 0.05 versus sham, # P < 0.05 versus vehicle)
Effects of Taurine on MAP-2 and αII-spectrin Levels
The MAP-2 and αII-spectrin protein levels in cytosolic fractions were determined by Western blot analysis. As illustrated in Fig. 5A–D, the MAP-2 and αII-spectrin protein levels in penumbra 24 h after MCAo decreased significantly in vehicle-treated rats (MAP-2: P < 0.05 versus sham-operated rats; αII-spectrin: P < 0.01 versus sham-operated rats), indicating the degradation of MAP-2 and αII-spectrin. Taurine treatment markedly increased the MAP-2 and αII-spectrin protein levels, indicating the reduction of the degradation of MAP-2 and αII-spectrin by taurine (both P < 0.01 versus vehicle-treated rats).
Effects of taurine on MAP-2 and αII-spectrin levels in cytosolic fractions of penumbras 24 h after MCAo in rats. The rats received 2-h ischemia and then reperfused. Taurine or saline was administrated intravenously 1 h after ischemia. (A) Western blot analysis using anti-MAP-2 antibody. (B) Western blot analysis using anti-αII-spectrin antibody. (C) The bar graph reflected the densitometric data from the experiment of MAP-2 Western blot (mean ± SEM, n = 8, *P < 0.05 versus sham, # P < 0.01 versus vehicle). (D) The bar graph reflected the densitometric data from the experiment of αII-spectrin Western blot (mean ± SEM, n = 8, *P < 0.01 versus sham, # P < 0.01 versus vehicle)
Effect of Taurine on Apoptotic Cell Death
The anatomic distribution of frontoparietal cortex motor area (cortical penumbra) and medial part of striatum (striatal penumbra) was shown in Fig. 6A (Memezawa et al. 1992). The apoptotic cell death was determined by TUNEL staining, and the results were illustrated in Fig. 6B–H. These TUNEL staining positive cells were densely labeled in the nuclei and showed morphologic signs of apoptosis. In the right frontoparietal cortex motor area (cortical penumbra) and medial part of striatum (striatal penumbra), a large number of cells showed a strong TUNEL staining in the vehicle-treated rats, while TUNEL staining was negative in sham-operated rats. Treatment with taurine significantly reduced the TUNEL staining positive score in the right frontoparietal cortex motor area and medial part of striatum 24 h after MCAo (both P < 0.05 versus vehicle-treated rats).
The representative feature of TUNEL-positive cells in the frontoparietal cortex motor area (cortical penumbra, B–D) and medial part of striatum (striatal penumbra, E–G) 24 h after MCAo in rats (Original magnification 400×). The rats received 2-h ischemia and then reperfused. Taurine or saline was administrated intravenously 1 h after ischemia. (A) Schematic representation of the distribution of neuronal damage in rat brain after transient focal ischemia delineated by TUNEL. Two areas subjected to analysis of TUNEL are illustrated: FPCM, frontoparietal cortex motor area (cortical penumbra); MS, medial part of striatum (striatal penumbra). (B and E) Sham-operated rats. (C and F) Vehicle-treated rats. (D and G) Taurine-treated rats. (H) The bar graph reflected the TUNEL positive staining score in each group (mean ± SEM, n = 9, *P < 0.05 versus vehicle)
Discussion
In the present study, we first investigate the dose–response of taurine to transient focal cerebral ischemia, and our results reveal that taurine treatment 1 h after ischemia improves neurological functions and decreases infarct volume in a dose-dependent manner, and taurine in dose of 5, 15, or 50 mg/kg has marked protection. Furthermore, we focus our attentions on the effects of 50 mg/kg of taurine on actions of calpain/calpastatin and caspase-3 after focal cerebral ischemia, and find that it reduces the m-calpain and caspase-3 activities, enhances the calpastatin protein levels and activities, suppresses the degradation of MAP-2 and αII-spectrin, and mitigates apoptotic cell death in penumbra. These results provide convincing evidences that this endogenous amino acid exerts neuroprotective effects against focal ischemia when administrated exogenously, and suggest that one of its protective mechanisms is blocking the m-calpain- and caspase-3-mediated apoptotic cell death pathways.
Taurine as a neurotransmitter, neuromodulator, and membrane stabilizer is used for experimental therapy against neuronal damages caused by neurotoxical substances, hypoxia, epilepsy, etc. (Birdsall 1998). Taurine has been reported to antagonize the seizures induced by different factors. For example, taurine suppresses the onset of convulsions caused by hypoxia in a dose-dependent manner, and 50 mg/kg of taurine (intraperitoneal injection) is most effective, but 100 mg/kg adversely affects its anti-convulsant action (Sanberg and Willow 1980), and all the taurine doses between 1 and 10 mg/kg effectively reduce the incidence of seizure due to cobalt, with 10 mg/kg giving the best results (Carruthers-Jones and van Gelder 1978). In mice seizure model induced by 4-aminopyridine, taurine at dose of 2.6 mg/kg (intraperitoneal injection) increases the latency of clonic seizures, reduces the incidence of tonic seizures and the postconvulsive mortality (Pasantes-Morales and Arzate 1981), and in the mice model of epileptic seizure caused by parenteral injection of kainic acid, taurine (43 mg/kg, subcutaneous injection) suppresses epileptic seizure (El Idrissi et al. 2003). These data indicate that taurine may protect brain against focal cerebral ischemia with a broad range of doses. Therefore, in this study, we evaluate the protection of 1, 2.5, 5, 15, or 50 mg/kg of taurine against focal cerebral ischemia, and find that taurine treatment at the dose of 5, 15, or 50 mg/kg have marked therapeutic effects, however, taurine at the dose of 1 or 2.5 mg/kg only has tendency to reduce ischemic damage. Our data demonstrate the dose-dependent neuroprotection of taurine against transient focal cerebral ischemia, and the range of valid dose is similar to that reported in above literatures. In contrast to our data, it has been reported that treatment with 100 mg/kg of taurine intraperitoneally at the onset of ischemia has a trend towards reducing infarct volume, however, it does not reach statistical significance (Shuaib 2003). The somewhat difference compared to our results possibly may be due to the different experimental conditions, such as animal model, timing and route of administration, particularly, a single different dose to be used without a dose–response study. On the other hand, it may be due to the biphasic action of taurine. For example, taurine improves the recovery of neuronal function following cerebral hypoxia in vitro (Schurr et al. 1987) and suppresses the onset of convulsions caused by hypoxia in vivo in a dose-dependent manner (Sanberg and Willow 1980), however, taurine administrated at relative high dose (5 mM in vitro, Schurr et al. 1987 and 100 mg/kg in vivo, Sanberg and Willow 1980) has no protection.
As a neuroprotectvie agent, taurine must pass through blood–brain barrier (BBB) and enter into brain under neuropathological conditions. Some researchers have found that the radioactive taurine increases in brain after radiolabeled taurine is administrated systemically (Urquhart et al. 1974; Pasantes-Morales and Arzate 1981). In vitro a Na+ and Cl− gradient-dependent transport system for taurine is identified in both the luminal and the anti-luminal membranes of bovine brain capillary endothelial cells, and the carrier-mediated transport found by in vitro experiments is confirmed to function for the translocation of the taurine molecule from the vascular space into the brain (Tamai et al. 1995). Moreover, a linear increase of taurine concentration in rat brain after intraperitoneal injection has been reported (Lallemand and De Witte 2004). In addition, taurine has been used with varying degrees of success in the experimental and clinical therapy of epilepsy and other seizure disorders (Sanberg and Willow 1980; Durelli and Mutani 1983; Birdsall 1998; El Idrissi et al. 2003). These researches provide convincing evidences that taurine may cross BBB and reach the ischemic area to exert its cytoprotection when it is administrated intravenously after brain ischemia.
In regard to the mechanism of taurine against brain ischemia, it is more complicated since both pathophysiological process of brain ischemia and cytoprotective property of taurine are more complex. In the study, we primarily investigate whether taurine exerts neuroprotection through blocking the actions of calpain and caspase-3 during focal cerebral ischemia, as taurine is a modulator of intracellular calcium homeostasis (Foos and Wu 2002; El Idrissi and Trenkner 2003).
It is well known that caspase-3 and calpain, two cysteine proteases, play central roles in neuronal death during focal cerebral ischemia (Lipton 1999). Caspase-3 acts as an executioner of the apoptosis, and the involvement of caspase-3 in apoptotic processes after ischemia is supported by the observation that treatment with caspase-3 inhibitors reduces ischemia-induced brain damage (Lipton 1999; Fink et al. 1998). Calpain, a calcium-dependent neutral protease, is activated by intracellular calcium during focal cerebral ischemia, which has been demonstrated through investigating the proteolysis of substrates and the protection of calpain inhibitors (Minger et al. 1998; Lipton 1999; Kambe et al. 2005). More recently, some reports have shown the involvement of calpain in the apoptotic cell death after brain ischemia through causing degradation of substrates such as spectrin, calcineurin (Rami et al. 2003; Shioda et al. 2006).
MAP-2 and spectrin, two cytoskeletal proteins, play important roles in maintenance of structural integrity, neuronal stability, normal functions and plasticity of neurons through interacting with neuronal cytoskeleton (Fitzpatrick et al. 1996; Ludin and Matus 1993; Goodman and Zagon 1986; Bennett 1990). Therefore, possible functional consequences of neuronal cytoskeletal loss could result in the disruption of structural integrity and stability of neurons, disturbances of axonal transport, neuronal dysfunction, leading to neuronal death. MAP-2 is a preferred substrate for calpain, and spectrin is very susceptible to calpain and to caspase-3-like proteases (Johnson et al. 1991; Nath et al. 1996). It has been reported that cytoskeletal degradation, represented by the loss of MAP-2 or spectrin shown by immunochemical methods, is a sensitive indicator of neuronal damage after focal cerebral ischemia (Dawson and Hallenbeck 1996; Kambe et al. 2005), and the degradation of MAP-2 or spectrin is involved in the cell apoptosis (Williams et al. 2003; Fifre et al. 2006).
Our results reveal that taurine treatment reduces the activities of m-calpain and caspase-3, suppresses the degradation of MAP-2 and αII-spectrin, and alleviates TUNEL staining positive score in penumbra, although it has no effect on μ-calpain activities. These results indicate that taurine may protect brain against transient focal cerebral ischemia through down-regulating m-calpain and caspase-3 activation, maintaining structural integrity and stability of cells, terminally, mitigating the apoptotic cell death. Our results provide multiple evidences, including the activation of proteases, degradation of substrates and apoptotic cell death, to demonstrate the neuroprotection of taurine, and suggest that the blockade of m-calpain- and caspase-3-mediated, but not μ-calpain-mediated apoptotic cell death pathway may be one of the neuroprotective mechanisms of taurine after transient focal cerebral ischemia. Figure 7 summarizes the calpain- and caspase-3-mediated apoptotic cell death pathways in penumbra after transient focal cerebral ischemia and the actions of taurine.
It is believed that intracellular calcium overload is induced through the opening of ionic channels gated by receptors and voltage, the release of calcium from intracellular calcium storage pools, and other mechanisms after brain ischemia. Subsequently, it initiates a series of intracellular events that impact the development of tissue damage profoundly, such as activation of proteolytic enzymes and endonucleases, production of reactive oxygen species (ROS), release of excitatory amino acids (EAAs), and apoptosis (Kristian and Siesjö 1998; Lipton 1999; Mattson et al. 2000). A number of reports have demonstrated the regulation of taurine on intracellular calcium homeostasis (Huxtable 1992; Foos and Wu 2002; El Idrissi and Trenkner 2003). Therefore, it is logical that taurine may block the calpain- or caspase-3-mediated ischemic cell death pathway through suppressing the intracellular calcium overload after focal cerebral ischemia, since the increases in intracellular calcium can directly activate calpain (Goll et al. 2003) and induce caspase-3-like activity by regulating the release of cytochrome c from mitochondria (Juin et al. 1998). This hypothesis has been demonstrated by our data that taurine down-regulating m-calpain and caspase-3 activation, although we do not directly investigate the effects of taurine on intracellular calcium in this study. The different effects of taurine on μ- and m-calpain activation may be due to the calcium concentration required for the activation of μ- and m-calpain, as μ-calpain which needs micromolar calcium for activation, and m-calpain which needs millimolar concentration of calcium (Goll et al. 2003).
As a neuromodulator, neurotransmitter, and membrane stabilizer, taurine may reduce the intracellular calcium overload and exert its neuroprotection against focal cerebral ischemia through one or more of a number of means: (1) by suppressing EAA-induced excitotoxicity through acting upon nerve terminals to reduce the release of EAAs (Kamisaki et al. 1996), and acting upon GABA-A receptors to enhance chloride currents (El Idrissi and Trenkner 2004), as membrane depolarization due to EAAs is contributed to the calcium influx through opening the ionic channels (Kristian and Siesjö 1998; Lipton 1999; Mattson et al. 2000); (2) by modulating mitochondrial calcium homeostasis and improve mitochondrial function (Foos and Wu 2002; El Idrissi and Trenkner 2003), since taurine may reduce the release of calcium from intracellular calcium storage pools through enhancing the sequestration of mitochondrial calcium (Palmi et al. 1999), and inhibiting the reversal of sodium/calcium exchangers (Foos and Wu 2002); and (3) by affecting the calcium influx through voltage-gated calcium channels (Huxtable 1992), for taurine may maintain the structural integrity of the membrane (Moran et al. 1987) and reduce the membrane damage due to ROS (Nakamura et al. 1993; Pokhrel and Lau-Cam 2000).
Calpastatin is an endogenous calpain inhibitor. It has been shown that calpastatin could be degraded by calpain and caspase-3 during apoptosis or cerebral hypoxia-ischemia (Blomgren et al. 1999; Wang et al. 1998), and an increase in expression of calpastatin is neuroprotective to several neurological diseases, such as Parkinson’s disease and cerebral ischemia (Rami et al. 2003; Han et al. 2006; Crocker et al. 2003). On the other hand, taurine is an endogenous neuroprotectant (Schurr et al. 1987; Wu et al. 1994; Saransaari and Oja 2000), and it reduces ischemic brain damage, as shown in the present study. Therefore, we are particularly interested in the effects of taurine on calpastatin during transient focal cerebral ischemia. Our study reveals that taurine treatment increases calpastatin protein levels and activities in penumbra 24 h after MCAo, suggesting that exogenous administration of taurine strengthens the endogenous protective mechanism of calpastatin, which is supported by the study that the expression of calpastatin mRNA and protein is induced by cerebral hypoxia-ischemia in immature rats (Blomgren et al. 1999). The calpastatin protein levels and activities in vehicle-treated rats have no statistical significance compared with sham-operated rats, which could be explained by (1) calpastatin is degraded by calpain and caspase-3 (Wang et al. 1998; Blomgren et al. 1999), as both enzymes are activated during cerebral ischemia (Lipton 1999), and (2) the levels of taurine released from cells during transient focal cerebral ischemia are not enough to initiate the calpastatin-mediated endogenous neuroprotection. However, our results do not exclude the increase in calpastatin expression during focal cerebral ischemia. The mechanism by which taurine increases calpastatin actions is unclear, which might be related to the up-regulation of gene expression by taurine (Park et al. 2006), or down-regulation of calpain and caspase-3 activation by taurine, as demonstrated in this study. Further study is needed to determine the mechanism by which taurine up-regulates the calpastatin actions during focal cerebral ischemia.
The taurine concentration is very high in brain. The intracellular:extracellular concentration ratio has been estimated at 600 in neurons (Jacobson and Hamberge 1984), and it is a functional equilibrium between active uptake, passive release, and biosynthesis from cysteine. Taurine is a key regulator of intracellular homeostasis or enantiostasis (Huxtable 1992, 2000; Michalk et al. 1996). Under cell-damaging conditions, the release of taurine is increased, and the uptake of taurine is inhibited (Saransaari and Oja 2000). During focal cerebral ischemia, the level of taurine in extracellular fluid increases and remains elevated, though somewhat attenuated, throughout the subsequent reperfusion phase (Lo et al. 1998). The increase in the extracellular level of taurine under cell-damaging conditions including brain ischemia may constitute an important endogenous protective mechanism against neuronal damage (Saransaari and Oja 2000). Meanwhile, the decrease in intracellular concentration of taurine and the loss of intracellular:extracellular concentration ratio due to the release of taurine may result in the disorder of intracellular homeostasis or enantiostasis, leading to neuronal damage. Hence, the release of taurine may be an obligatory self-protective mechanism under ischemic stress. It is reasonable that exogenous administration of adequate amount of taurine after brain ischemia may be contributed to the recovery of intracellular homeostasis or enantiostasis and the reduction of ischemic damage. This speculation has been supported by our results in this study. Further studies are needed to elucidate the detailed mechanisms of taurine against focal cerebral ischemia, which are beneficial to understanding the modulation of taurine on intracellular homeostasis or enantiostasis after focal cerebral ischemia.
In summary, this study has demonstrated that taurine in dose-dependent manner protects brain against transient focal cerebral ischemia in rats, and one of protective mechanisms of taurine against transient focal cerebral ischemia is blocking the m-calpain- and caspase-3-mediated apoptotic cell death pathway. These data provide evidences to support the hypothesis that exogenous administration of taurine may reduce ischemic brain damage through modulating intracellular homeostasis or enantiostasis after focal cerebral ischemia.
References
Ashwal S, Tone B, Tian HR, Cole DJ, Pearce WJ (1998) Core and penumbral nitric oxide synthase activity during cerebral ischemia and reperfusion. Stroke 29:1037–1047
Benchoua A, Guegan C, Couriaud C, Hosseini H, Sampaio N, Morin D, Onteniente B (2001) Specific caspase pathways are activated in the two stages of cerebral infarction. J Neurosci 21:7127–7134
Bennett V (1990) Spectrin: a structural mediator between diverse plasma membrane proteins and the cytoplasm. Curr Opin Cell Biol 2:51–56
Birdsall TC (1998) Therapeutic applications of taurine. Altern Med Rev 3:128–136
Blomgren K, Hallin U, Andersson AL, Puka-Sundvall M, Bahr BA, McRae A, Saido TC, Kawashima S, Hagberg H (1999) Calpastatin is up-regulated in response to hypoxia and is a suicide substrate to calpain after neonatal cerebral hypoxia-ischemia. J Biol Chem 274:14046–14052
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Carruthers-Jones DI, van Gelder NM (1978) Influence of taurine dosage on cobalt epilepsy in mice. Neurochem Res 3:115–123
Cheng YD, Al-Khoury L, Zivin JA (2004) Neuroprotection for ischemic stroke: two decades of success and failure. NeuroRx 1:36–45
Crocker SJ, Smith PD, Jackson-Lewis V, Lamba WR, Hayley SP, Grimm E, Callaghan SM, Slack RS, Melloni E, Przedborski S, Robertson GS, Anisman H, Merali Z, Park DS (2003) Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson’s disease. J Neurosci 23:4081–4091
Davoli MA, Fourtounis J, Tam J, Xanthoudakis S, Nicholson D, Robertson GS, Ng GY, Xu D (2002) Immunohistochemical and biochemical assessment of caspase-3 activation and DNA fragmentation following transient focal ischemia in the rat. Neuroscience 115:125–136
Dawson DA, Hallenbeck JM (1996) Acute focal ischemia-induced alterations in MAP2 immunostaining: description of temporal changes and utilization as a marker for volumetric assessment of acute brain injury. J Cereb Blood Flow Metab 16:170–174
Dirnagl U, Simon RP, Hallenbeck JM (2003) Ischemic tolerance and endogenous neuroprotection. Trends Neurosci 26:248–254
Durelli L, Mutani R (1983) The current status of taurine in epilepsy. Clin Neuropharmacol 6:37–48
El Idrissi A, Trenkner E (2003) Taurine regulates mitochondrial calcium homeostasis. Adv Exp Med Biol 526:527–536
El Idrissi A, Trenkner E (2004) Taurine as a modulator of excitatory and inhibitory neurotransmission. Neurochem Res 29:189–197
El Idrissi A, Messing J, Scalia J, Trenkner E (2003) Prevention of epileptic seizures by taurine. Adv Exp Med Biol 526:515–525
Fifre A, Sponne I, Koziel V, Kriem B, Yen Potin FT, Bihain BE, Olivier JL, Oster T, Pillot T (2006) Microtubule-associated protein MAP1A, MAP1B, and MAP2 proteolysis during soluble amyloid beta-peptide-induced neuronal apoptosis. Synergistic involvement of calpain and caspase-3. J Biol Chem 281:229–240
Fink K, Zhu J, Namura S, Shimizu-Sasamata M, Endres M, Ma J, Dalkara T, Yuan J, Moskowitz MA (1998) Prolonged therapeutic window for ischemic brain damage caused by delayed caspase activation. J Cereb Blood Flow Metab 18:1071–1076
Fitzpatrick MO, Dewar D, Teasdale GM, Graham DI (1996) The neuronal cytoskeleton: an insight for neurosurgeons. Brit J Neurosurg 10:483–487
Foos TM, Wu JY (2002) The role of taurine in the central nervous system and the modulation of intracellular calcium homeostasis. Neurochem Res 27:21–26
Gavrieli Y, Sherman Y, Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119:493–501
Gladstone DJ, Black SE, Hakim AM, Heart and Stroke Foundation of Ontario Centre of Excellence in Stroke Recovery (2002) Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke 33:2123–2136
Goll DE, Thompson VF, Li H, Wei W, Cong J (2003) The calpain system. Physiol Rev 83:731–801
Goodman SR, Zagon IS (1986) The neural cell spectrin skeleton: a review. Am J Physiol 250:C347–C360
Han F, Shirasaki Y, Fukunaga K (2006) 3-[2-[4-(3-Chloro-2-methylphenylmethyl)-1-piperazinyl]ethyl]-5,6-dimethoxy-1-(4-imidazolylmethyl)-1H-indazole dihydro-chloride 3.5 hydrate (DY-9760e) is neuroprotective in rat microsphere embolism: role of the cross-talk between calpain and caspase-3 through calpastatin. J Pharmacol Exp Ther 317:529–536
Hoffman GE, Merchenthaler I, Zup SL (2006) Neuroprotection by ovarian hormones in animal models of neurological disease. Endocrine 29:217–232
Huxtable RJ (1992) Physiological action of taurine. Physiol Rev 72:101–163
Huxtable RJ (2000) Expanding the circle 1975–1999: sulfur biochemistry and insights on the biological functions of taurine. Adv Exp Med Biol 483:1–25
Jacobson I, Hamberger A (1984) Veratridine-induced release in vivo and in vitro of amino acids in the rabbit olfactory bulb. Brain Res 299:103–112
Johnson GV, Litersky JM, Jope RS (1991) Degradation of microtubule-associated protein 2 and brain spectrin by calpain: a comparative study. J Neurochem 56:1630–1638
Juin P, Pelletier M, Oliver L, Tremblais K, Gregoire M, Meflah K, Vallette FM (1998) Induction of a caspase-3-like activity by calcium in normal cytosolic extracts triggers nuclear apoptosis in a cell-free system. J Biol Chem 273:17559–17564
Kambe A, Yokota M, Saido TC, Satokata I, Fujikawa H, Tabuchi S, Kamitani H, Watanabe T (2005) Spatial resolution of calpain-catalyzed proteolysis in focal cerebral ischemia. Brain Res 1040:36–43
Kamisaki Y, Wada K, Nakamoto K, Itoh T (1996) Release of taurine and its effects on release of neurotransmitter amino acids in rat cerebral cortex. Adv Exp Med Biol 403:445–454
Kristian T, Siesjö BK (1998) Calcium in ischemic cell death. Stroke 29:705–718
Labiche LA, Grotta JC (2004) Clinical trials for cytoprotection in stroke. NeuroRx 1:46–70
Lallemand F, De Witte P (2004) Taurine concentration in the brain and in the plasma following intraperitoneal injections. Amino Acids 26:111–116
Lin TN, He YY, Wu G, Khan M, Hsu CY (1993) Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke 24:117–121
Lipton P (1999) Ischemic cell death in brain neurons. Physiol Rev 79:1431–1568
Lizasoain I, Cardenas A, Hurtado O, Romera C, Mallolas J, Lorenzo P, Castillo J, Moro MA (2006) Targets of cytoprotection in acute ischemic stroke: present and future. Cerebrovasc Dis 21(Suppl 2):1–8
Lo EH, Pierce AR, Matsumoto K, Kano T, Evans CJ, Newcomb R (1998) Alterations in K+ evoked profiles of neurotransmitter and neuromodulator amino acids after focal ischemia-reperfusion. Neuroscience 83:449–458
Louzada PR, Lima AC, Mendonca-Silva DL, Noel F, De Mello FG, Ferreira ST (2004) Taurine prevents the neurotoxicity of beta-amyloid and glutamate receptor agonists: activation of GABA receptors and possible implications for Alzheimer’s disease and other neurological disorders. FASEB J 18:511–518
Ludin B, Matus A (1993) The neuronal cytoskeleton and its role in axonal and dendritic plasticity. Hippocampus 3:61–71
Mattson MP, Culmsee C, Yu ZF (2000) Apoptotic and antiapoptotic mechanisms in stroke. Cell Tissue Res 301:173–187
Memezawa H, Minamisawa H, Smith ML, Siesjö BK (1992) Ischemic penumbra in a model of reversible middle cerebral artery occlusion in the rat. Exp Brain Res 89:67–78
Michalk DV, Wingenfeld P, Licht C, Ugur T, Siar LF (1996) The mechanisms of taurine mediated protection against cell damage induced by hypoxia and reoxygenation. Adv Exp Med Biol 403:223–232
Minger SL, Geddes JW, Holtz ML, Craddock SD, Whiteheart SW, Siman RG, Pettigrew LC (1998) Glutamate receptor antagonists inhibit calpain-mediated cytoskeletal proteolysis in focal cerebral ischemia. Brain Res 81:181–199
Moran J, Salazar P, Pasantes-Morales H (1987) Effect of tocopherol and taurine on membrane fluidity of retinal rod outer segments. Exp Eye Res 45:769–776
Nakamura T, Ogasawara M, Koyama I, Nemoto M, Yoshida T (1993) The protective effect of taurine on the biomembrane against damage produced by oxygen radicals. Biol Pharm Bull 16:970–972
Nath R, Raser KJ, Stafford D, Hajimohammadreza I, Posner A, Allen H, Talanian RV, Yuen P, Gilbertsen RB, Wang KK (1996) Non-erythroid alpha-spectrin breakdown by calpain and interleukin 1 beta-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem J 319:683–690
Palmi M, Youmbi GT, Fusi F, Sgaragli GP, Dixon HB, Frosini M, Tipton KF (1999) Potentiation of mitochondrial Ca2+ sequestration by taurine. Biochem Pharmacol 58:1123–1131
Park SH, Lee H, Park KK, Kim HW, Park T (2006) Taurine-responsive genes related to signal transduction as identified by cDNA microarray analyses of HepG2 cells. J Med Food 9:33–41
Pasantes-Morales H, Arzate ME (1981) Effect of taurine on seizures induced by 4-aminopyridine. J Neurosci Res 6:465–474
Pokhrel PK, Lau-Cam CA (2000) Protection by taurine and structurally related sulfur-containing compounds against erythrocyte membrane damage by hydrogen peroxide. Adv Exp Med Biol 483:411–429
Rami A, Volkmann T, Agarwal R, Schoninger S, Nurnberger F, Saido TC, Winckler J (2003) Beta2-Adrenergic receptor responsiveness of the calpain–calpastatin system and attenuation of neuronal death in rat hippocampus after transient global ischemia. Neurosci Res 47:373–382
Raser KL, Posner A, Wamg KKW (1995) Casein zymography: a method to study μ-calpain, m-calpain and their inhibitory agents. Arch Biochem Biophys 319:211–216
Salvesen GS, Dixit VM (1997) Caspases: intracellular signaling by proteolysis. Cell 91:443–446
Sanberg PR, Willow M (1980) Dose-dependent effects of taurine on convulsions induced by hypoxia in the rat. Neurosci Lett 16:297–300
Saransaari P, Oja SS (2000) Taurine and neural cell damage. Amino Acids 19:509–526
Schmid-Elsaesser R, Zausinger S, Hungerhuber E, Baethmann A, Reulen, HJ (1998) A critical reevalution of the intraluminal thread model of focal cerebral ischemia. Evidence of inadvertent premature reperfusion and subarachnoid hemorrhage in rats by laser-Doppler flowmetry. Stroke 29:2162–2170
Schuller-Levis GB, Park E (2004) Taurine and its chloramine: modulators of immunity. Neurochem Res 29:117–126
Schurr A, Tseng MT, West CA, Rigor BM (1987) Taurine improves the recovery of neuronal function following cerebral hypoxia: an in vitro study. Life Sci 40:2059–2066
Shioda N, Moriguchi S, Shirasaki Y, Fukunaga K (2006) Generation of constitutively active calcineurin by calpain contributes to delayed neuronal death following mouse brain ischemia. J Neurochem 98:310–320
Shuaib A (2003) The role of taurine in cerebral ischemia: studies in transient forebrain ischemia and embolic focal ischemia in rodents. Adv Exp Med Biol 526:421–431
Sorimachi Y, Harada K, Saido TC, Ono T, Kawashima S, Yoshida K (1997) Downregulation of calpastatin in rat heart after brief ischemia and reperfusion. J Biochem(Tokyo) 122:743–748
Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR (1990) A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab 10:290–293
Tamai I, Senmaru M, Terasaki T, Tsuji A (1995) Na+- and Cl−-dependent transport of taurine at the blood–brain barrier. Biochem Pharmacol 50:1783–1793
Urquhart N, Perry TL, Hansen S, Kennedy J (1974) Passage of taurine into adult mammalian brain. J Neurochem 22:871–872
Wang KKW, Posmantur R, Nadimpalli R, Nath R, Mohan P, Nixon RA, Talanian RV, Keegan M, Herzog L, Allen H (1998) Caspase-mediated fragmentation of calpain inhibitor protein calpastatin during apoptosis. Arch Biochem Biophys 356:187–196
Williams ST, Smith AN, Cianci CD, Morrow JS, Brown TL (2003) Identification of the primary caspase 3 cleavage site in alpha II-spectrin during apoptosis. Apoptosis 8:353–361
Wu JY, Lin CT, Johansen FF, Liu JW (1994) Taurine neurons in rat hippocampal formation are relatively inert to cerebral ischemia. Adv Exp Med Biol 359:289–298
Yoshida K, Yamasaki Y, Kawashima S (1993) Calpain activity alters in rat myocardial subfractions after ischemia or reperfusion. Biochim Biophys Acta 1182:215–220
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, M., Xu, C. Neuroprotective Mechanism of Taurine due to Up-regulating Calpastatin and Down-regulating Calpain and Caspase-3 during Focal Cerebral Ischemia. Cell Mol Neurobiol 28, 593–611 (2008). https://doi.org/10.1007/s10571-007-9183-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-007-9183-8