Abstract
Stroke is one of the leading causes of mortality and disability worldwide. There is no effective treatment for stroke despite extensive research. Taurine is a free amino acid which is present at high concentrations in a range of organs including the brain, heart, and retina in mammalian systems. It had been shown that taurine can significantly increase cell survival under stroke conditions using both in vivo and in vitro models. Recently, we have found that several agents including granulocyte colony-stimulating factor (G-CSF), a stem cell enhancer and facilitator;S-methyl-N-diethylthiolcarbamate sulfoxide (DETC-MeSO), an NMDA receptor partial antagonist; sulindac, a potent antioxidant; and taurine, a neuroprotectant and calcium regulator, are effective in protecting against stroke-induced neuronal injury when used alone or in combination in both animal and tissue/cell culture models. In this chapter, we demonstrate that taurine can protect human neuroblastoma cells measured by ATP assay under conditions of hypoxia or oxygen/glucose deprivation (OGD). In addition, we found that taurine exerts its protective function by suppressing the OGD-induced upregulation of endoplasmic reticulum (ER) stress markers and proapoptotic proteins. A model depicting the mode of action of taurine in protecting neuroblastoma cells under OGD conditions is presented.
Po-Chih Chen and Chunliu Pan contributed equally to this work.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Endoplasmic Reticulum Stress
- Oxygen Glucose Deprivation
- Hypoxia Group
- Taurine Treatment
- Oxygen Glucose Deprivation Group
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Taurine, 2-aminoethanesulfonic acid, is one of the most abundant amino acids in mammals and is found at high concentrations in various tissues, including brain, heart, and kidney (for review, Huxtable1992). Taurine plays important physiological functions in the brain including serving as a neurotransmitter/modulator and trophic factor, and in neuronal migration in the cerebellum and visual cortex (for review, Wu and Prentice2010).
In addition, taurine has been shown to have protective functions in various systems including the nervous system, heart, lung, and kidney. presumably through its regulation of calcium homeostasis and its antiapoptotic property (Takatani et al.2004; Wu et al. 2010). In animal studies, taurine had been shown to be effective in reducing the infarct size in a rat stroke model (Ghandforoush-Sattari et al.2011). Taurine has also been applied clinically in several disorders, including cardiovascular diseases, metabolic disease, alcoholism, retinal degeneration, and hepatic and renal diseases (Birdsall1998; Bidri and Choay2003).
In our previous study, we have demonstrated that taurine exerts a protective effect on PC 12 cells under oxidative stress (Pan et al.2010). Here we report that taurine also has protective effects on a neuroblastoma cell line under hypoxia or oxygen/glucose deprivation conditions.
2 Methods
The neuroblastoma SH-SY5Y cell line, F-12 media, EMEM media withl-glutamine, and trypsin-EDTA solution were purchased from ATCC (Manassas, VA, USA). Fetal bovine serum and penicillin–streptomycin were purchased from Sigma (St. Louis, MO, USA).
Rabbit anti-ATF4, rabbit anti-XBP-1, rabbit, rabbit anti-PUMA, and rabbit anti-IRE1 antibodies were purchased from Abcam (Cambridge, MA, USA). RIPA buffer was purchased from Thermo Scientific (Rockford, IL, USA). Rabbit anti-p-eIF2α antibody, was purchased from Cell Signaling Technology (Boston, MA, USA). Rabbit anti-GADD34 antibody and secondary mouse and rabbit antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Adenosine 5′-triphosphate (ATP) Bioluminescent Assay Kit was purchased from Promega (Madison, WI, USA).
2.1 Cell Culture
SH-SY-5Y human neuroblastoma cells were maintained at 37°C 5% CO2in complete medium (Eagle’s Minimum Essential Medium (EMEM) 44.5% and F12 medium 44.5%, fetal bovine serum to a final concentration of 10%, penicillin–streptomycin 1%). Cultured cells were plated in 6 (cell density 2 × 106) or 96-well dishes (5 × 105cell/ml). Dishes contained complete medium at 2 ml/well for 6-well dishes and 100 μl/well for 96-well dishes. After plating 1–2 days, the medium was replaced with incomplete medium (50% EMEM, 50%F12 medium plus 10 μM retinoic acid) to induce cell differentiation.
2.2 Glucose Deprivation
After 5–7 days in complete medium, cells were changed to medium without glucose (154 mM NaCl, 5.6 mM KCl, 2.3 mM CaCl2, 1.0 mM MgCl2, 3.6 mM NaHCO3, 5 mM Hepes, pH 7.2). Taurine was added to each well to a final concentration of 10 mM, and 1 h later, cells were subjected to 20 h of hypoxia.
2.3 Hypoxia/Reoxygenation
To generate hypoxic conditions, neuroblastoma SH-SY5Y cells in 6- or 96-well plates were placed in the hypoxia chamber with oxygen levels maintained at 0.3–0.4%. The level of oxygen was continuously monitored using an oxygen electrode. Neuroblastoma cells with or without taurine treatment were subjected to 20 h of hypoxia. Reoxygenation was performed by removing cultured plates from the hypoxic chamber and transferring them into normal culture incubator for 20 h.
2.4 Measurement of Cell Viability: ATP Assay
Neuroblastoma cells in 96-well plates were incubated with and without 10 mM taurine and then exposed to hypoxic conditions for 24 h to induce cell death. ATP solution (Promega) was added to each well, and cells were incubated for 10 min, after which the amount of ATP was quantified by a luciferase reaction. The luminescence intensity was determined using a luminometer (SpectraMax, Molecular Devices) after transferring the lysate to a standard opaque wall multi-well plate. The ATP content was determined by running an internal standard and expressed as a percentage of untreated cells (control).
2.5 Western Blot Analysis
Neuroblastoma cells were lysed in RIPA buffer (25 mM Tris–HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) containing 1% (v/v) mammalian protease inhibitor cocktail from Sigma. Cellular proteins were separated on SDS-PAGE and then transferred to a nitrocellulose membrane. The membrane was blocked in blocking buffer (20 mM Tris–HCl, 150 mM NaCl, 0.1% Tween-20, 5% milk) for 1.5 h at room temperature. After blocking, the primary antibody was incubated for 1 h, followed by 1 h incubation with the appropriate HRP-conjugated secondary antibody at room temperature. Extensive washes with a blocking buffer were performed between each step. The protein immunocomplex was visualized by ECL detection reagents.
3 Results
3.1 Taurine Protects Neuroblastoma SH-SY5Y Cells Against Hypoxic Stress
Using the ATP assay it was found that hypoxia conditions elicited approximately a 50% decrease in viability compared to the control group. This decrease was substantially reversed in the 10 mM taurine-treated group which showed approximately 75% cell survival (Fig.14.1).
Effect of taurine on the viability of neuroblastoma cells under hypoxic conditions. The percentage of cell viability in the neuroblastoma SH-SY5Y cell line under hypoxic (0.3% O2, 20 h) conditions is shown (1normal control,2hypoxia,310 mM taurine + hypoxia). 10 mM taurine was administered by preincubation for 1 h followed by hypoxic exposure for 20 h
3.2 Taurine Restored the Expression of PUMA Under Oxygen/Glucose Deprivation Conditions
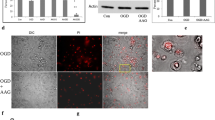
In the oxygen glucose deprivation (OGD) study, we analyzed the expression of PUMA (p53 upregulated modulator of apoptosis), a Bcl-2 family member originally identified in differential gene expression studies as a p53-inducible gene (Yu et al.2001). Two BH3-containing proteins are encoded from the puma gene, Puma-a and Puma-b, both of which are induced by p53, bind Bcl-2, and Bcl-xL; localize to the mitochondria; and promote cytochrome c release and apoptosis. Western blot analysis shows the expression of PUMA was markedly increased in the OGD group and decreased in the OGD plus taurine treatment group (Fig.14.2).
Effect of taurine on the expression of PUMA (p53 upregulated modulator of apoptosis) in neuroblastoma cells under oxygen/glucose deprivation (OGD) conditions. Neuroblastoma cells were treated with or without taurine under OGD conditions. Western blot analysis was conducted with an anti-PUMA antibody.Ccontrol,OGDoxygen/glucose deprivation,tau + OGDoxygen/glucose deprivation plus treatment with 10 mM taurine
3.3 Taurine Treatment Had No Significant Effect on the Levels of p-eIF2α ATF4 and GADD34 Expression Under Hypoxic Conditions
In hypoxic conditions, we analyzed levels of expression of phosphorylated-eukaryotic initiation factor 2α (p-eIF2α), a downstream component of the PERK pathway which plays a role in inhibition of protein synthesis. The results showed markedly increased levels of p-eIF2-alpha both with and without taurine treatment compared with the control group (Fig.14.3).
Effect of taurine on the expression of p-eIF2α, GADD 34, and ATF4 in neuroblastoma cells under hypoxic conditions. Neuroblastoma cells were preincubated with 10 mM taurine before with or without 20 h hypoxia condition followed by Western blot analysis. Phosphorylated-eukaryotic initiation factor 2α (p-eIF2α), growth arrest and DNA damage-inducible protein 34 (GADD 34), and activating transcription factor 4 (ATF4) revealed no difference between any of the groups.Ccontrol,tau + hypohypoxia with taurine treatment
Activating transcription factor 4 (ATF4), which is translated as a compensatory response during a block of expression of eIF-2-alpha, showed no significant change in either the hypoxia condition or hypoxia plus taurine condition. In a previous study, the autoregulatory loop in PKR-like endoplasmic reticulum kinase (PERK) phosphorylates eIF-2α and in turn inhibits protein synthesis and allows ATF4 translation, subsequently leading to growth arrest and DNA damage-inducible protein-34 (GADD34) increases under cellular stress (Ma and Hendershot2003). Our data showed p-eIF 2α was increased markedly both in the hypoxia group, compatible with conditions of cell stress, whereas GADD 34 expression was increased by 30% in the hypoxia group but not in the taurine plus hypoxia group compared with control.
3.4 Taurine Reversed the Increased Expression of XBP-1 and pIRE-1 Under Hypoxic Conditions
We further analyzed inositol-requiring kinase-1 (IRE-1), a ser/thr protein kinase that possesses endonuclease activity. IRE-1 is important for altering gene expression as a response to ER stress signals. IRE-1 senses and responds to unfolded proteins in the lumen of the endoplasmic reticulum via its N-terminal domain, leading to enzyme autoactivation. The active endoribonuclease domain induces splicing of X-box binding protein 1 (XBP-1) mRNA. XBP-1 is a transcription factor that has been shown to be the target of the endonuclease activity of IRE-1 in mammals (Yoshida et al.2003). Spliced XBP-1 then generates a new C-terminus, converting it into a potent unfolded-protein response (UPR) transcriptional activator and subsequently triggering growth arrest and apoptosis. The current results also demonstrated an increase in XBP-1 and p-IRE1 under hypoxic conditions. The increase in p-IRE1 and XBP-1 expression was reversed in the hypoxia plus taurine treatment condition (Fig.14.4).
Effect of taurine on the expression of p-IRE1 and XBP-1 in neuroblastoma cells under hypoxic conditions. Neuroblastoma cells were exposed to normoxic conditions or subjected to 20 h of hypoxia with or without a preexposure to 10 mM taurine. Cells were harvested for western blot analysis using antibodies to phosphorylated inositol-requiring kinase-1 (pIRE-1) and X-box binding protein 1 (XBP-1).Ccontrol,tau + hyphypoxia with taurine treatment
4 Discussion
Taurine has been shown to exert protective effects against neuronal damage by inhibiting the reverse mode Na+/Ca2+exchanger (Buddhala et al.2012) and by decreasing calcium influx through L-, P/Q-, and N-type voltage-gated calcium channels as well asN-methyl-d-aspartic acid (NMDA) receptors (Wu et al.2005).
Taurine treatment also decreases expression of caspase-3 and calpains and increases the ratio of levels of the antiapoptic protein Bcl-2 and proapoptotic protein Bax. In the ischemic hypothalamic nucleus of mice, taurine also attenuated the expression of caspase-8 and caspase-9 (Taranukhin et al.2008; Leon et al.2009).
Taurine has also been found to prevent mitochondrial dysfunction and subsequent apoptosis in hypoxic retinal ganglion cells in culture (Chen et al.2009). The effects of neuroprotection by taurine have also been seen in in vivo studies including models of epilepsy and of stroke (Sun et al.2011). In addition, taurine has been shown to reduce cell swelling under conditions of oxygen–glucose deprivation and reoxygenation-induced damage in rat brain cortical slices (Ricci et al.2009).
ER stress occurs when misfolded or unfolded proteins accumulate in the ER, and the cell is capable of triggering caspase-12 or CHOP-mediated apoptosis if it is unable to repair these misfolded or unfolded proteins. ER stress is known to be activated in various neurodegenerative diseases, including Alzheimer’s disease, Huntington’s chorea, Parkinson’s disease, and amyotrophic lateral sclerosis (Lindholm et al.2006; Reijonen et al.2008).
There are at least three known signaling pathways of ER stress identified by the components double-stranded RNA-activated protein kinase 1 (PKR)-like endoplasmic reticulum kinase (PERK), activating transcription factor 6 (ATF6), and IRE-1, respectively. In our previous study, we demonstrated that taurine decreases the expression of ATF6 and IRE-1 while exerting no effect on the PERK pathway in primary neuronal cultures (Pan et al.2011).
In the current study, we employed the SH-SY5Y neuroblastoma cell line under OGD and hypoxic conditions, and our data revealed a robust pro-survival effect of taurine that was similar to that of our previous cell culture studies. After hypoxia and reoxygenation, neuronal viability without taurine treatment dropped to about 50% (percentage of control). The presence of 10 mM taurine improved the cell viability to greater than 70% (percentage of control neurons). This finding is compatible with our previous studies indicating that taurine decreases cell apoptosis. We also demonstrate that taurine attenuates the ER stress produced by hypoxia, but this protection did not involve the PERK-eIF2-ATF 4 pathway. In our ongoing studies, we will further characterize the role of the p-IRE1 pathway in mediating protection by taurine against hypoxia OGD-induced cell damage as well as examine the potential involvement of the ATF6 pathway in mediating protection in the neuroblastoma cell line.
5 Conclusion
In this study, we found taurine exerts a protective effect on the SH-SY5Y neuroblastoma cell line under OGD and hypoxic conditions. Taurine attenuates OGD and hypoxia-induced apoptosis and ER stress. Our understanding of the mechanisms is depicted schematically in Fig.14.5. The full mechanism of neuroprotective function of taurine is still not fully understood, and further studies will characterize in more detail the components of the apoptotic and ER stress pathways that are regulated by taurine under conditions of OGD and hypoxia.
Diagram showing the site of action of taurine in improving survival of neuroblastoma SH-SY5Y cells under conditions of oxygen/glucose deprivation or hypoxia. Taurine decreases the expression of IRE-1 and XPB-1 under hypoxic conditions (left). Taurine also decreases the expression of PUMA under oxygen/glucose deprivation conditions (right)
References
Birdsall TC (1998) Therapeutic applications of taurine. Altern Med Rev 3(2):128–136
Bidri M, Choay P (2003) Taurine: a particular aminoacid with multiple functions. Ann Pharm Fr 61(6):385–391
Buddhala C, Prentice H, Jang-Yen W (2012) Modes of action of taurine and granulocyte colony-stimulating factor in neuroprotection. J Exp Clin Med 4(1):1–7
Chen K, Zhang Q, Wang J, Liu F, Mi M, Xu H, Chen F, Zeng K (2009) Taurine protects transformed rat retinal ganglion cells from hypoxia-induced apoptosis by preventing mitochondrial dysfunction. Brain Res 1279:131–138
Ghandforoush-Sattari M, Mashayekhi SO, Nemati M, Ayromlou H (2011) Changes in plasma concentration of taurine in stroke. Neurosci Lett 496(3):172–175
Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72(1):101–163
Leon R, Wu H, Jin Y, Wei J, Buddhala C, Prentice H, Wu JY (2009) Protective function of taurine in glutamate-induced apoptosis in cultured neurons. J Neurosci Res 87(5):1185–1194
Lindholm D, Wootz H, Korhonen L (2006) ER stress and neurodegenerative diseases. Cell Death Differ 13(3):385–392
Ma Y, Hendershot LM (2003) Delineation of a negative feedback regulatory loop that controls protein translation during endoplasmic reticulum stress. J Biol Chem 278(37):34864–34873
Pan C, Giraldo GS, Prentice H, Wu JY (2010) Taurine protection of PC12 cells against endoplasmic reticulum stress induced by oxidative stress. J Biomed Sci 17(Suppl 1):S17
Pan C, Prentice H, Price AL, Wu JY (2011) Beneficial effect of taurine on hypoxia- and glutamate-induced endoplasmic reticulum stress pathways in primary neuronal culture. Amino Acids 43:845–855
Reijonen S, Putkonen N, Nørremølle A, Lindholm D, Korhonen L (2008) Inhibition of endoplasmic reticulum stress counteracts neuronal cell death and protein aggregation caused by N-terminal mutant huntingtin proteins. Exp Cell Res 314(5):950–960
Ricci L, Valoti M, Sgaragli G, Frosini M (2009) Protection by taurine of rat brain cortical slices against oxygen glucose deprivation- and reoxygenation-induced damage. Eur J Pharmacol 621(1–3):26–32
Sun M, Gu Y, Zhao Y, Xu C (2011) Protective functions of taurine against experimental stroke through depressing mitochondria-mediated cell death in rats. Amino Acids 40(5):1419–1429
Takatani T, Takahashi K, Uozumi Y, Shikata E, Yamamoto Y, Ito T, Matsuda T, Schaffer SW, Fujio Y, Azuma J (2004) Taurine inhibits apoptosis by preventing formation of the Apaf-1/caspase-9 apoptosome. Am J Physiol Cell Physiol 287(4):C949–C953
Taranukhin AG, Taranukhina EY, Saransaari P, Djatchkova IM, Pelto-Huikko M, Oja SS (2008) Taurine reduces caspase-8 and caspase-9 expression induced by ischemia in the mouse hypothalamic nuclei. Amino Acids 34(1):169–174
Wu H, Jin Y, Wei J, Jin H, Sha D, Wu JY (2005) Mode of action of taurine as a neuroprotector. Brain Res 1038(2):123–131
Wu JY, Prentice H (2010) Role of taurine in the central nervous system. J Biomed Sci 17(Suppl 1):S1
Yang J, Wu G, Feng Y, Lv Q, Lin S, Hu J (2010) Effects of taurine on male reproduction in rats of different ages. J Biomed Sci 17(Suppl 1):S9
Yoshida H, Matsui T, Hosokawa N, Kaufman RJ, Nagata K, Mori K (2003) A time-dependent phase shift in the mammalian unfolded protein response. Dev Cell 4(2):265–271
Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B (2001) PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell 7(3):673–682
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this paper
Cite this paper
Chen, PC., Pan, C., Gharibani, P.M., Prentice, H., Wu, JY. (2013). Taurine Exerts Robust Protection Against Hypoxia and Oxygen/Glucose Deprivation in Human Neuroblastoma Cell Culture. In: El Idrissi, A., L'Amoreaux, W. (eds) Taurine 8. Advances in Experimental Medicine and Biology, vol 775. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6130-2_14
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6130-2_14
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6129-6
Online ISBN: 978-1-4614-6130-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)