Abstract
It has been suggested that oxidative stress plays an important role in the pathophysiology of traumatic brain injury (TBI). N-acetylcysteine (NAC) and selenium (Se) display neuroprotective activities mediated at least in part by their antioxidant and anti-inflammatory properties although there is no report on oxidative stress, antioxidant vitamin, interleukin-1 beta (IL)-1β and IL-4 levels in brain and blood of TBI-induced rats. We investigated effects of NAC and Se administration on physical injury-induced brain toxicity in rats. Thirty-six male Sprague–Dawley rats were equally divided into four groups. First and second groups were used as control and TBI groups, respectively. NAC and Se were administrated to rats constituting third and forth groups at 1, 24, 48 and 72 h after TBI induction, respectively. At the end of 72 h, plasma, erythrocytes and brain cortex samples were taken. TBI resulted in significant increase in brain cortex, erythrocytes and plasma lipid peroxidation, total oxidant status (TOS) in brain cortex, and plasma IL-1β values although brain cortex vitamin A, β-carotene, vitamin C, vitamin E, reduced glutathione (GSH) and total antioxidant status (TAS) values, and plasma vitamin E concentrations, plasma IL-4 level and brain cortex and erythrocyte glutathione peroxidase (GSH-Px) activities decreased by TBI. The lipid peroxidation and IL-1β values were decreased by NAC and Se treatments. Plasma IL-4, brain cortex GSH, TAS, vitamin C and vitamin E values were increased by NAC and Se treatments although the brain cortex vitamin A and erythrocyte GSH-Px values were increased through NAC only. In conclusion, NAC and Se caused protective effects on the TBI-induced oxidative brain injury and interleukin production by inhibiting free radical production, regulation of cytokine-dependent processes and supporting antioxidant redox system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxidative injury has been causally linked to a variety of neurodegenerative diseases including spinal cord and brain injuries. Oxidative stress is mediated by reactive oxygen species (ROS) [1, 2] and cellular antioxidant defense systems [3]. Generation of ROS is ubiquitous since ROS are generated during aerobic metabolism i.e. mitochondrial oxidations, respiratory burst function of phagocytes (inflammation) and cell lyses during the injuries such as spinal cord injury and traumatic brain injury (TBI) [4, 5]. In order to scavenge ROS various antioxidant defense systems exist in brain. The selenium is an essential dietary trace element which plays an important role in a number of biological processes [3]. Selenium dependent GSH-Px and catalase are responsible for the reduction hydrogen peroxide in the presence of reduced glutathione (GSH) [6, 7]. Glutathione (GSH) reduces disulfide bonds formed within cytoplasmic proteins to cysteines by serving as an electron donor. GSH induces also an antioxidant role and it is preventing damage to cellular components caused by ROS [8, 9]. Vitamin E is a donor fat soluble antioxidant that reacts with and reduces peroxyl radicals and, thus, inhibits the propagation cycle of lipid peroxidation. Vitamin C detoxifies free radicals by reducing them [1]. Scavenging of ROS by vitamin C occurs in the aqueous phase, which is in contrast to the site of action of vitamin E, within membranes [8, 9]. Retinoic acid, an essential factor derived from vitamin A, has been shown to have a variety of functions including roles as an antioxidant and in cellular differentiation [11]. Given differences in mechanism and site of action, there are differences in antioxidant free radical scavenging ability as well as synergistic interaction of hydrophilic and lipophilic antioxidants.
Under physiological conditions ROS play a critical role in the control enzyme activities and cell signaling process through modulation of cellular redox status. Oxidant and antioxidant balances are often disrupted when cells are exposed to environmental factors such as radiation and injuries. ROS generated by activated phagocytes in injured and inflamed cells are also exacerbating oxidative cell injury [12]. Brain is extremely susceptible to oxidative damage induced by these ROS because it generates very high levels of ROS due to its very high aerobic metabolism and blood perfusion and it has relatively poor enzymatic antioxidant defense [1, 2]. Brain contains polyunsaturated fatty acids (PUFAs) which can readily be peroxidized [13]. Lipid peroxidation causes toxicity to cell and intracellular membranes and may lead to cell destruction and subsequently cell dead. Brain is protected by enzymatic and non-enzymatic antioxidants form oxidative damage [1, 2].
TBI from physical impact frequently occurs in human beings involved in sports and automobile accidents. Over one million TBI are treated in United States hospitals each year [14]. Other than giving relief care, there are no treatments for TBI. The degree of TBI is determined by the severity of primary injury, a direct result of the physiological impact, and secondary injury, which involves biochemical and cellular oxidative changes that eventually lead to neuronal degeneration surrounding the site of primary injury [15]. The principal auto-destructive processes that have been thus far postulated are secondary ischemic changes, ROS-induced lipid peroxidation which damages the lipid membranes in neuron of brain [12]. All of these processes are believed to induce damage to the brain; they are started by the mechanisms of initial TBI, but the detailed biocascades inherent to this process remain incompletely clear. Certain antioxidant balance is crucial for a health nervous system and neuronal susceptibility to excitability. Several reports suggested that body selenium levels and GSH-Px activity play a vital role in brain injury condition to develop [16, 17] although there is no report role of selenium supplementation on antioxidant values in TBI-induced animal.
It has not been studied whether NAC and selenium modify the alterations in antioxidant enzyme system, lipid peroxidation and antioxidant vitamin concentrations in the brain cortex and blood of TBI-induced rat. Hence, we aimed to evaluate whether there would be protective effect of NAC and selenium on oxidative stress, antioxidants, IL-1β and IL-4 values in TBI-induced brain injury in rats.
Materials and Methods
Animals
All experimental procedures were approved by the Medical Faculty Experimentation Ethics Committee of Süleyman Demirel University (SDU). Thirty-six adult (4 months old) male adult Sprague–Dawley rats were used in the current study. Animals were kept and used in accordance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory animals prepared by the SDU. The animals allowed acclimating to their environmentally controlled rooms (20 °C and 12 h light/dark cycles). The animals fed rodent chow and water ad libitum.
Induction of TBI
Marmarou’s weight drop model is one of the most frequently used constrained rodent models of impact acceleration traumatic head injury [18]. The trauma device consists of a column of brass weights falling freely by gravity from a designated height through a Plexiglas tube, causing a contusional head trauma. After exposing the animal’s skull by a midline incision, a stainless steel disc (10 mm in diameter and 1 mm in depth) is rigidly fixed with dental cement to the animal’s skull centrally between lambda and bregma fissures. The rats are then placed on a 10-cm deep foam bed and the impact generated by dropping the brass weight onto the stainless steel disc. This method has been shown to produce graded brain injury in rats.
Study Groups
The animals were randomly divided into four groups as follows;
- Group I:
-
Control group (n = 9): Placebo was supplemented to the first group.
- Group II:
-
TBI group (n = 9): TBI was performed on each animal through a 200 g weight causing a head trauma [18].
- Group III:
-
TBI plus NAC group (n = 9): NAC (150 mg/kg body weight) was orally (via gastric gavage) given to animals consisting the group at 1, 24, 48 and 72 h after brain trauma [19].
- Group IV:
-
TBI plus selenium group (n = 9): Selenium (sodium selenite and 1.5 mg/kg body weight) was intraperitoneally administrated to the animals at 1, 24, 48 and 72 h after brain trauma [20].
Anesthesia and Preparation of Brain and Blood Samples
Rats were anesthetized with ether before sacrifice of each rats and removal of the cortex brain and blood samples. Cortex brain tissues were washed twice with cold saline solution, placed into glass bottles, labeled and stored in a deep freeze (−85 °C) until processing (maximum 4 weeks). After weighing, half of the cortex were placed on ice, cut into small pieces, using scissors, and homogenized (2 min at 5,000 rpm) in a five volumes (1:5, w/v) of ice-cold Tris–HCl buffer (50 mM, pH 7.4), by using an ultrasonic homogenizer (Bandelin-2070, BANDELIN electronic, GmbH & Co. KG, Berlin, Germany). All preparation procedures were performed on ice.
The blood (3–5 ml) was taken from the heart, using a sterile injector, into tubes, protected against light. Blood sample was separated into plasma and erythrocytes by centrifugation at 1,500×g for 10 min at +4 °C. The erythrocytes samples were washed three times in cold isotonic saline (0.9 %, v/w), then hemolyzed with a nine-fold volume of phosphate buffer (50 mM, pH 7.4). The brain homogenate, hemolyzed erythrocytes and plasma samples were stored at −85 °C for <4 weeks pending measurement of enzymatic activity. The remaining brain homogenate, hemolyzed erythrocytes and plasma samples were used for immediate lipid peroxidation and vitamin assay.
Lipid Peroxidation Level Determinations
Lipid peroxidation levels in the brain homogenate, hemolyzed erythrocytes and plasma samples were assayed based on the method of Placer et al. [21]. Briefly, the principle of the method depends on the determination of the pink color that is produced by the interaction of thiobarbituric acid with malondialdehyde (MDA) to form a colored MDA–TBA adduct. Levels of lipid peroxidation as MDA were determined spectrophotometrically (Shimadzu UV-1800, Shimadzu Corp., Kyoto, Japan) at a wavelength of 532 nm. The values of LP in the brain homogenate, hemolyzed erythrocytes and plasma samples were expressed as μmol/gram protein and μmol/gram protein, respectively.
Reduced Glutathione (GSH), Glutathione Peroxidase (GSH-Px) and Protein Assay
The GSH content of the brain and erythrocytes was measured at 412 nm using the method of Sedlak and Lindsay [22] as described own studies [23, 24]. GSH-Px activities of brain and erythrocytes were measured spectrophotometrically (Shimadzu UV-1800, Shimadzu Corp., Kyoto, Japan) at 37 °C and 412 nm according to the Lawrence and Burk [25]. GSH-Px catalyzes the oxidation of GSH by cumene hydroperoxide. In the presence of GSH reductase and NADPH, the oxidized glutathione is immediately converted to the reduced form with a concomitant oxidation of NADPH to NADP+. The enzymatic reaction was stopped at 10 min by using trichloroacetic acid. The decrease in absorbance at 412 nm against blank was measured spectrophotometrically. One international unit (IU) of GSH-Px activity was defined as the amount of enzyme that converts 1 μmol of NADPH to NADP+ per minute. The GSH-Px activity was expressed as unit per g of tissue or erythrocyte protein (IU/g protein). The total protein concentration in the brain cortex and erythrocytes was determined according to Lowry et al. [26] with bovine serum albumin as the standard.
Brain Cortex β-Carotene, Vitamins A, C and E Analyses
Vitamins A (retinol) and E (α-tocopherol) were determined in the brain cortex and plasma samples by a modification of the method described by Desai [27] and Suzuki and Katoh [28]. A 0.5-ml n-hexane extract aliquot was measured at 325 nm for the vitamin A concentration. Next, reactants were added, and the hexane absorbance value was measured at 535 nm in the spectrophotometer for the measurement of the vitamin A concentration. Calibrations were performed using standard solutions of all-trans retinol and α-tocopherol in hexane.
The β-carotene concentration in the brain cortex was determined as described previously [28]. We mixed 2 ml of hexane with 0.25 g of brain and 0.25 ml plasma. The β-carotene concentration in the hexane mixture was measured at 453 nm in the spectrophotometer.
Vitamin C (ascorbic acid) concentration in the brain cortex samples was spectrophotometrically determined at 760 nm according to the method of Jagota and Dani [29] and it is expressed in micromoles per gram tissue.
Total Antioxidant Status (TAS) and Total Oxidant Status (TOS) Analyses
The brain cortex TAS and TOS levels were measured calorimetrically using the TAS and TOS commercial kit (Mega Tıp Inc, Gaziantep, Turkey) [30]. The results in the brain were expressed in μmol H2O2 equivalent/g protein (μmol H2O2 equiv/g prot).
Cytokine Determinations in Plasma
Plasma cytokine (IL-1β and IL-4) levels were measured using a commercial enzyme-linked immunosorbent assay (ELISA), following the manufacturer’s instructions as described in previous studies [31]. All ELISA kits were purchased from DRG Inc. (Marburg, Germany). Determinations were made in duplicate, and cytokine results are expressed in pictogram (pg) per milliliter.
Statistical Analyses
All results are expressed as mean ± standard deviation (SD). p values <0.05 were regarded as significant. Significant values were assessed with the Mann–Whitney U test. Data was analyzed using the SPSS statistical program (version 17.0 software, SPSS Inc. Chicago, IL, USA).
Results
Lipid Peroxidation and TOS Results
Lipid peroxidation level as MDA is widely used in acting as a biomarker of oxidative stress. The mean brain cortex, plasma and erythrocytes lipid peroxidation and brain cortex TOS levels in four groups were shown in Tables 1, 2 and 3. The results showed that LP levels in the brain cortex (p < 0.05), plasma (p < 0.05) and erythrocyte (p < 0.01) samples, and TOS levels (p < 0.001) in the brain cortex in TBI group were significantly higher than the control. Hence, oxidative stress level in blood and brain of the rats was increased by induction of TBI. However, administrations of NAC and selenium caused decrease the lipid peroxidation level of the brain cortex, plasma and erythrocytes in the rats. The lipid peroxidation levels in the brain cortex (p < 0.05), plasma (p < 0.05) and erythrocyte (p < 0.01) in the TBI + NAC and TBI + Se groups were significantly lower than the TBI group, respectively.
Glutathione Peroxidase (GSH-Px) Activities and Reduced Glutathione (GSH) Results
The mean GSH-Px activities in the brain cortex and erythrocytes in four groups were shown in Tables 1 and 3, respectively. The results showed that the brain cortex and erythrocytes GSH-Px activities were significantly lower in TBI group as compared to control. GSH-Px activity in the erythrocyte was markedly (p < 0.05) increased by NAC treatment although their activities did not increase in brain cortex and erythrocyte by selenium administration.
The mean GSH concentrations in the brain cortex and erythrocytes in four groups were shown in Tables 1 and 3, respectively. The GSH concentrations in the brain cortex and erythrocytes were significantly (p < 0.05) lower in TBI group than the control. However, the decreased brain GSH concentrations improved by NAC and selenium treatments. On the other word, the brain cortex GSH concentrations in TBI + NAC (p < 0.05) and TBI + Se (p < 0.01) groups were significantly higher than in control group.
Results of Antioxidant Vitamin and TAS Concentrations
The mean vitamin A, β-carotene, vitamin C, vitamin E and TAS concentrations in brain cortex and plasma of four groups were shown in Tables 1 and 2, respectively. The brain cortex vitamin A (p < 0.001), β-carotene (p < 0.01), vitamin C, vitamin E (p < 0.05) and TAS concentrations, and plasma vitamin E (p < 0.01) concentration were significantly lower in TBI group than in control. The decreases of vitamin E concentrations in brain cortex and plasma, TAS levels in brain cortex were increased either by NAC (p < 0.05) or selenium (p < 0.01) administrations However, plasma vitamin A concentrations in the four groups did not affect by NAC and selenium treatments.
Results of Cytokine
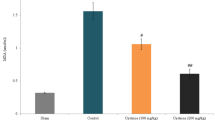
The mean IL-1β and IL-4 levels in plasma of four groups were shown in Figs. 1 and 2, respectively. The plasma IL-1β level is increased by TBI (p < 0.01) although IL-4 level is decreased by TBI (p < 0.001). However, the plasma IL-4 level was significantly (p < 0.001) increased both by NAC and selenium treatments although the plasma IL-1β level was decreased through NAC and selenium treatments (p < 0.001). In addition, plasma IL-4 level was also significantly (p < 0.001) higher in TBI + Se group than in TBI + NAC group.
The effects of NAC and selenium (Se) on plasma IL-4 levels in traumatic brain injury (TBI)-induced rats (mean ± SD and n = 9). a p < 0.001 versus control. b p < 0.001 versus TBI group. c p < 0.001 versus TBI + NAC group. Se and NAC combination was given to the rats 1, 24, 48 and 72 h (four doses) after brain trauma
Discussion
We found that lipid peroxidation and TOS in the brain cortex, erythrocyte and plasma, and IL-1β level in plasma were increased by TBI although brain cortex vitamin A, β-carotene, vitamin C, vitamin E, TAS and GSH, and plasma vitamin E concentrations, plasma IL-4 level and brain cortex and erythrocyte GSH-Px activities decreased by the TBI. Hence, TBI induction in the animals are characterized by increased lipid peroxidation and decreased IL-4, GSH, GSH-Px, and antioxidant vitamin values. Administration of NAC and selenium caused decrease in lipid peroxidation and IL-1β levels although GSH, vitamin E and IL-4 values increased. Hence, we have shown that treatment with NAC and selenium-based treatments modulated the balance of oxidant and antioxidant, pro- and anti-inflammatory cytokines in rats by down-regulating the levels of pro-inflammatory (IL-1β) cytokine and up-regulating the levels of anti-inflammatory (IL-4) cytokine. To the best of our knowledge, the current study is the first to compare the medicine NAC and selenium with particular reference to its effects on oxidative stress, cytokine production and antioxidant redox system in TBI-induced oxidative injury in rats.
The current study indicated that TBI produced a significant increase in lipid peroxidation and TOS levels of the brain cortex, erythrocytes and plasma levels although the lipid peroxidation levels were decreased by NAC and selenium treatments. Our results are in accordance with the previous reports of lipid peroxidation increment in brain, erythrocytes and plasma after TBI [4, 5]. On the other hand, current study is first report regarding effects of NAC and selenium on the oxidative stress in the the brain cortex, erythrocytes and plasma in TBI-induced rats. It well known that TBI-induced increases of ROS in brain in causing secondary brain injury. Overproduction of ROS in brain is a result of over stimulation of glutamate receptors such as N-methyl-d-aspartate receptors by the excessive release of glutamate from presynaptic nerve terminals and astrocytes after primary injury [12]. However, NAC and selenium modulates the brain injury through their antioxidant and anti-inflammatory effects.
Inactivation of ROS can be carried out in neuronal cells by antioxidant vitamins [32, 33]. Vitamin E, alpha-tocopherol, is the most important antioxidant in the lipid phase of cells [10]. Vitamin E acts to protect cells against the effects of free radicals, which are potentially damaging byproducts of the body’s metabolism [23]. Therefore, low antioxidant levels and high content of PUFA, results in limited antioxidant defense in brain. Vitamin A, β-carotene and vitamin E concentrations in the brain cortex were decreased in the TBI group although vitamin E concentrations in the brain cortex and plasma were increased in the NAC and selenium treatment groups. The increased concentrations of the antioxidant vitamins could be due to its depletion or inhibition as a result of the increased production of free radicals. The increase in the brain cortex GSH and vitamin E values in animals during NAC and selenium treatments has been attributed to the inhibition of free radicals and lipid peroxidation [23, 34, 35]. In the current study, brain cortex and erythrocyte antioxidant values were more affected than in plasma by the induction of TBI. Concentrations of the antioxidant vitamins in brain and erythrocytes are relatively low as compared to plasma [36]. In addition, oxidation occurs in nucleic acids, lipids and proteins [1, 36] and it is well known that protein concentrations of the erythrocytes and brain were higher in erythrocyte and brain than in plasma. Hence, significance differences of the oxidant and antioxidant changes were lesser in plasma than in the brain and erythrocytes. Therefore, a combination of the high oxygen consumption, low antioxidant vitamin activities and high content of PUFA and iron, results in limited antioxidant defense in erythrocytes and brain cortex.
GSH-Px enzyme is, a selenium containing protein, is a member of the group of thiol group antioxidant enzyme and it functions in reductive detoxification of hydrogen peroxide and alkyl hydroperoxides [8, 9]. GSH plays an important role in protecting cells against oxidative damage as a non-enzymatic antioxidant and most abundant non protein thiol source in the cells [37]. GSH-Px enzyme uses GSH as substrate during the detoxification of hydrogen peroxide and alkyl hydroperoxides. Xiong et al. [16] demonstrated that TBI caused a significant decrease in hipocampus total GSH-Px levels, suggesting that oxidative stress could occur during inflammation. Xiong et al. [38] demonstrated that NAC treatment given within 1 h greatly restored brain GSH levels from 1 h to 14 days and mitochondrial GSH levels from 12 h to 14 days post-TBI. In the current study, the brain cortex GSH and GSH-Px values, and erythrocyte GSH-Px activities decreased in TBI-induced rats although their values increased by NAC and selenium administrations. Hence, result of the study supported the idea that TBI-induced mitochondrial oxidative stress was reduced by NAC and selenium treatments.
Within minutes of a traumatic impact, a robust inflammatory response is increased in the injured brain. The complexity of this post-traumatic squeal involves a cellular component, comprising the activation of resident glial cells, microglia, and astrocytes, and the infiltration of blood leukocytes. IL-1β is a pro-inflammatory cytokine although IL-4 is an anti-inflammatory cytokine [39]. Increase of IL-1β level is induced by clinical and experimental TBI. IL-1β levels reach a maximum in hippocampus within 1-2 h after TBI [39–41]. Perfusion into the brain of the recombinant mouse IL-1β receptor antagonist reduced lesion volume and nitro-oxidative stress in mice brain [42]. Conversely, IL-1β infusion increased lesion volume in the fluid percussion model of TBI [40]. These observations suggest a key role for IL-1β in the pathophysiology of TBI. NAC has known antioxidant activity, but it decreases pro-inflammatory cytokine expression and oxidative stress after experimental TBI and blocks microglial activation in vitro [43]. In the current study, NAC and selenium treatments reduced these effects via antioxidant and anti-inflammatory properties. On the other word, the increase in the plasma IL-1β levels of TBI exposure groups was decreased by NAC and selenium treatment although the IL-4 levels were increased by the treatments. It was reported that NAC treatment reduced a variety of neurological symptoms and IL-1β expression in rats after mild TBI when dosed within 24 h after TBI suggesting its efficacy as a single drug [44]. Marini et al. [45] reported that lipid peroxidation inhibition reduces IL-1β gene expression and protects against kainic acid-induced brain damage.
In conclusion, our brain and blood results in TBI group are consistent with a generalized antioxidant abnormality in different tissues of clinical and experimental TBI. However, NAC and selenium supplementation have protective effect on oxidative stress and antioxidant redox system in the brain cortex and blood. The beneficial effect of NAC and selenium was regulation of GSH-Px, GSH, antioxidant vitamins, lipid peroxidation, IL-1β and IL-4 values in the brain cortex and blood of TBI-induced rats. Hence, use of the selenium and NAC could be potential approach in arresting or inhibiting the TBI-induced oxidative injury caused by excitotoxic agents.
Abbreviations
- GSH:
-
Glutathione
- GSH-Px:
-
Glutathione peroxidase
- LP:
-
Lipid peroxidation
- MDA:
-
Malondialdehyde
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TAS:
-
Total antioxidant status
- TOS:
-
Total oxidant status
References
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658
Nazıroğlu M (2007) New molecular mechanisms on the activation of TRPM2 channels by oxidative stress and ADP-ribose. Neurochem Res 32:1990–2001
Nazıroğlu M, Yürekli VA (2013) Effects of antiepileptic drugs on antioxidant and oxidant molecular pathways: focus on trace elements. Cell Mol Neurobiol 33:589–599
Mustafa AG, Singh IN, Wang J, Carrico KM, Hall ED (2010) Mitochondrial protection after traumatic brain injury by scavenging lipid peroxyl radicals. J Neurochem 114:271–280
Khan M, Sakakima H, Dhammu TS, Shunmugavel A, Im YB, Gilg AG, Singh AK, Singh I (2011) S-nitrosoglutathione reduces oxidative injury and promotes mechanisms of neurorepair following traumatic brain injury in rats. J Neuroinflamm 8:78
Nazıroğlu M, Yıldız K, Tamtürk B, Erturan İ, Flores-Arce M (2012) Selenium and psoriasis. Biol Trace Elem Res 150:3–9
Nazıroğlu M (2012) Molecular role of catalase on oxidative stress-induced Ca(2+) signaling and TRP cation channel activation in nervous system. J Recept Signal Transduct Res 32:134–141
Nazıroğlu M (2009) Role of selenium on calcium signaling and oxidative stress-induced molecular pathways in epilepsy. Neurochem Res 34:2181–2191
Özgül C, Nazıroğlu M (2012) TRPM2 channel protective properties of N-acetylcysteine on cytosolic glutathione depletion dependent oxidative stress and Ca2+ influx in rat dorsal root ganglion. Physiol Behav 106:122–128
Joshi YB, Praticò D (2012) Vitamin E in aging, dementia, and Alzheimer’s disease. BioFactors 38:90–97
Lee HP, Casadesus G, Zhu X, Lee HG, Perry G, Smith MA, Gustaw-Rothenberg K, Lerner A (2009) All-trans retinoic acid as a novel therapeutic strategy for Alzheimer’s disease. Expert Rev Neurother 9:1615–1621
Bains M, Hall ED (2012) Antioxidant therapies in traumatic brain and spinal cord injury. Biochim Biophys Acta 1822:675–684
Özmen I, Naziroğlu M, Alici HA, Sahin F, Cengiz M, Eren I (2007) Spinal morphine administration reduces the fatty acid contents in spinal cord and brain by increasing oxidative stress. Neurochem Res 32:19–25
Moore M (2013) Mild traumatic brain injury: implications for social work research and practice with civilian and military populations. Soc Work Health Care 52:498–518
Freire MA (2012) Pathophysiology of neurodegeneration following traumatic brain injury. West Indian Med J 61:751–755
Xiong Y, Shie FS, Zhang J, Lee CP, Ho YS (2004) The protective role of cellular glutathione peroxidase against trauma-induced mitochondrial dysfunction in the mouse brain. J Stroke Cerebrovasc Dis 13:129–137
Fang KM, Cheng FC, Huang YL, Chung SY, Jian ZY, Lin MC (2013) Trace element, antioxidant activity, and lipid peroxidation levels in brain cortex of gerbils after cerebral ischemic injury. Biol Trace Elem Res 152:66–74
Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K (1994) A new model of diffuse brain injury in rats. Part I: pathophysiology and biomechanics. J Neurosurg 80:291–300
Oksay T, Nazıroğlu M, Ergün O, Doğan S, Özatik O, Armağan A, Özorak A, Çelik Ö (2013) N-acetylcysteine attenuates diazinon exposure-induced oxidative stress in rat testis. Andrologia 45:171–177
Nazıroğlu M, Kutluhan S, Yilmaz M (2008) Selenium and topiramate modulates brain microsomal oxidative stress values, Ca2+-ATPase activity, and EEG records in pentylentetrazol-induced seizures in rats. J Membr Biol 225:39–49
Placer ZA, Cushman L, Johnson BC (1966) Estimation of products of lipid peroxidation (malonyl dialdehyde) in biological fluids. Anal Biochem 16:359–364
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Nazıroğlu M, Karaoğlu A, Aksoy AO (2004) Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology 195:221–230
Calışkan AM, Naziroğlu M, Uğuz AC, Ovey IS, Sütçü R, Bal R, Calişkan S, Ozcankaya R (2010) Acamprosate modulates alcohol-induced hippocampal NMDA receptors and brain microsomal Ca2+-ATPase but induces oxidative stress in rat. J Membr Biol 237:51–58
Lawrence RA, Burk RF (1976) Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 71:952–958
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Desai ID (1984) Vitamin E analysis methods for animal tissues. Methods Enzymol 105:138–147
Suzuki J, Katoh N (1990) A simple and cheap method for measuring vitamin A in cattle using only a spectrophotometer. Jpn J Vet Sci 52:1282–1284
Jagota SK, Dani HM (1982) A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal Biochem 127:178–182
Erel O (2004) A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem 37:277–285
Paredes SD, Bejarano I, Terrón MP, Barriga C, Reiter RJ, Rodríguez AB (2009) Melatonin and tryptophan counteract lipid peroxidation and modulate superoxide dismutase activity in ringdove heterophils in vivo. Effect of antigen-induced activation and age. Age (Dordr) 31:179–188
Nazıroğlu M (2011) TRPM2 cation channels, oxidative stress and neurological diseases: where are we now? Neurochem Res 36:355–366
Nazıroğlu M, Dikici DM, Dursun S (2012) Role of oxidative stress and Ca(2+) signaling on molecular pathways of neuropathic pain in diabetes: focus on TRP channels. Neurochem Res 37:2065–2075
Yeo JE, Kang SK (2007) Selenium effectively inhibits ROS-mediated apoptotic neural precursor cell death in vitro and in vivo in traumatic brain injury. Biochim Biophys Acta 1772:1199–1210
Yeo JE, Kim JH, Kang SK (2008) Selenium attenuates ROS-mediated apoptotic cell death of injured spinal cord through prevention of mitochondria dysfunction; in vitro and in vivo study. Cell Physiol Biochem 21:225–238
Cimen MY (2008) Free radical metabolism in human erythrocytes. Clin Chim Acta 390:1–11
Nazıroğlu M, Ciğ B, Ozgül C (2013) Neuroprotection induced by N-acetylcysteine against cytosolic glutathione depletion-induced Ca2+ influx in dorsal root ganglion neurons of mice: role of TRPV1 channels. Neuroscience 242:151–160
Xiong Y, Peterson PL, Lee CP (1999) Effect of N-acetylcysteine on mitochondrial function following traumatic brain injury in rats. J Neurotrauma 16:1067–1082
Woodcock T, Morganti-Kossmann MC (2013) The role of markers of inflammation in traumatic brain injury. Front Neurol 4:18
Utagawa A, Truettner JS, Dietrich WD, Bramlett HM (2008) Systemic inflammation exacerbates behavioral and histopathological consequences of isolated traumatic brain injury in rats. Exp Neurol 211:283–291
Jones NC, Prior MJ, Burden-Teh E, Marsden CA, Morris PG, Murphy S (2005) Antagonism of the interleukin-1 receptor following traumatic brain injury in the mouse reduces the number of nitric oxide synthase-2-positive cells and improves anatomical and functional outcomes. Eur J Neurosci 22:72–78
Clausen F, Hånell A, Israelsson C, Hedin J, Ebendal T, Mir AK, Gram H, Marklund N (2011) Neutralization of interleukin-1β reduces cerebral edema and tissue loss and improves late cognitive outcome following traumatic brain injury in mice. Eur J Neurosci 34:110–123
Lijia Z, Zhao S, Wang X, Wu C, Yang J (2012) A self-propelling cycle mediated by reactive oxide species and nitric oxide exists in LPS-activated microglia. Neurochem Int 61:1220–1230
Haber M, Abdel Baki SG, Grin’kina NM, Irizarry R, Ershova A, Orsi S, Grill RJ, Dash P, Bergold PJ (2013) Minocycline plus N-acetylcysteine synergize to modulate inflammation and prevent cognitive and memory deficits in a rat model of mild traumatic brain injury. Exp Neurol 249:169–177
Marini H, Altavilla D, Bellomo M, Adamo EB, Marini R, Laureanti F, Bonaccorso MC, Seminara P, Passaniti M, Minutoli L, Bitto A, Calapai G, Squadrito F (2004) Modulation of IL-1 beta gene expression by lipid peroxidation inhibition after kainic acid-induced rat brain injury. Exp Neurol 188:178–186
Acknowledgments
M.N. and N.Ş. formulated the present hypothesis and was responsible for writing the report. N.Ş. and V.Y. were responsible for the induction of TBI. M.N. was responsible for the analyses. All authors approved the final manuscript. Chemical expenses of the current study were supported by N.Ş. The study was performed in Neuroscience Research Center, Suleyman Demirel University, Isparta, Turkey.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Şenol, N., Nazıroğlu, M. & Yürüker, V. N-Acetylcysteine and Selenium Modulate Oxidative Stress, Antioxidant Vitamin and Cytokine Values in Traumatic Brain Injury-Induced Rats. Neurochem Res 39, 685–692 (2014). https://doi.org/10.1007/s11064-014-1255-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1255-9