Abstract
Sevoflurane (Sev) might cause neurotoxicity in elderly rats. However, the role of Lin28A in Sev-induced neurotoxicity remains unclear in elderly rats. In this study, elderly rats were used to construct an Sev-induced nerve injury model. Learning and memory abilities were assessed by Morris water maze (MWM) trainings; pathological alterations in hippocampal region were assessed by HE staining; neuronal apoptosis was assessed by TUNEL; related protein expression was analyzed by immunofluorescence, immunohistochemistry, and Western blotting. Results of this study showed that Sev treatment caused nerve injury and cognitive dysfunction in elderly rats, with increased neuronal apoptosis and decreased Lin28A levels. Pathological impairment and learning and memory abilities of elderly rats were significantly improved after forced overexpression of Lin28A using AAV, accompanied by decreased expression of CD68, Iba-1, and GFAP. TUNEL analysis showed that Lin28A overexpression significantly reversed Sev-induced neuronal apoptosis. Further mechanistic analysis showed that Lin28A significantly promoted SIRT1 expression, which further reversed Sev-induced Tau acetylation at lysine 280 and 686 and Tau hyperphosphorylation, thereby alleviating nerve injury and cognitive dysfunction in elderly rats. The introduction of SIRT1 inhibitor EX527 further confirmed the involvement of SIRT1 in the regulation of Lin28A in elderly rats. In conclusion, our findings demonstrated that Lin28A reduced sevoflurane-induced nerve injury and cognitive dysfunction by inhibiting Tau acetylation and phosphorylation via activating SIRT1 in elderly rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hippocampus is a high-level center for cognitive functions such as learning and memory (Kutlu and Gould 2016). The hippocampal pyramidal neurons are dense and relatively simple in structure, and are also one of the least tolerant sites in the entire central nervous system to various injuries such as ischemia, hypoxia, and inflammation (Pimentel et al. 2011; Sunabori et al. 2016). Inhalation of anesthetics can lead to hippocampal nerve damage, which can result in postoperative cognitive dysfunction (PPCD) (Micha et al. 2016; Qiu et al. 2021). Sevoflurane (Sev) is a volatile anesthetic with fast onset, short recovery time, sweet odor, and non-flammability, and is commonly used in pediatric anesthesia (Apai et al. 2021). Sev has been used clinically for decades (Liu and Yu 2021), and most studies support its safety in adults (Landoni et al. 2019, 2011). However, studies have shown that Sev causes stress and neurotoxicity in the developing brains of rodents and non-human primates (Sun 2010). In addition, clinical observations have confirmed that exposure to anesthesia from a young age can cause long-term cognitive impairment (Shen et al. 2018). However, the mechanism of action of Sev exposure leading to hippocampal damage is unrevealed.
Neurofibrillary tangles in neurons of the brain, especially the hippocampus, can lead to microtubule instability, dysfunction of substance transport, synaptic signal transmission, etc., which triggers neuronal degeneration and loss (Mamun et al. 2020). Tau is a neuronal protein that assembles and stabilizes microtubules. It is now generally accepted that neurogenic fiber tangles may be due to hyperphosphorylation of intra-neuronal tau proteins that are highly susceptible to polymerization to form double-stranded helical filaments (Ma et al. 2017). Tau lesions, i.e., pathological aggregation of Tau protein in neurogenic or glial protofibrillary tangles in the brain, are a hallmark of the neuropathogenesis of Alzheimer’s disease (Naseri et al. 2019). Repeated inhalation of Sev has been reported to lead to brain Tau phosphorylation in neonatal mice, causing cognitive impairment of neurological function (Yu et al. 2020). In addition to phosphorylation, other pathological alterations including acetylation, glycosylation, and nitration are also involved. Among them, Tau acetylation is found in physiological aging and occurs prior to Tau hyperphosphorylation (Lucke-Wold et al. 2017). A series of studies have confirmed that neuronal Tau deacetylation is directly mediated by silent information-regulated transcription factor 1 (SIRT1), and activation of SIRT1 could reduce Tau acetylation and phosphorylation and improve Sev-induced cognitive dysfunction (Yan et al. 2020).
Lin28 is a highly conserved RNA-binding protein composed of two homologs, Lin28A and Lin28B, which share similar structural and functional characteristics. Lin28 is involved in cell proliferation and differentiation in embryonic cells, stem cells, cancer, skeletal myogenesis, gliogenesis, lymphangiogenesis, and glucose metabolism (Jung et al. 2020). Reports have shown that Lin28A exhibited a role in neurodegenerative diseases. For example, overexpression of Lin28A in ventral midbrain NSCs maintains dopamine neurogenic potential as well as expression of midbrain-specific markers (Rhee et al. 2016). In addition, it was shown that Lin28A has a role in neuronal differentiation (Nowak et al. 2014) and that Lin28A is also a protective factor against ischemia-induced injury in the brain, alleviating ischemia/reperfusion-induced nerve injury (Chen et al. 2021). However, the role of Lin28A in Sev-induced cognitive dysfunction is less studied, and the relevant-related mechanisms are unclear.
This study showed that Sev-induced cognitive dysfunction in elderly rats and downregulated Lin28A expression in brain hippocampal tissue; overexpression of Lin28A alleviated Sev-induced neuronal damage and cognitive dysfunction in elderly rats by inhibiting Tau phosphorylation and acetylation via activating SIRT1.
Material and Methods
Animal Modeling and Grouping
Animal study protocol was approved by the Animal Welfare Ethics Committee of The affiliated Huai’an No.1 People’s Hospital of Nanjing Medical University (Approval No. TJBB03121202). Twenty healthy elderly Sprague–Dawley (SD) rats (Male, 18 months of age, 550–750 g) were purchased from SJA Laboratory Animal Company (Hunan, China) and then raised at 24 °C with a constant humidity in light/dark environment (12 h) for 48 h, with free access to water and food. The rats were randomly divided into 2 groups: control group and Sev group. To induce general anesthesia, rats in the Sev group were exposed to 2% SEV delivered in a wetted 30% O2 carrier gas for 5 h, while rats in the control group were exposed to N2 delivered in the same carrier gas without Sev. In addition, saline was injected intraperitoneally once during sevoflurane inhalation. The condition of the rats was continuously monitored by Arterial Blood Gas Analyzer, with a conductance catheter placed in the left carotid artery. Following sevoflurane inhalation, the animals were maintained on pure oxygen until fully conscious and observed for 2 h with free access to food, and arterial blood was collected after sevoflurane inhalation for analysis of PaO2. Three hours after the rats in the Sev group were awakened, the rats were subjected to Morris water maze (MWM) trainings, and then sacrificed by decapitation. Specifically, the back of the rat was fixed and the right axilla and the left forelimb were clamped. We cut the rat neck vertically with scissors, and then, the rat died due to cerebrospinal cord dissection and massive bleeding. Hippocampal tissue was removed for subsequent studies.
Lin28A Overexpression
Forty elderly rats were randomly divided into 4 groups (10 rats per group) using a secure random number generator: control + AAV-empty group, control + AAV-Lin28A group, Sev + AAV-empty group, and Sev + AAV-Lin28A group. Overexpression of Lin28A was achieved by adeno-associated virus (AAV), which was provided by Genechem (Shanghai, China). Each group of rats was injected with AAV-empty or AAV-Lin28A in tail vein, respectively. Two weeks after AAV injection via tail vein, some rats were subjected to Morris water maze (MWM) trainings, and the other rats were executed and brain tissues were taken for follow-up studies.
Morris Water Maze (MWM) Trainings
One day before MWM training, rats in each group were placed in water to swim freely for 2 h to familiarize themselves with the water maze environment. The rats’ learning ability and memory ability were then assessed by navigation test and spatial exploration test, respectively. In the navigation test, rats were successively put into water from the marked entrance, and the time of finding and climbing the platform within 2 min was collected, namely, the escape latency (stable on the platform for 10 s is counted as finding the platform, otherwise continue record). In the spatial detection test, the platform was removed and the rats were put into the pool. The time spent by the rats in the target quadrant placed on the platform and the times of crossing the area of the platform within 2 min were recorded, and the clutter of the path was observed.
HE Staining
Brain tissues were removed from three random rats after adequate anesthesia, and the hippocampal tissues were separated on ice and soaked in 4% paraformaldehyde, followed by dehydration, wax immersion, and embedding, and the wax blocks were cut into 5 μm per section, followed by HE staining according to the instructions as follows: the sections were treated with xylene for 20 min and then sequentially with graded ethanol (100%, 95%, and 80%). After rinsing with distilled water for 5 min, the sections were stained with hematoxylin for 15 min, then stained with eosin for 5 min. Finally, the sections were treated with xylene for 5 min, mounted with neutral gum, and then subsequently observed and photographed under a light microscope.
Terminal Deoxynucleotidyl Transferase-Mediated Deoxyuridine Triphosphate-Biotin Nick End Labeling (TUNEL) Staining
Paraffin-embedded hippocampal tissue sections were deparaffinized to water and incubated with hydrogen peroxide for 40 min to eliminate endogenous peroxidase activity. The sections were incubated with proteinase K for 10 min and TUNEL reaction solution for 1 h. Afterwards, the sections were incubated with POD-conversion solution for 30 min, developed with DAB for 10 min, and counterstained with hematoxylin. After being dehydrated, transparent, and mounted, the sections were observed under a high-power microscope (× 400) for staining. TUNEL-positive cells were pyknotic and stained yellowish-brown or brownish-yellow, and the apoptotic index of hippocampal neurons was calculated.
Western Blotting
RIPA lysis buffer was added to hippocampal tissues in the EP tubes and ground evenly to extract the total protein. The protein concentration was determined by the BCA method. Lin28A, CD68, iba-1, GFAP, Bax, Bcl-2, cleaved caspase 3, cleaved PARP, SIRT1, ac-Tau (k280), ac-Tau (k686), and p-Tau (AT8) levels in the hippocampal tissues were determined by Western blotting. The extracted proteins (20 μg) were separated by SDS-PAGE, and subsequently, the target proteins were fully transferred to PVDF membranes, and anti-Lin28A (ab279647, abcam, 1:1000, Cambridge, UK), anti-CD68 (ab283654, abcam, 1:1000, Cambridge, UK), anti-iba1 (ab178846, abcam, 1:1000, Cambridge, UK), anti-GFAP (ab279647, abcam, 1/10000, Cambridge, UK), anti-Bax (ab32503, abcam, 1:3000, Cambridge, UK), anti-Bcl-2 (ab32124, abcam, 1:1000, Cambridge, UK), anti-cleaved caspase 3 (ab32042, abcam, 1:500, Cambridge, UK), anti-cleaved PARP (#9532, Cell Signaling Technology, 1:1000), anti-SIRT1 (ab189494, abcam, 1:1000, Cambridge, UK), anti-ac-Tau (k280), anti-ac-tau (k280) (1:1000, Anaspec, CA, USA), anti-ac-Tau (k686) (1:1000, EnoGene, Nanjing, China), and anti-p-tau (AT8) (1:1000, Thermo Fisher Scientific, MA, USA) primary antibodies were added, respectively. GAPDH was used as an internal reference and goat anti-rabbit antibody (HRP-labeled) was used as a secondary antibody. The PVDF membrane was sealed with 5% skim milk for 2 h and then washed with PBS. After developed with ECL for 60 s, band scanning was conducted to obtain OD value.
Immunohistochemistry (IHC)
Paraffin sections were deparaffinized and washed three times with PBS for 5 min each. After antigen retrieval, they were washed three times for 5 min with PBS. Endogenous peroxidase activity was removed by dropping 3% hydrogen peroxide. Then, non-specific antigen blocking was performed with 10% serum, followed by incubation with primary antibodies overnight at 4 °C in a cold room and secondary antibodies for 50 min at room temperature, respectively. After incubation was complete, color was developed with DAB and counterstained with hematoxylin. Dehydrate, mount, dry, and observe under a microscope for photography. Following incubation, the sections were visualized with DAB and counterstained with hematoxylin, followed by dehydration, mounting, and drying. The sections were observed and photographed under a microscope.
Immunofluorescence Assay
Hippocampal tissues from rats in each group were fixed with 4% neutral formaldehyde for 12 h. After dehydrated with graded ethanol and cleared with xylene, the tissues were embedded in paraffin. The tissues embedded in paraffin were cut into 5 mm. After that, the sections were rinsed with PBS and permeabilized with 0.5% Triton X- 100 X for 20 min at room temperature. After blocking with 10% BSA for 30 min at 37 °C, primary antibodies were added and cultured overnight at 4 °C. The next day, the sections were incubated with fluorescently labeled secondary antibodies for 1 h at 37 °C in the dark. Nuclei were stained with diimino-2-phenylindole (DAPI) for 5 min in the dark and sealed with a fluorescent quencher. The percentage of Lin28A, Iba-1 and GFAP-positive cells in the total number of cells was observed and recorded under a fluorescence microscope (Olympus, Japan).
Statistical Analysis
Data were represented as the mean ± SD. Statistical Analysis was carried out using GraphPad Prism 7.0 (GraphPad Software, CA, USA). Comparison between 2 groups was performed using two-sided Student’s t test, while comparison among groups was performed using one-way analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
Results
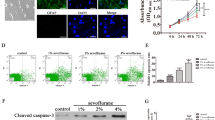
Lin28A Is Lowly Expressed in the Hippocampal Tissues of Rat Treated with Sev
To assess spatial learning and memory associated with the hippocampus, we performed the MWM. water maze pathways are shown in Fig. 1A. As shown in Fig. 1A, water maze pathways were more complex for rats in the control groups. However, SEV treatment made the pathway simpler in the water maze. Besides, SEV exposure increased the escape latency of the rats and decreased the number of platform crossings and time spent in the target quadrant, as well as the distance spent in the target quadrant. In addition, HE staining showed no pathological changes in the hippocampal tissue of rats in the control group. In contrast, hippocampal neuron nuclei in the SEV group showed vacuolation, with disorganized and loose hippocampal cell arrangement, and smaller cells (Fig. 1B). TUNEL staining showed that apoptotic cells in hippocampal tissue increased after SEV exposure. These results suggest that SEV exposure caused hippocampal tissue damage and loss of spatial memory in rats (Fig. 1C). Notably, WB and IFA showed that the expression of Lin28A in hippocampal tissues was decreased after Sev treatment (Fig. 1D–E), indicating that the low expression of Lin28A might be involved in Sev-induced hippocampal tissue damage and spatial memory loss in rats.
Lin28A is lowly expressed in the hippocampal tissues of rat treated with Sev. Rats were randomly divided into 2 groups: control group and Sev group. A Morris water maze (MWM) trainings in each group. B Histopathological changes of hippocampus were determined by HE staining. C Apoptosis was monitored by TUNEL staining. D The expression of Lin28A was monitored by WB. E The level of Lin28A was monitored by IFA (**p < 0.05, ***p < 0.001 vs control group)
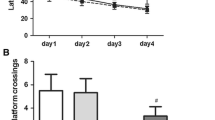
Lin28A Ameliorated Sev-Induced Nerve Injury and Cognitive Dysfunction
To investigate whether Lin28A was involved in the effect of Sev on nerve injury, we overexpressed Lin28A using AAV. As shown in Fig. 2A, after rats were injected with Lin28A-containing AAV, the level of Lin28A in vivo was significantly higher compared to that in the Sev + AAV-empty group, indicating that Lin28A had been successfully overexpressed. To further investigate the effect of Lin28A on nerve injury, we performed HE staining and MWM. As shown in Fig. 2B, compared with the Sev + AAV-empty group, in the Sev + AAV-Lin28A group, high Lin28A expression significantly ameliorated Sev-induced nerve injury (Fig. 2B). In addition, MWM revealed that high Lin28A expression decreased the escape latency of the rats, while increasing the number of platform crossings and time spent in the target quadrant, as well as the distance spent in the target quadrant (Fig. 2C). Together, these findings suggested that high Lin28A expression reversed Sev-induced nerve injury and cognitive dysfunction.
Lin28A ameliorated Sev-induced nerve injury and cognitive dysfunction. Rats were randomly divided into 4 groups: control + AAV-empty group, control + AAV-Lin28A group, Sev + AAV-empty group, and Sev + AAV-Lin28A group. A The expression of Lin28A was monitored by WB. B Histopathological changes of hippocampus were determined by HE staining. C Morris water maze (MWM) trainings in each group (***p < 0.001 vs control + AAV-empty group; ###p < 0.001 vs control + AAV-Lin28A group; &&p < 0.01, &&&p < 0.001 vs Sev + AAV-empty group)
Lin28A Ameliorated Sev-Induced Microglia Activation
To determine the effect of Lin28A on microglia activation, we detected the expression of CD68, Iba-1, and GFAP. As shown in Fig. 3A and B, there was no significant change in the expression of CD68, Iba-1, and GFAP in control + AAV-empty and control + AAV-Lin28A groups. Notably, the expression of CD68, Iba-1, and GFAP increased dramatically after Sev treatment, but retracted after high Lin28A expression, indicating that high expression of Lin28A ameliorated Sev-induced microglia activation.
Lin28A ameliorated Sev-induced microglia activation. Rats were randomly divided into 4 groups: control + AAV-empty group, control + AAV-Lin28A group, Sev + AAV-empty group, and Sev + AAV-Lin28A group. A The levels of Iba-1 and GFAP were monitored by IFA. B The expression of CD68, Iba-1, and GFAP was measured by WB. (***p < 0.001 vs control + AAV-empty group; #p < 0.05, ###p < 0.001 vs control + AAV-Lin28A group; &&&p < 0.001 vs Sev + AAV-empty group)
Lin28A Ameliorated Sev-Induced Neuronal Apoptosis
To determine the effect of Lin28A on neuronal apoptosis, we performed TUNEL and WB. As shown in Fig. 4A, high Lin28A expression had no effect on nerve cells not treated with Sev, but significantly reversed Sev-induced neuronal apoptosis. WB further revealed that Sev treatment increased the expression of pro-apoptotic proteins (Bax, cleaved caspase 3, and cleaved PARP) but decreased the expression of anti-apoptotic protein Bcl-2. However, high expression of Lin28A had no effect on the expression of apoptosis-related proteins without Sev treatment, but significantly inhibited the expression of pro-apoptotic proteins (Bax, cleaved caspase 3, and cleaved PARP) and significantly promoted the expression of anti-apoptotic protein Bcl-2. In conclusion, these results suggested that Lin28A ameliorated Sev-induced neuronal apoptosis.
Lin28A ameliorated Sev-induced neuronal apoptosis. Rats were randomly divided into 4 groups: control + AAV-empty group, control + AAV-Lin28A group, Sev + AAV-empty group, and Sev + AAV-Lin28A group. A Apoptosis was monitored by TUNEL staining. B The number of apoptotic cells. C–D The expression of Bcl-2, Bax, cleaved caspase 3, and cleaved PARP was measured by WB. (***p < 0.001 vs control + AAV-empty group; #p < 0.05, ##p < 0.01, ###p < 0.001 vs control + AAV-Lin28A group; &p < 0.05, &&p < 0.01, &&&p < 0.001 vs Sev + AAV-empty group)
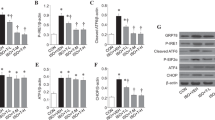
Lin28A Activated SIRT1 and Inhibited Tau Phosphorylation and Acetylation
To further elucidate the mechanism of action of Lin28A, we evaluated the effect of Lin28A on SIRT1, ac-Tau (k280), ac-Tau (k686), and ac-Tau (AT8) using immunofluorescence and WB. As shown in Fig. 5A and B, Sev treatment significantly decreased the expression of SIRT1. High expression of Lin28A had no significant effect on the expression of SIRT1 in nerve cells without Sev treatment, but increased the expression of SIRT1 after Sev treatment. WB revealed similar results. In addition, Sev treatment significantly increased the expression of ac-Tau (k280), ac-Tau (k686), and ac-Tau (AT8). Similarly, high Lin28A expression had no significant effect on the expression of ac-Tau (k280), ac-Tau (k686), and ac-Tau (AT8) in nerve cells without Sev treatment, but reversed the expression of ac-Tau (k280), ac-Tau (k686), and ac-Tau (AT8) after Sev treatment (Fig. 5C and D). Overall, these findings demonstrated that Lin28A activated SIRT1 and inhibited Tau phosphorylation and acetylation.
Lin28A activated SIRT1 and inhibited Tau phosphorylation and acetylation. Rats were randomly divided into 4 groups: control + AAV-empty group, control + AAV-Lin28A group, Sev + AAV-empty group, and Sev + AAV-Lin28A group. A The levels of SIRT1 were monitored by IFA. B SIRT1 positive cells (%). C–D The expression of SIRT1, ac-Tau (k280), ac-Tau (k686), and ac-Tau (AT8) was measured by WB. (***p < 0.001 vs control + AAV-empty group; #p < 0.05, ###p < 0.001 vs control + AAV-Lin28A group; &p < 0.05, &&&p < 0.001 vs Sev + AAV-empty group)
Lin28A Ameliorated Sev-Induced Hippocampal Damage in Rats via SIRT1
From the above findings, it is obvious that Sev-induced tissue damage in the hippocampal region, Tau phosphorylation and acetylation, and neuronal apoptosis. To further confirm the involvement of SIRT1 in Sev-induced nerve injury, we introduced the SIRT1 inhibitor Ex527 and investigated the role of Ex527. As shown in Fig. 6A, Ex527 significantly reversed the nerve injury ameliorated by high Lin28A expression (Fig. 6A). In addition, IFA revealed that Ex527 significantly increased the expression of Iba-1 and GFAP (Fig. 6B). Furthermore, Ex527 treatment significantly reversed the decrease of pro-apoptotic related proteins (Bax, cleaved caspase 3, and cleaved PARP) and the increase of anti-apoptotic proteins due to Lin28A (Fig. 6C). In conclusion, our results suggested that Lin28A ameliorated Sev-induced hippocampal damage in rats via SIRT1.
Lin28A ameliorated Sev-induced hippocampal damage in rats via SIRT1. Rats were randomly divided into 3 groups: Sev + AAV-empty group, Sev + AAV-Lin28A group, and Sev + AAV-Lin28A + Ex527 (microinjected into the ventrolateral orbital cortex with a dosage of 1 μmol/L (dissolved in 10% DMSO)). A Histopathological changes of hippocampus were determined by HE staining. B The levels of Iba-1 and GFAP were monitored by IFA. C The expression of Bcl-2, Bax, cleaved caspase 3, and cleaved PARP was measured by WB (**p < 0.01, ***p < 0.001 vs Sev + AAV-empty group; #p < 0.05, ###p < 0.001 vs Sev + AAV-Lin28A group)
Discussion
The brain is the target organ of anesthetic drugs, and the function of the central nervous system is suppressed when patients receive anesthesia (Li et al. 2019a). The use of anesthetic drugs during surgery may induce postoperative cognitive dysfunction (POCD) (Zhong and Xu 2019). Studies have found that anesthesia and surgery can induce allergies, inflammation, and oxidative stress, which can produce neurotoxicity and ultimately damage hippocampal tissue (Chen et al. 2019; Trapasso et al. 2019; Selmanoglu et al. 2021). While the hippocampus is an important brain region in the brain for functions such as learning and memory, hippocampal damage can lead to impairment in learning and cognition (Aksoz et al. 2019). Sev is a general anesthetic drug that is more widely used in clinical practice. In the present study, we examined the pathological changes of brain tissue in elderly rats by HE staining, and the results showed that the rats in the Sev group suffered significant pathological damage compared with the normal group, which is consistent with the results of previous studies (Li et al. 2019b; Engelhard et al. 2007). MWM training is a typical behavioral experiment, which can reflect the spatial learning memory ability of animals (D’Hooge and Deyn 2001). In the present study, the rats in the Sev group had a significantly higher latency than the control group and crossed the platform less often than the control group, indicating that Sev anesthesia significantly impaired the cognitive function of elderly rats. Neuronal apoptosis after stress, trauma, and anesthesia processes in the elderly is an important influencing factor for cognitive dysfunction; therefore, the apoptosis of neuronal cells after Sev anesthesia was measured by TUNEL staining in this study. Our results showed that SEV treatment induced apoptosis of neuronal cells, which caused cognitive dysfunction in the central nervous system (Kang et al. 2020). Notably, Sev treatment was found to inhibit the expression of Lin28A in this study, which may be related to nerve damage.
Lin28A gene can be recognized by its unique pairing of the cold shock domain (CSD) and the zinc finger domain of cysteine cysteine histidine cysteine (CCHC) zinc finger protein and plays an important role in sequence-specific mRNA binding, miRNA binding, miRNA preprocessing, and miRNA-regulated gene silencing (Venugopal et al. 2018). Lin28A has been shown to have an important role in neural differentiation and cerebral ischemia–reperfusion injury (Nowak et al. 2014; Chen et al. 2021; Xia et al. 2018). As reported by Xia et al., Lin28A promoted and antagonized neuronal and glial cell generation, respectively, by repressing glial-specific genes and differentiating neuroglia directly along the neuronal lineage (Xia et al. 2018). However, the role of Lin28A in neural injury remains unclear. In the present study, we found that Lin28A was lowly expressed in Sev-treated elderly rats, indicating that Lin28A might be associated with Sev-induced nerve injury in elderly rats. Therefore, we evaluated the effect of Lin28A on Sev-induced nerve injury by overexpression by AAV, MWM trainings, and HE staining. The results showed that Lin28A significantly reversed Sev-induced cognitive dysfunction and nerve injury in elderly rats. GFAP is a unique cytoskeletal protein of astrocytes in the central nervous system and plays an important role in maintaining the morphology and function of astrocytes (Middeldorp and Hol 2011). GFAP is also a specific marker of astrocytes. Wang et al. showed that the number of microglia (MG) marker Iba-1-and GFAP-positive cells increased after epilepsy (Wang et al. 2019). As expected, we also found that Lin28A suppressed the expression of CD68, GFAP, and Iba-1, thus exerting neuroprotective effects. Furthermore, Lin28A could alleviate Sev-induced cognitive dysfunction in elderly rats by inhibiting neuronal apoptosis (Kang et al. 2020). These results have also been reported in the current study. As for the mechanism of action of Lin28A, we believe that the action of Lin28A might be related to SIRT1.
SIRT1 has been reported to be a nicotinamide adenine dinucleotide (NAD) + -dependent class III histone deacetylase. It is widely expressed in the brain and is involved in various physiological activities such as aging, stress, and energy metabolism (Lanzillotta et al. 2013; Cho and Chen 2015). Depression of SIRT1 could cause neurodegeneration in aged mice, and promoting the expression of SIRT1 protein significantly alleviated the central neuroinflammatory response induced by injurious stimuli (Kim et al. 2007). SIRT1 is expressed in both neurons and glial cells. In neurons, SIRT1 is involved in regulating synaptic plasticity and deacetylating Tau proteins, thus reducing pathological Tau protein diffusion, and maintaining synaptic and neuronal function (Zhou et al. 2022; Chen et al. 2005). Similarly, the present study found that Lin28A promoted SIRT1 expression, and Lin28A-induced high SIRT1 expression inhibited Tau acetylation at lysine (K) 280 and 686 and Tau hyperphosphorylation in the brain, thereby reducing pathological Tau protein spreading. However, the introduction of SIRT1 inhibitor EX527 exacerbated pathological damage in the hippocampal region and promoted the expression of Iba-1 and GFAP as well as apoptosis-related proteins. These results suggest that Lin28A ameliorates Sevo-induced hippocampal damage in rats via SIRT1. Nevertheless, the pathological mechanisms of Sev-induced cognitive dysfunction have not been fully elucidated, and the mechanism of action of Lin28A in Sev-induced cognitive dysfunction is quite limited. Previous studies have shown that Lin28A had anti-inflammatory and antioxidant effects (Guo and Li 2022), but inflammation and oxidative stress factors were not investigated in this study. Besides, this study only assessed the SIRT1/Tau pathway, and a possible contribution of other signaling pathways to Lin28A-induced hippocampal injury protection cannot be excluded. In addition, this study only assessed aged rats, and no studies were performed in neonatal or adult rats, and knockdown of Lin28A on hippocampal injury are required as supplements. Furthermore, additional non-clinical and clinical studies are still required to further define the role of Lin28A.
In conclusion, in this study, we investigated the effects and potential molecular mechanisms of Lin28A by constructing a rat model of neurological injury. Our study showed that Lin28A attenuated Sev-induced nerve injury and cognitive dysfunction in elderly rats, and these effects may be achieved by activating SIRT1, thereby inhibiting Tau phosphorylation and acetylation.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Aksoz E, Gocmez SS, Sahin TD, Aksit D, Aksit H, Utkan T (2019) The protective effect of metformin in scopolamine-induced learning and memory impairment in rats. Pharmacol Rep PR 71:818–825. https://doi.org/10.1016/j.pharep.2019.04.015
Apai C, Shah R, Tran K, Pandya Shah S (2021) Anesthesia and the developing brain: a review of sevoflurane-induced neurotoxicity in pediatric populations. Clin Ther 43:762–778. https://doi.org/10.1016/j.clinthera.2021.01.024
Chen D, Zheng K, Wu H, Zhang X, Ye W, Tan X, Xiong Y (2021) Lin28a attenuates cerebral ischemia/reperfusion injury through regulating Sirt3-induced autophagy. Brain Res Bull 170:39–48. https://doi.org/10.1016/j.brainresbull.2021.01.022
Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L (2005) SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem 280:40364–40374. https://doi.org/10.1074/jbc.M509329200
Chen X, Ren X, Ma Y, Ge L, Hu Z, Yan W (2019) Research progress of the role of postoperative pain in the development of postoperative cognitive dysfunction in geriatric patients. Nan fang yi ke da xue xue bao = J Southern Med Univ 39:1122–1126. https://doi.org/10.12122/j.issn.1673-4254.2019.09.20
Cho SH, Chen JA (2015) SIRT1 deficiency in microglia contributes to cognitive decline in aging and neurodegeneration via epigenetic regulation of IL-1β. 35:807–818. https://doi.org/10.1523/jneurosci.2939-14.2015
D’Hooge R, De Deyn PP (2001) Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev 36:60–90. https://doi.org/10.1016/s0165-0173(01)00067-4
Engelhard K, Winkelheide U, Werner C, Kluge J, Eberspächer E, Hollweck R, Hutzler P, Winkler J, Kochs E (2007) Sevoflurane affects neurogenesis after forebrain ischemia in rats. Anesth Analg 104:898–903. https://doi.org/10.1213/01.ane.0000255730.73018.31
Guo L, Li L (2022) LIN28A alleviates inflammation, oxidative stress, osteogenic differentiation and mineralization in lipopolysaccharide (LPS)-treated human periodontal ligament stem cells. Exp Ther Med 23:411. https://doi.org/10.3892/etm.2022.11338
Jung GS, Hwang YJ, Choi JH, Lee KM (2020) Lin28a attenuates TGF-β-induced renal fibrosis. BMB Rep 53:594–599. https://doi.org/10.5483/BMBRep.2020.53.11.153
Kang W, Lu D, Yang X, Ma W, Chen X, Chen K, Xu X, Zhou X, Zhou L, Feng X (2020) Sevoflurane Induces Hippocampal Neuronal Apoptosis by Altering the Level of Neuropeptide Y in Neonatal Rats 45:1986–1996. https://doi.org/10.1007/s11064-020-03028-9
Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH (2007) SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J 26:3169–3179. https://doi.org/10.1038/sj.emboj.7601758
Kutlu MG, Gould TJ (2016) Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: contributions to development and maintenance of addiction. Learning & Memory (cold Spring Harbor, NY) 23:515–533. https://doi.org/10.1101/lm.042192.116
Lanzillotta A, Pignataro G, Branca C, Cuomo O, Sarnico I, Benarese M, Annunziato L, Spano P, Pizzi M (2013) Targeted acetylation of NF-kappaB/RelA and histones by epigenetic drugs reduces post-ischemic brain injury in mice with an extended therapeutic window. Neurobiol Dis 49:177–189. https://doi.org/10.1016/j.nbd.2012.08.018
Landoni G, Lomivorotov VV, Nigro Neto C, Monaco F, Pasyuga VV, Bradic N, Lembo R, Gazivoda G, Likhvantsev VV, Lei C, Lozovskiy A, Di Tomasso N, Bukamal NAR, Silva FS, Bautin AE, Ma J, Crivellari M, Farag A, Uvaliev NS, Carollo C, Pieri M, Kunstýř J, Wang CY, Belletti A, Hajjar LA, Grigoryev EV, Agrò FE, Riha H (2019) Volatile Anesthetics versus Total Intravenous Anesthesia for Cardiac Surgery 380:1214–1225. https://doi.org/10.1056/NEJMoa1816476
Landoni G, Guarracino F, Baldassarri R, Cariello C, Gerli C, Fano G, Simone Fd, Cassarà L, Frati E, Pittarello D, Tritapepe L, Zangrillo A (2011) Sevoflurane vs propofol in high risk cardiac surgery: design of the randomized trial “Sevo-Aifa”. Signa Vitae 6:36–40. https://doi.org/10.22514/sv61.042011.6
Li X, Sun Y, Jin Q, Song D, Diao Y (2019a) Kappa opioid receptor agonists improve postoperative cognitive dysfunction in rats via the JAK2/STAT3 signaling pathway. Int J Mol Med 44:1866–1876. https://doi.org/10.3892/ijmm.2019.4339
Li Y, Liu L, Tian Y, Zhang J (2019b) Rapamycin improves sevoflurane-induced cognitive dysfunction in aged rats by mediating autophagy through the TLR4/MyD88/NF-κB signaling pathway. Mol Med Rep 20:3085–3094. https://doi.org/10.3892/mmr.2019.10541
Liu C-W, Yu J (2021) Elaborate anesthetic management of cesarean section followed by abdominal radical hysterectomy for uterine cervical cancer: case report. EJGO 42:595–597. https://doi.org/10.31083/j.ejgo.2021.03.2229
Lucke-Wold B, Seidel K, Udo R, Omalu B, Ornstein M, Nolan R, Rosen C, Ross J (2017) Role of Tau acetylation in Alzheimer’s disease and chronic traumatic encephalopathy: the way forward for successful treatment. J Neurol Neurosurg 4
Mamun AA, Uddin MS, Mathew B, Ashraf GM (2020) Toxic tau: structural origins of tau aggregation in Alzheimer’s disease. Neural Regen Res 15:1417–1420. https://doi.org/10.4103/1673-5374.274329
Ma RH, Zhang Y, Hong XY, Zhang JF, Wang JZ, Liu GP (2017) Role of microtubule-associated protein tau phosphorylation in Alzheimer’s disease. J Huazhong Univ Sci Technol Med Sci = Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban = Huazhong keji daxue xuebao Yixue Yingdewen ban 37:307–312. https://doi.org/10.1007/s11596-017-1732-x
Micha G, Tzimas P, Zalonis I, Kotsis K, Papdopoulos G, Arnaoutoglou E (2016) Propofol vs Sevoflurane anaesthesia on postoperative cognitive dysfunction in the elderly. A randomized controlled trial. Acta Anaesthesiol Belg 67:129–137
Middeldorp J, Hol EM (2011) GFAP in health and disease. Prog Neurobiol 93:421–443. https://doi.org/10.1016/j.pneurobio.2011.01.005
Naseri NN, Wang H, Guo J, Sharma M, Luo W (2019) The complexity of tau in Alzheimer’s disease. Neurosci Lett 705:183–194. https://doi.org/10.1016/j.neulet.2019.04.022
Nowak JS, Choudhury NR, de Lima AF, Rappsilber J, Michlewski G (2014) Lin28a regulates neuronal differentiation and controls miR-9 production. Nat Commun 5:3687. https://doi.org/10.1038/ncomms4687
Pimentel VC, Pinheiro FV, De Bona KS, Maldonado PA, da Silva CR, de Oliveira SM, Ferreira J, Bertoncheli CM, Schetinger MR, Da Luz SC, Moretto MB (2011) Hypoxic-ischemic brain injury stimulates inflammatory response and enzymatic activities in the hippocampus of neonatal rats. Brain Res 1388:134–140. https://doi.org/10.1016/j.brainres.2011.01.108
Qiu J, Zhang Y, Xie M (2021) Chrysotoxine attenuates sevoflurane-induced neurotoxicity in vitro via regulating PI3K/AKT/GSK pathway. Signa Vitae 17:185–191. https://doi.org/10.22514/sv.2021.107
Rhee YH, Kim TH, Jo AY, Chang MY, Park CH, Kim SM, Song JJ, Oh SM, Yi SH, Kim HH, You BH, Nam JW, Lee SH (2016) LIN28A enhances the therapeutic potential of cultured neural stem cells in a Parkinson’s disease model. Brain : a J Neurol 139:2722–2739. https://doi.org/10.1093/brain/aww203
Selmanoglu A, Güvenir H, Celik IK, Karaatmaca B, Toyran M, Civelek E, Misirlioglu ED (2021) Immediate local anesthetic reactions and diagnostic test results in pediatric patients. Allergol Immunopathol (Madr) 49:108–114. https://doi.org/10.15586/aei.v49i3.87
Shen FY, Song YC, Guo F, Xu ZD, Li Q, Zhang B, Ma YQ, Zhang YQ, Lin R, Li Y, Liu ZQ (2018) Cognitive impairment and endoplasmic reticulum stress induced by repeated short-term sevoflurane exposure in early life of rats. Front Psych 9:332. https://doi.org/10.3389/fpsyt.2018.00332
Sunabori T, Koike M, Asari A, Oonuki Y, Uchiyama Y (2016) Suppression of ischemia-induced hippocampal pyramidal neuron death by hyaluronan tetrasaccharide through inhibition of toll-like receptor 2 signaling pathway. Am J Pathol 186:2143–2151. https://doi.org/10.1016/j.ajpath.2016.03.016
Sun L (2010) Early childhood general anaesthesia exposure and neurocognitive development. Br J Anaesth 105(Suppl 1):i61-68. https://doi.org/10.1093/bja/aeq302
Trapasso S, Visconti F, Cello AD, Rocca ML, Gallucci A, Zullo F, Venturella R (2019) Obturator nerve injury during gynecological surgery: our experience. EJGO 40:667–670. https://doi.org/10.12892/ejgo4527.2019
Venugopal P, Koshy T, Lavu V, Ranga Rao S (2018) Differential expression of microRNAs let-7a, miR-125b, miR-100, and miR-21 and interaction with NF-kB pathway genes in periodontitis pathogenesis. 233:5877–5884. https://doi.org/10.1002/jcp.26391
Wang Z, Zhou L, An D, Xu W, Wu C, Sha S, Li Y, Zhu Y, Chen A, Du Y, Chen L (2019) TRPV4-induced inflammatory response is involved in neuronal death in pilocarpine model of temporal lobe epilepsy in mice. 10:386. https://doi.org/10.1038/s41419-019-1612-3
Xia X, Teotia P, Ahmad I (2018) Lin28a regulates neurogliogenesis in mammalian retina through the Igf signaling. Dev Biol 440:113–128. https://doi.org/10.1016/j.ydbio.2018.05.007
Yan J, Luo A, Sun R, Tang X, Zhao Y, Zhang J, Zhou B, Zheng H, Yu H, Li S (2020) Resveratrol mitigates hippocampal Tau acetylation and cognitive deficit by activation SIRT1 in aged rats following anesthesia and surgery. 2020:4635163. https://doi.org/10.1155/2020/4635163
Yu Y, Yang Y, Tan H, Boukhali M, Khatri A, Yu Y, Hua F, Liu L, Li M, Yang G, Dong Y, Zhang Y, Haas W, Xie Z (2020) Tau contributes to sevoflurane-induced neurocognitive impairment in neonatal mice. Anesthesiology 133:595–610. https://doi.org/10.1097/aln.0000000000003452
Zhong J, Xu W (2019) Characterization of DNA hydroxymethylation in the hypothalamus of elderly mice with post-operative cognitive dysfunction. Exp Ther Med 18:4002–4010. https://doi.org/10.3892/etm.2019.8056
Zhou Q, Deng Y, Hu X, Xu Y (2022) Resveratrol ameliorates neuronal apoptosis and cognitive impairment by activating the SIRT1/RhoA pathway in rats after anesthesia with sevoflurane. Bosn J Basic Med Sci 22:110–117. https://doi.org/10.17305/bjbms.2021.5997
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and the experiments were performed by Yingjun Zhu. Data collection and analysis were performed by Min Zhang and Jiayu Wang. The first draft of the manuscript was written by Qingxiu Wang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Ethical approval was obtained from the Ethics Committee of The affiliated Huai’an No.1 People’s Hospital of Nanjing Medical University. The animal experiment complies with the ARRIVE guidelines and in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (Approval No. TJBB03121202).
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, Y., Zhang, M., Wang, J. et al. Lin28A Reduced Sevoflurane-Induced Nerve Injury and Cognitive Dysfunction by Inhibiting Tau Acetylation and Phosphorylation via Activating SIRT1 in Elderly Rats. Neurotox Res 40, 1913–1923 (2022). https://doi.org/10.1007/s12640-022-00594-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-022-00594-4