Abstract
Question
Which imaging techniques most accurately differentiate true tumor progression from pseudo-progression or treatment related changes in patients with previously diagnosed glioblastoma?
Target population
These recommendations apply to adults with previously diagnosed glioblastoma who are suspected of experiencing progression of the neoplastic process.
Recommendations
Level II
Magnetic resonance imaging with and without gadolinium enhancement is recommended as the imaging surveillance method to detect the progression of previously diagnosed glioblastoma.
Level II
Magnetic resonance spectroscopy is recommended as a diagnostic method to differentiate true tumor progression from treatment-related imaging changes or pseudo-progression in patients with suspected progressive glioblastoma.
Level III
The routine use of positron emission tomography to identify progression of glioblastoma is not recommended.
Level III
Single-photon emission computed tomography imaging is recommended as a diagnostic method to differentiate true tumor progression from treatment-related imaging changes or pseudo-progression in patients with suspected progressive glioblastoma.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Imaging rationale
Primary malignant brain tumors (malignant glioma, anaplastic astrocytoma and glioblastoma multiforme) carry a nearly uniformly dismal outcome. Even the most optimistic predictions of long term survival of greater than 5 years after diagnosis only approach 1 or 2 %, although higher 5-year survival rates have been recently published in one Phase 3 study. The median survival of patients with newly diagnosed glioblastoma has improved slightly in the last decade and may approach 15–18 months [1, 2]. Due to the dismal prognosis, patients with these tumors are treated early in the course of the disease with aggressive multimodality regimens including surgical resection, radiotherapy and chemotherapy [3–5]. Serial imaging has been the mainstay of assessing treatment response, and therefore the decision to alter or even abandon therapy in favor of supportive care is guided heavily by non-invasive radiographic imaging techniques.
However, differentiating treatment effect from true tumor progression is challenging and has been approached with a variety of strategies [6–9].
Traditional gadolinium-enhanced magnetic resonance imaging (MRI) is confounded by overlap of findings as progressive tumor and post-radiation or treatment related effects can both show changes at or near the original tumor site, including increased contrast enhancement, mass effect, and edema. Attempts to utilize the powerful imaging capability of MRI have resulted in alternative advanced MRI techniques, including diffusion-weighted imaging (DWI), dynamic susceptibility contrast-enhanced perfusion imaging (DSC), and MR spectroscopy (MRS). [10] It is theorized that these advanced techniques may provide physiologic information not assessed using conventional anatomic MRI alone. For example the use of proton-based MRS (or 1H-MRS) yields information on the metabolic composition within a selected target area of tissue, conceptually similar to an “electronic biopsy”. Comparison of the relative concentration of these metabolites provides an indication of factors such as cellular membrane turnover and neuronal viability that assists in the assessment of whether viable tumor is present in the sampled region. MRS adds little additional time (15–30 min) to the traditional MRI technique routinely utilized in malignant glioma patient management and therefore is an appealing non-diagnostic technique.
The purpose of this guideline is to assess the ability of the most widely attempted imaging techniques, primarily magnetic imaging based and radiotracer techniques, to accurately differentiate recurrent or progressive tumor (true tumor progression) from imaging artifact and treatment effect (false tumor progression), also referred to more recently as “pseudo-progression” in the setting of suspected progressive glioblastoma. This review has been structured based on the techniques involved and divided broadly into MRI based techniques and radiotracers as outlined below.
Magnetic resonance imaging techniques (MRI)
-
1.
Contrast patterns
-
2.
Perfusion
-
(a)
DSC (dynamic susceptibility contrast)
-
(b)
DCE (dynamic contrast enhanced)
-
(a)
-
3.
DWI/ADC (diffusion weighted imaging/apparent diffusion coefficients).
-
4.
Spectroscopy
Radiotracers
(PET Scanning—Positron Emission Tomography and SPECT—Single Photon Emission Computed Tomography).
-
5.
FDG PET (fluoro-deoxy-glucose positron emission tomography)
-
6.
MET PET (methionine positron emission tomography)
-
7.
SPECT
The overall objectives of this guideline are:
-
1.
To systematically review the evidence available for the imaging of adult patients with previously diagnosed glioblastoma suspected of having progression following initial treatment.
-
2.
To make recommendations based on this evidence for the role of imaging in the management of these patients.
Imaging methodology
Literature review
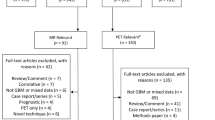
A broad search strategy was used due to the relative small number of studies on each specific topic. PubMed (National Library of Medicine, http://www.ncbi.nlm.nih.gov) was searched using Endnote® (Thomas Reuters, Inc. http://www.endnote.com) using “ALL FIELDS” and entering “RECURRENT GLIOBLASTOMA” AND “IMAGING” without date limits for a broad initial search. The results were then hand searched based on the titles and abstracts to exclude laboratory only studies and titles not on topic. Electronic versions of relevant studies were obtained via secure access to the University of Iowa College of Medicine Hardin Library and when not available were obtained through inter-library loan with the assistance of the medical library at Covenant Medical Center, Waterloo, IA, USA. Two foreign literature papers referenced in a 2006 systematic review by Hollingworth et al. [10], one in Japanese with English abstract and one in German with English abstract were included as there was an English abstract, the abstracts could be reviewed for relevance and the data required to construct a 2 by 2 Bayesian table was available. Other foreign language papers if there was no English abstract or the table data could not be abstracted the study was excluded.
Article inclusion and exclusion criteria
All studies addressing imaging of adult patients with a known diagnosis of a glioblastoma containing information on diagnostic results of correctly identifying whether the tumor has progressed were selected for review. Exclusion criteria included: pediatric population, newly diagnosed patients as the study focus, laboratory only studies, non-human studies, and the focus of paper was a clinical trial rather than a diagnostic study.
Data collection and review
Two independent reviewers abstracted data for each article and the two sets of data were compared for agreement by a third party. Inconsistencies were re-reviewed and disagreements were resolved by consensus. Data collected included the following: author year, age of study population, study addressed previously diagnosed patients with glioblastoma, study included imaging data on the diagnosis of progressive or progressive glioblastoma, number of patients in each group, image results either positive or negative, clinical results indicating the presence of absence of progressive malignant glioblastoma, and, when possible, study allows completion of a Bayesian table for calculation of sensitivity and specificity.
Study selection and quality assessment
Following broad screening for relevance, two independent reviewers evaluated citations and full text screening of potentially relevant papers using a priori criteria for data extraction on a standardized form. Disagreements were resolved with the involvement of a third reviewer, followed by primary re-review until agreement was achieved. Both the quality of the evidence and the eventual strength of the recommendations generated by this evidence were graded according to a three-tiered system for assessing studies addressing diagnostic testing as approved by the American Association of Neurological Surgeons (AANS)/Congress of Neurological Surgeons (CNS) Joint Committee on Guidelines criteria [4, 5, 11].
Imaging scientific foundation
Overall, 57 publications met the eligibility criteria and are included in the evidentiary tables below (see Tables 1, 2, 3, 4, 5 and 6). These included 11 focused on MRI contrast techniques (see Table 1), 8 on MRI perfusion techniques (see Table 2), 5 on MRI diffusion techniques (see Table 3), 13 on MRS (see Table 4), 10 on PET techniques (see Table 5) and 10 on SPECT techniques (see Table 6).
In addition, data extracted from 4 papers provides information on combination imaging techniques and multi-parametric analysis (see Table 7).
MRI techniques: contrast patterns
MRI with and without gadolinium contrast administration has been cited as the imaging modality of choice in previous evidence based reviews both for newly diagnosed and progressive glioblastoma, although the direct evidence supporting these recommendations is surprisingly small [3, 12]. At the present time we agree that MRI with gadolinium represents the “gold standard” as there is also little evidence to the contrary. The Level 1 recommendation made in the previously published guideline for imaging in newly diagnosed glioblastoma is based on a single blinded histopathological study including a total of 72 patients, 36 with glioblastoma, undergoing MRI imaging with pathological correlation which found a sensitivity of 0.89, specificity of 0.82, positive predictive value of 0.88, negative predictive value (NPV) of 0.88 and an overall accuracy of 0.86 for the ability of MRI to accurately diagnose glioblastoma [13]. The evidence based review of recurrent malignant glioma by Easaw et al. [3], states a Level 1 recommendation for the role of MRI, but does not cite any specific references to support this and presumably this recommendation was arrived at by consensus.
Johnson et al. [14] published the results of a study comparing T2-weighted post mortem magnetic resonance (MR) images of glioma patients and the histologic findings from whole-brain sections [15]. This study included 10 patients with recurrent glioblastoma. In general there was a high correlation of the MRI images with the pathologic findings, but resulted in overestimations (24 %) or underestimations (28 %) of tumor extent in the cases of recurrent tumor. However, the authors concluded that MRI accurately identified the presence of tumor with a high-degree of sensitivity (no false negatives were described).
The value of serial imaging in the management of glioblastoma is in early detection of treatment failure to guide intervention. The fact that progression free survival at 6 months has been shown to correlate with overall survival, to the point that is has been recommended as a primary trial endpoint, increases the importance of diagnosing early tumor progression [15–17].
Several points are of relevance when considering the situation of primary glioblastoma progression and treatment response. Although it is generally assumed that increased enhancement, edema and mass effect will accompany all cases of true tumor progression, a pooled study examining outcome measures from Phase II clinical trials for progressive glioblastoma did find that 14 of 375 (3.7 %) of patients progressed clinically without radiographic changes [18].
Pseudo-progression and pseudo-response are recent phenomena that are causing increasing discussion. Pseudo-progression has been defined as an increase in contrast enhancement and/or edema on MRI without true tumor progression and has most often been associated with the combination or radiotherapy and temozolamide [7, 8, 19, 20]. In contrast pseudo-response, refers to a decrease in enhancement on MRI without a true antitumor effect, generally associated with anti-angiogenic targeted therapy [21–25]. Both of these radiographic based phenomena are having an increasing impact on treatment planning due to the increasing use of both temozolomide and the anti-angiogenic agents, bevacizumab or cediranib. The traditional Macdonald response assessment generally based on MRI data interpretation is particularly prone to problems as a result of the variable pathological progression and treatment response of the glioblastoma [26]. Irregular growth patterns, irregular contrast-enhancement and infiltration all complicate methodology based on cross-sectional expansion alone. The more recently proposed RANO criteria have been proposed specifically to address this shortcoming and provide a more realistic set of parameters to determine true progression. These proposed criteria take into account not just MR enhanced response but T2 changes, steroid use and clinical status. It is assumed that as new studies are designed, these potentially more relevant criteria will be utilized and therefore it will be possible to assess whether they truly represent an improved response assessment. They are, however, currently not validated [27–29].
The pattern of MRI contrast enhancement has been investigated in a number of studies [30–38].
This set of publications includes one Class II study using criteria for diagnostic testing and is listed in evidentiary Table 1 below [31]. In the study by Aiken et al., the authors describe the differentiation of true progression from pseudo-progression with a sensitivity of 0.95 and specificity of 0.67 using the pattern of recurrence as the differentiating criteria with nodular enhancement strongly suggesting tumor progression and feathery enhancement suggesting radiation necrosis (seen in the group receiving brachytherapy).
Supporting Class III studies are also listed in evidentiary Table 1 below [14, 17, 30]. The study by Taal et al. [37] found a sensitivity and specificity of 0.67 in determining true from pseudo-progression and conclude that pseudo-progression is common and needs to be high in the differential diagnosis of increasing enhancement on serial imaging. Of note factors suggestive of pseudoprogression included T1 enhancement without edema [34], appearance of necrosis in a previously non-enhancing area after treatment, enhancing focus developing at a distance from the original occurrence, enhancement near periventricular white matter tracts, and soap bubble or Swiss cheese pattern [35]. Other authors noted that 80–90 % of all progression occurred with 2 cm of the original tumor bed [30, 36] and that increasing surface tumor volume was associated with decreased survival [38].
Based on the Class II and supporting Class III data discussed above and summarized in Evidentiary Table 1, the following Level 2 recommendation is made: MRI with and without gadolinium enhancement is recommended as an imaging surveillance method to detect the progression of previously diagnosed glioblastoma, with a sensitivity of 0.95, a specificity of 0.67 and an overall accuracy of 0.83.
MRI techniques: perfusion DSC and on DCE
Developing MRI techniques include the ability to study the perfusion of central nervous system tissue using DSC and dynamic contrast enhancement. Eight studies were reviewed and included in evidentiary Table 2 below [39–46]. All were considered Class III studies, primarily due to lack of control groups, inability to create Bayesian tables and lack of relevant validation groups.
Barajas et al. [40] retrospectively evaluated 57 patients with progressive glioblastoma to investigate whether cerebral blood volume (CBV), peak height (PH), and percentage of signal intensity recovery (PSR), all measurements derived from the results of T2-weighted dynamic susceptibility-weighted contrast material-enhanced (DSC) MRI performed after external beam radiation therapy (EBRT) could be used to distinguish progressive glioblastoma multiforme from pseudo-progression or radiation necrosis. Both PH and CBV were significantly higher (p < 0.01) in patients with recurrent GBM than in patients with radiation necrosis. Additionally, the PSR values were significantly lower (p < 0.05) in patients with recurrent GBM than in patients with radiation necrosis. Although a non-validated study and no specific guidelines were proposed, the authors conclude that the use of DSC perfusion MRI may allow differentiation of recurrent GBM from pseudo-progression.
Vrabec et al. [46] retrospectively reviewed 32 imaging studies in 8 patients with glioblastoma to evaluate patients at various stages of follow-up: stable, prior to progression and following clear progression. They evaluated the regional CBV (rCBV) and the apparent diffusion coefficient (ADC—see also next section) and concluded that maximum rCBV and minimum ADC correspond to tumor recurrence or progression. Ringelstein et al. [42] prospectively studied 12 patients with progressive high-grade glioma (number of GBM patients not specified) and found that ADC mapping was predictive of tumor response to treatment in over 80 % of the patients and gave an indication of which patients were at highest risk of progression. In a similar prospective study of 17 patients undergoing treatment for high grade glioma (including 11 patients with GBM), Al Sayyari et al. [39] concluded that an increase in enhancement volume (as well as a reduction in ADC, see further discussion below) supports a diagnosis of progressive tumor. Although these studies are small and non-validated, the high rate of identifying responding versus progressive tumor was encouraging.
Several authors have proposed new metrics using MRI perfusion data. Hu et al. [41] published a prospective series of 25 patients with suspected progression of GBM at craniotomy and defined a new metric called pMRI fractional tumor burden (see entry in Evidentiary Table 2 for definition) showing that when compared to other blood volume parameters this variable demonstrated a significant correlation with overall survival (r = 0.82, p < 0.0001). Sawlani et al. [43] also describe a novel imaging metric, the hyperperfusion volume (HPV), which reflects local perfusion change in progressive GBM and correlated significantly with time to progression. Sorenson et al. [44] used three parameters (changes in vascular permeability/flow, in microvessel volume, and circulating collagen IV level) to create a vascular normalization index (VNI) that correlated with overall survival (R = 0.54; p = 0.004) and progression-free survival (R = 0.6; p = 0.001). Stenberg et al. [45] that elevated rCBV suggests progressive tumor; however, rapidly growing lesions can be misinterpreted as reactive non-tumor changes (pseudoprogression).
While potentially significant metrics, incorporation into recommendations will await further study and validation in independent relevant populations. The planning and execution of well-designed clinical trials for these promising techniques is strongly encouraged.
MRI techniques: perfusion DWI and ADC
Additional MRI based techniques include diffusion weighted imaging (DWI) and ADC mapping, as introduced along with CBV determination in the section above. All data reviewed was Class III, but promises with additional study to provide valuable information in the patient with progressive glioblastoma [39, 47–50] The details of these studies are included in evidentiary Table 3 and the conclusions are highlighted below.
Paldino et al. [47] retrospectively reviewed images obtained from 15 patients with progressive GBM evaluating the prognostic significance of diffusion tensor imaging (DTI) following treatment with bevacizumab (BEV) and irinotecan. DTI detected changes in ADC within the flair signal abnormality after therapy, which correlated with a significantly shorter overall survival (p = 0.032) and progression free survival (p = 0.046).
Al Sayyari et al. [39] prospectively studied 17 patients previously treated for high-grade glial neoplasms (including 11 patients with GBM) presenting with new enhancing lesions to evaluate the relationship between contrast enhancement and ADC mapping. The presence of new enhancement coupled with a reduction in ADC supports a diagnosis of tumor progression.
Zeng et al.’s prospective single-center study of 55 patients with suspected recurrent GBM examined a variety of imaging techniques. ADC value and ADC ratios (ADC of contrast-enhancing lesion to the matching structure in the contralateral hemisphere) were significantly higher in radiation injury regions than in recurrent tumor (p < 0.01). The authors summarize by indicating that the ADC value can be added to discriminant analysis, to improve the ability to differentiate recurrent glioma and radiation injury [50].
Rollin et al. published their prospective study of 28 patients with intraaxial brain tumors who underwent conventional MR imaging (T2- and T1-weighted sequences after gadolinium injection), diffusion imaging and T2 weighted echo-planar perfusion imaging. Determinations of rCBV and ADC were performed in the solid parts of each tumor; peritumoral region and contra-lateral white matter. They found that rCBV values were increased in all recurrent tumors. Diffusion and perfusion imaging, even with relatively short imaging and data processing times, provided important information for lesion characterization [48].
Sundgren et al. conducted a retrospective review of 28 patients with progressive malignant glioma (including 4 patients with GBM) using mean ADC, fractional anisotropy (FA) and eigenvalues to assess the use of DTI in the evaluation of new contrast-enhancing lesions and peri-lesional edema to differentiate progressive neoplasm from treatment-related injury. The mean ADC was significantly greater comparing progressive to non-progressive groups (1.27 ± 0.15 × 10−3 mm2/s vs 1.12 ± 0.14 × 10−3 mm2/s, p = 0.01). ADC ratios in the white matter tracts in peri-lesional edema trended higher (p = 0.09) in treatment-related injury than in recurrent neoplasm (mean ± SD = 1.85 ± 0.30 vs 1.60 ± 0.27, respectively). FA ratios were significantly higher in normal-appearing white matter) tracts adjacent to the edema in the non-recurrence group (mean ± SD = 0.89 ± 0.15) than in those in the recurrence group (mean ± SD = 0.74 ± 0.14; p = 0.03). The authors conclude that the assessment of diffusion properties, (ADC values and ADC ratios), in contrast-enhancing lesions, peri-lesional edema and normal white matter adjacent to the edema can differentiate radiation injury from tumor progression [49].
While these studies represent intriguing possibilities, incorporation into recommendations will await further study and validation in independent relevant populations. As with the MRI perfusion techniques, the planning and execution of well-designed clinical trials for these promising techniques is strongly encouraged.
MRI techniques: spectroscopy
One of the earliest specialized MRI techniques was MRS, which has been increasingly utilized to monitor treatment effect and tumor growth. The use of proton-based MRS (or 1H-MRS) yields information on the metabolic composition within a selected target area of tissue, conceptually similar to an “electronic biopsy”. Comparison of the relative concentration of these metabolites provides an indication of factors such as cellular membrane turnover and neuronal viability that assists in the assessment of whether viable tumor is present in the sampled region. MRS adds little additional time (15–30 min) to the traditional MRI technique routinely utilized in progressive glioblastoma patient management and therefore is an appealing non-diagnostic technique. Multiple publications addressing the role of MRS are available for review in Evidentiary Table 4) [10, 50–61].
Only one previous systematic review on the topic was identified [10]. Hollingworth et al. [10] evaluated the role of MRS in characterizing brain tumors and included differentiating radiation necrosis from progressive tumor in patients with primary brain tumors in papers published between 2002 and 2004. This paper included four studies that are included in this guideline. Although no combined summary statistic was calculated the results indicated a range of sensitivity of 64–89 % and specificity of 82–89 % as follows:
Traber et al. [58] sensitivity 72 % (95 % CI 53–86 %) specificity 82 % (95 % CI 48–98 %). Ando et al. [51] sensitivity 64 % (95 % CI 35–87 %) specificity 83 % (95 % CI 36–100 %). Lichy et al. [62] sensitivity 87 % (95 % CI 60–98 %) specificity 89 % (95 % CI 52–100 %). Plotkin et al. [63] 89 % sensitivity 83 % specificity (no CI). Three studies containing Class II data are included in evidentiary Table 4 below, [58–60] only one of which is included in the above systematic review [58].
In the study by Weybright et al. [60], the ability of MRS to differentiate progressive tumor from radiation necrosis was evaluated in 28 patients in a single institution non-blinded retrospective study. Cho/Cr (choline/creatine) and Cho/NAA (choline/N-acetyl aspartate) ratios were significantly higher, and the NAA/Cr ratios significantly lower, in tumor than in radiation injury (all three comparisons, p < 0.0001). Cho/Cr and Cho/NAA ratios were significantly higher in radiation injury than in normal-appearing white matter (p < 0.0003 and p < 0.0001, respectively), whereas NAA/Cr ratios were not (p = 0.075). Using a cutoff value of 1.8 for Cho/Cr and/or Cho/NAA, 27 of 28 patients were correctly classified (accuracy 0.96). The authors conclude MRS can differentiate tumor from radiation injury in patients with suspected progressive glioblastoma with a high degree of sensitivity and specificity (0.94 and 0.96 respectively).
Traber et al. [58] described a prospective single-institution study of 42 patients with suspected progressive glioma including 34 patients with progressive GBM and 8 patients with anaplastic astrocytoma evaluated with MRS. In this study the sensitivity was 0.72, the specificity was 0.82 and the overall accuracy was 0.74. This study is a well-conducted prospective study that has an unrestricted study population that is predominately GBM. It is downgraded to Class 2 only because it is not possible to create a completely separate Bayesian analysis that excludes the 8 anaplastic astrocytoma patients from the data presented.
Finally, in an older study Wald et al. [59] published their small series of 12 patients with progressive GBM evaluated with serial MRS with sensitivity of 0.55 and specificity of 1.00 and an overall accuracy of 0.58. This is an older study and small in size but well-conducted. A well conducted meta-analysis creating a summary statistic for sensitivity and specificity would address this issue more appropriately.
Three high-quality studies provide additional supportive data that would be at least Class II for imaging in progressive glioblastoma but contains either a less uniform patient population or the results specifically for GBM are not clear and are therefore lowered to Class III for the purposes of this study [51, 55, 56], Ando et al. [51] published a retrospective single institution study of 20 patients with progressive glioblastoma evaluated with MRS using Cho/Cr ratio of 1.5 or greater used as an indicator of tumor presence. This small series (published in Japanese) found a sensitivity of 0.64 and a specificity of 0.83 with an overall accuracy of 0.70. The authors conclude that Cho/Cr ratio of 1H-MRS differentiate residual/recurrent gliomas from non-neoplastic lesions.
Plotkin et al. [63] published a prospective single-institute non-blinded study of MRS (Cho/Cr and Cho/NAA ratio of 1.11 and 1.17, respectively). MRS resulted in sensitivity of 0.89, specificity 1.00 and overall accuracy of 92 %. The authors conclude that MRS was successful in differentiating progressive tumor from radiation necrosis although in this study SPECT yielded slightly more favorable results, as discussed below. Rabinov et al. [56] reported a prospective single institution study of 17 patients with recurrent glioma evaluated with MRS using a Cho/Cr cutoff ratio of 1.3 to differentiate progressive tumor from inactive tumor or treatment effect. In this study the sensitivity was 1.00, the specificity was 0.88 and the overall accuracy was 0.94.
An additional six papers containing Class 3 data are summarized in Evidentiary Table 4 below [50, 52, 54, 57, 61, 62], This additional Class 3 data indicates that MRS identifies progressive tumor from treatment effect/necrosis with a sensitivity ranging from 0.55 to 1.00, a specificity ranging from 0.50 to 1.00 and an overall accuracy ranging from 0.58 to 1.00. The two largest series had sensitivities of 0.9 and 0.93, specificity of 1.00 and accuracy of 0.95 and 0.96, providing additional supporting evidence for the ability of MRS to discriminate progression from treatment effect.
Based on the Class II and supporting Class III data discussed above and summarized in Evidentiary Table 4, the following Level 2 recommendation is made: MRS is recommended as a diagnostic method to differentiate true tumor progression from treatment-related imaging changes or pseudo-progression in patients with suspected progressive glioblastoma with a sensitivity ranging from 0.72 to 0.94, a specificity ranging from 0.82 to 1.00 and an overall accuracy ranging from 0.58 to 0.96.
Radiotracers
FDG PET (fluoro deoxy-glucose positron emission tomography) and MET PET (methionine positron emission tomography)
PET is used widely in non-CNS cancer paradigms to differentiate progressive tumor from treatment effect due to the metabolic incorporation of the tracer molecule into the dividing tumor cells. From review of the available studies the role of radiolabelled tracers (fluoro-deoxy glucose (FDG) and MET) has yet to establish a role in the routine evaluation of primary central nervous system tumor progression, however, further work will determine if this technique will add significantly to the discrimination of progression from treatment effect. Petrirena et al. [64] provide an optimistic view of the continuing role of PET in brain imaging but provides no usable data for the purposes of this guideline.
There are several papers evaluating the role of PET included in the evidence Table 5 providing Class II data [65–67]. Of note, however, is the fact that the Class II nature of this data addresses the choice of radiotracer and provides information at that level if the choice has already been made to use PET in this type of imaging. They are not high-level comparative studies to other techniques such as MRS or SPECT. Therefore, they do not support a Level II recommendation for or against the overall role of PET imaging. Taken together there is insufficient data to support the routine use of PET imaging in the evaluation of progressive glioblastoma and therefore the routine use is not recommended. The strength of this recommendation is Level 3.
Enslow et al. [65] prospectively evaluated 15 patients with suspected recurrent glioma. The study compared the role of F-fluorodeoxyglucose (FDG) and F-fluorothymidine (FLT) PET in differentiating radiation necrosis from progressive glioblastoma, and while this provides Class 2 data on the comparison (and found that the use of FLT did not improved the predictive ability of PET over the more established FDG), it found a sensitivity of 0.91, a specificity of 0.50, a positive predictive value (PPV) of 0.80 and a NPV of 0.20. Despite the prospective study design, the low specificity and very low NPV argue against recommendation and the small size reduces the value of the study for either technique alone and underscores concerns over the routine role of PET techniques for recurrent primary brain tumors.
Tripathi et al. [67] reported their prospective comparative study of F-18 flurodeoxyglucose (FDG) PET and C-11 MET PET for the evaluation of in 35 patients with recurrent primary brain tumors. FDG predicted recurrence in 15/35 (42 %) and MET predicted recurrence in 24/34 (70.5 %) with sensitivities of 0.81 and 0.95 and specificities of 0.89 and 0.89 respectively. The authors conclude that if PET imaging is employed then MET should be the radiotracer of choice.
Potzi et al. [66] also published a prospective comparative study of F-18 FDG PET and C-11 MET PET for the evaluation of 28 patients with suspected progressive brain tumors and added an evaluation of the results predicting survival. Neither FDG nor MET uptake correlated with either survival time or disease duration, although similar to the Tripathi study, these authors found the sensitivity of MET uptake was better than FDG.
Additional Class III data is provided in the evidence Table 5 evaluating the role of PET imaging in recurrent malignant glioma including the role of monitoring chemotherapy or treatment response [68–70], improved targeting of biopsy with radiolabelled peptides [71], the role of glucose improving fluoro-deoxy-glucose (FDG) [72] and failure of fluoro-thymidine (FLT) to correlate with MRI gadolinium uptake (Table 5) [73].
In 1991, Chin et al. [68], described in a retrospective report of 2 patients followed with serial PET imaging concluded that, “PET has a promising role in neuroradiology for accurate diagnosis and prognostication of malignant tumors as well as differential diagnosis of radiation necrosis and recurrent tumors. Particularly, PET has proven its ability to accurately differentiate radiation necrosis from recurrent brain tumor.” From a review of the subsequent available studies, this statement appears to have been overly optimistic.
Based on Class III data discussed above and summarized in Evidentiary Table 5, high-quality comparative studies of PET with other techniques such as MRS or SPECT are not available resulting in a Level 3 recommendation that the routine use of PET imaging in the evaluation of progressive glioblastoma is not recommended.
Radiotracers: CT SPECT
A systematic review published in 2007 by Vos et al. [74], determined the diagnostic accuracy of 201Tl SPECT in the detection of recurrence in patients with previous radiotherapy for supratentorial glioma. Studies included a minimum of 6 patients with 201Tl SPECT study and pathological confirmation. Eight studies met the inclusion criteria for this systematic review but only one was considered high quality and a meta-analysis could not be performed due to methodological variability [73, 75–81] The sensitivities reported ranged from 0.43 to 1.0, specificities ranged from 0.25 to 1.00 and the diagnostic odds ratio was significant in all eight studies ranging from 2 to over 350, for detecting tumor progression for supratentorial glioma. However, in seven of the eight studies it was not possible to differentiate the patients with progressive glioblastoma and therefore only one of the studies is included in this review [79]. In follow up of this review, Vos et al. [82] published a prospective trial in 2012 involving 46 patients undergoing both MRI and (201)Tl SPECT imaging procedures to compare their prognostic value. Both strongly related to OS (p < 0.02) but the addition of one imaging modality to the other did not contribute to the prediction of OS. This was considered Class II data based on prognosis but not on diagnosis but is included as important data supportive of both MRI and SPECT.
Several studies provide Class 3 data in support of SPECT imaging in progressive glioblastoma [63, 79], Plotkin et al. [63] reported their prospective single institute non-blinded study including 25 patients undergoing SPECT imaging (cut-off value 1.62) with suspected progressive malignant glioma and found a sensitivity of 0.95, a specificity of 1.00 and an accuracy of 1.00 which was slightly superior to the MRS in the same population (described above). The authors conclude that both SPECT and MRS were successful in differentiating recurrent tumor from radiation necrosis. 123I-IMT SPECT yielded slightly more favorable results in this study.
Schwartz et al. [79] published a prospective study of 47 patients undergoing reoperation for suspected progressive glioblastoma imaged with 201Tl SPECT and 99mTc-hexamethypropyleneamine oxime (HMPAO). The patients were divided into three groups based on pathological findings and imaging as indicated in the evidentiary table. The authors conclude that SPECT data correlates with pathological findings and survival.
Additional Class III data is included in the evidentiary tables as supporting information including data on supporting the role of SPECT differentiating progressive tumor from radiation necrosis [76, 83], comparison with FDG PET, [77], impact of steroid use reducing uptake [84] and impact on prognostic ability [85].
Based on the Class III data discussed above and summarized in Evidentiary Table 6, the following Level III recommendation is made: SPECT imaging is recommended as a diagnostic method to differentiate true tumor progression from treatment-related imaging changes or pseudo-progression in patients with suspected progressive glioblastoma with expected sensitivity ranging from 0.94 to 0.95 and an expected specificity ranging from 0.63 to 1.00. One study reported a positive predictive value of 0.92.
Combination studies and multi-parametric analysis
Several investigators have described methodologies to combine or model the combination of multiple imaging modalities to improve the diagnostic and prognostic accuracy of available techniques. All of these studies represent Class 3 data and lack validation and therefore are not suitable for use in recommendations at this time (see Table 7). They are all exciting possible techniques to foster additional study.
Zeng et al. [61] analyzed the combination of various MRS generated values including N-acetylaspartate (NAA), choline (Cho), creatine (Cr), lipid (Lip), and lactate (Lac) in conjunction with the ADC and correlated with histopathology (gold standard) using discriminant analysis. After analyzing various combinations, the authors described a predictive accuracy of over 96 % in predicting tumor progression. If this type of analysis were validated in larger independent study populations, it would be of potential benefit in redefining the optimal imaging protocol for detecting tumor progression.
Similarly, in a pilot study, Matsusue et al. [54] describe using a multiparametric scoring system including MR DWI, DSC-enhanced perfusion imaging, and MRS based on a retrospective single center non-blinded study that included 6 patients with recurrent GBM. The optimum thresholds for ADC ratio (1.30), rCBV ratio (2.10), and either combined Cho/Cr (1.29) and Cho/NAA (1.06) yielded diagnostic accuracies of 86.7, 86.7, and 84.6 %, respectively (p < 0.05). In this study the accuracy of MRS alone was only 67 % whereas the combined multiparametric score improved diagnostic accuracy to 93.3 % (p < 0.05). As with the study by Zeng et al., this model remains non-validated but does offer the promise of improving diagnostic accuracy to the point of limiting the need for invasive diagnostic procedures.
Galban et al. prospectively studied 45 patients with high grade glioma who underwent surgical resection followed by radiotherapy and concurrent temozolamide treatment with MRI using DWI and DSC-MRI at 3 and 10 weeks after treatment and found that a composite of ADC and rCBV, as analyzed by parametric response map enhanced the sensitivity of the biomarker for predicting those patients resistant to chemo-radiation at 3 weeks post treatment initiation [86].
The combination of multiple imaging results and multi-parametric analysis may well represent the solution to improving the diagnostic accuracy of imaging for progressive glioblastoma. However, as stated for the advanced MRI techniques, these studies represent intriguing possibilities; incorporation into recommendations will await validation in independent relevant populations.
Imaging summary and discussion
The current data on the role of imaging in progressive or recurrent glioblastoma available is lacking in high levels of evidence due primarily to poor study design, heterogeneity of the patient population, and variability in practices at the time of progression and general lack of prospectively collected data with comparable groups in this challenging patient population. Despite these shortcomings, recommendations can be formulated from the available data.
Based on our current review, Class II data supports a recommendation for MRI with and without gadolinium enhancement in the evaluation of patients with suspected progressive glioblastoma with a reasonable diagnostic accuracy. In order to improve the sensitivity, specificity and diagnostic accuracy of imaging, based on Class II data, MRS be recommended to further differentiate true tumor progression from treatment-related imaging changes or pseudo-progression in patients with suspected progressive malignant glioma. Similarly, Class III data supports the role of SPECT, but with a larger range of sensitivity and specificity. The reviewed data on PET indicates that the routine use of PET to identify progression of glioblastoma is not recommended. Additional high-quality studies to clarify the appropriate role of MRI-based perfusion and diffusion techniques, and to evaluate the combination of imaging results are strongly encouraged.
Although the current status of non-invasive imaging in progressive glioblastoma remains controversial, a detailed review of the relevant literature suggests that available imaging techniques are of benefit in the follow up care of glioblastoma patients and may add additional prognostic information.
A primary goal of serial imaging is to establish the time point when progression occurs in order to appropriately guide subsequent clinical decision-making. While the current recommendations are made on the best available information, the goal of future studies and updates to these recommendations will be to more accurately determine the optimal technique, timing and interpretation of non-invasive imaging in the management of patients with progressive glioblastoma.
Conclusions and key issues for future investigation
The recent review of the phenomena of “pseudoprogression” published by Sanghera et al. [87], highlights the importance of this area of study. The ability to detect early progression is critical to responding early with a change of treatment strategy in this deadly progressive tumor. In contrast, it is also critically important to identify treatment strategies which may benefit the patient but which may simultaneously also result in radiographic changes such as additional contrast enhancement not indicative of true progression. Being aware of such treatment effects whenever possible prevents the clinician from discarding a treatment strategy too early in the course of therapy as there are limited options available and each need to be used to its maximal potential benefit in order to have any impact on overall survival. Although the pathophysiology of pseudoprogression is poorly understood, it is important that it be recognized promptly. Current recommendations for imaging follow-up are based on less than optimal data and increase the rationale to provide the best quality studies and meta-analyses available to guide future recommendations. A series of well-designed studies would greatly clarify the issue of the diagnostic accuracy of current and future imaging techniques in identifying progressive tumor. Larger and more diverse study populations and studies performed in prospective fashion using validated criteria would address two major concerns in study design. A more specific definition of the “gold standard” (either tissue diagnosis or clinical signs of progression or some combination of the two) and a more specified definition of a “positive” versus “negative” test result would reduce heterogeneity. Attempts to blind the interpretation of results would also improve the quality of the design. In the future, this study design could be used to facilitate comparison between non-invasive imaging techniques of potential benefit in identifying true tumor progression. If all techniques prove roughly equivalent, a cost-effective analysis would be of significant impact and benefit.
The level of recommendation is based on the best available evidence, so the quality of the studies and data collected requires continued improvement and education of the investigators. Several investigators have described methodologies to combine or model the combination of multiple imaging modalities to improve the diagnostic and prognostic accuracy of available techniques.
References
Darefsky AS, King JT Jr, Dubrow R (2012) Adult glioblastoma multiforme survival in the temozolomide era: a population-based analysis of surveillance, epidemiology, and end results registries. Cancer 118(8):2163–2172
Koshy M, Villano JL, Dolecek TA et al (2012) Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J Neurooncol 107(1):207–212
Easaw JC, Mason WP, Perry J et al (2011) Canadian recommendations for the treatment of recurrent or progressive glioblastoma multiforme. Current Oncol 18(3):e126–e136
Olson JJ, Fadul CE, Brat DJ, Mukundan S, Ryken TC (2009) Management of newly diagnosed glioblastoma: guidelines development, value and application. J Neurooncol 93(1):1–23
Olson JJ, Ryken T (2008) Guidelines for the treatment of newly diagnosed glioblastoma: introduction. J Neurooncol 89(3):255–258
Brandes AA, Tosoni A, Spagnolli F et al (2008) Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: pitfalls in neurooncology. Neuro Oncol 10(3):361–367
Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ (2008) Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9(5):453–461
Brandsma D, van den Bent MJ (2009) Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol 22(6):633–638
Sanghera P, Perry J, Sahgal A et al (2010) Pseudoprogression following chemoradiotherapy for glioblastoma multiforme. Can J Neurol Sci 37(1):36–42
Hollingworth W, Medina LS, Lenkinski RE et al (2006) A systematic literature review of magnetic resonance spectroscopy for the characterization of brain tumors. AJNR Am J Neuroradiol 27(7):1404–1411
Matz PG, Anderson PA, Kaiser MG et al (2009) Introduction and methodology: guidelines for the surgical management of cervical degenerative disease. J Neurosurg Spine 11(2):101–103
Mukundan S, Holder C, Olson JJ (2008) Neuroradiological assessment of newly diagnosed glioblastoma. J Neurooncol 89(3):259–269
Dean BL, Drayer BP, Bird CR et al (1990) Gliomas: classification with MR imaging. Radiology 174(2):411–415
Johnson PC, Hunt SJ, Drayer BP (1989) Human cerebral gliomas: correlation of postmortem MR imaging and neuropathologic findings. Radiology 170(1 Pt 1):211–217
Ballman KV, Buckner JC, Brown PD et al (2007) The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro Oncol 9(1):29–38
Lamborn KR, Yung WK, Chang SM et al (2008) Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol 10(2):162–170
Wick W, Stupp R, Beule AC et al (2008) A novel tool to analyze MRI recurrence patterns in glioblastoma. Neuro Oncol 10(6):1019–1024
Hess KR, Wong ET, Jaeckle KA et al (1999) Response and progression in recurrent malignant glioma. Neuro Oncol 1(4):282–288
Brandes AA, Franceschi E, Tosoni A et al (2008) MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 26(13):2192–2197
Gerstner ER, McNamara MB, Norden AD, Lafrankie D, Wen PY (2009) Effect of adding temozolomide to radiation therapy on the incidence of pseudo-progression. J Neurooncol 94(1):97–101
Batchelor TT, Duda DG, di Tomaso E et al (2010) Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol 28(17):2817–2823
Vredenburgh JJ, Desjardins A, Herndon JE 2nd et al (2007) Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25(30):4722–4729
Friedman HS, Prados MD, Wen PY et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27(28):4733–4740
Kreisl TN, Kim L, Moore K et al (2009) A phase I trial of enzastaurin in patients with recurrent gliomas. Clin Cancer Res Off J Am Assoc Cancer Res 15(10):3617–3623
Kreisl TN, Kim L, Moore K et al (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27(5):740–745
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8(7):1277–1280
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28(11):1963–1972
Wen PY, Norden AD, Drappatz J, Quant E (2010) Response assessment challenges in clinical trials of gliomas. Curr Oncol Rep 12(1):68–75
van den Bent MJ, Vogelbaum MA, Wen PY, Macdonald DR, Chang SM (2009) End point assessment in gliomas: novel treatments limit usefulness of classical Macdonald’s Criteria. J Clin Oncol 27(18):2905–2908
Agbi CB, Bernstein M, Laperriere N, Leung P, Lumley M (1992) Patterns of recurrence of malignant astrocytoma following stereotactic interstitial brachytherapy with iodine-125 implants. Int J Radiat Oncol Biol Phys 23(2):321–326
Aiken AH, Chang SM, Larson D, Butowski N, Cha S (2008) Longitudinal magnetic resonance imaging features of glioblastoma multiforme treated with radiotherapy with or without brachytherapy. Int J Radiat Oncol Biol Phys 72(5):1340–1346
Curnes JT, Laster DW, Ball MR, Moody DM, Witcofski RL (1986) MRI of radiation injury to the brain. AJR. Am J Roentgenol 147(1):119–124
Dooms GC, Hecht S, Brant-Zawadzki M, Berthiaume Y, Norman D, Newton TH (1986) Brain radiation lesions: MR imaging. Radiology 158(1):149–155
Kleinberg L, Yoon G, Weingart JD et al (2009) Imaging after GliaSite brachytherapy: prognostic MRI indicators of disease control and recurrence. Int J Radiat Oncol Biol Phys 75(5):1385–1391
Kumar AJ, Leeds NE, Fuller GN et al (2000) Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 217(2):377–384
Sneed PK, Gutin PH, Larson DA et al (1994) Patterns of recurrence of glioblastoma multiforme after external irradiation followed by implant boost. Int J Radiat Oncol Biol Phys 29(4):719–727
Taal W, Brandsma D, de Bruin HG et al (2008) Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer 113(2):405–410
Valery CA, Marro B, Boyer O et al (2001) Extent of tumor-brain interface: a new tool to predict evolution of malignant gliomas. J Neurosurg 94(3):433–436
Al Sayyari A, Buckley R, McHenery C, Pannek K, Coulthard A, Rose S (2010) Distinguishing recurrent primary brain tumor from radiation injury: a preliminary study using a susceptibility-weighted MR imaging-guided apparent diffusion coefficient analysis strategy. AJNR Am J Neuroradiol 31(6):1049–1054
Barajas RF Jr, Chang JS, Segal MR et al (2009) Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 253(2):486–496
Hu LS, Eschbacher JM, Heiserman JE et al (2012) Reevaluating the imaging definition of tumor progression: perfusion MRI quantifies recurrent glioblastoma tumor fraction, pseudoprogression, and radiation necrosis to predict survival. Neuro Oncol 14(7):919–930
Ringelstein A, Turowski B, Gizewski ER et al (2010) Evaluation of ADC mapping as an early predictor for tumor response to chemotherapy in recurrent glioma treated with bevacizumab/irinotecan: proof of principle. RoFo : Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 182(10):868–872
Sawlani RN, Raizer J, Horowitz SW et al (2010) Glioblastoma: a method for predicting response to antiangiogenic chemotherapy by using MR perfusion imaging—pilot study. Radiology 255(2):622–628
Sorensen AG, Batchelor TT, Zhang WT et al (2009) A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res 69(13):5296–5300
Stenberg L, Englund E, Wirestam R, Siesjo P, Salford LG, Larsson EM (2006) Dynamic susceptibility contrast-enhanced perfusion magnetic resonance (MR) imaging combined with contrast-enhanced MR imaging in the follow-up of immunogene-treated glioblastoma multiforme. Acta Radiol (Stockholm, Sweden: 1987) 47(8):852–861
Vrabec M, Van Cauter S, Himmelreich U et al (2011) MR perfusion and diffusion imaging in the follow-up of recurrent glioblastoma treated with dendritic cell immunotherapy: a pilot study. Neuroradiology 53(10):721–731
Paldino MJ, Desjardins A, Friedman HS, Vredenburgh JJ, Barboriak DP (2011) A change in the apparent diffusion coefficient after treatment with bevacizumab is associated with decreased survival in patients with recurrent glioblastoma multiforme. Br J Radiol 13(12):1349–1363
Rollin N, Guyotat J, Streichenberger N, Honnorat J, Tran Minh VA, Cotton F (2006) Clinical relevance of diffusion and perfusion magnetic resonance imaging in assessing intra-axial brain tumors. Neuroradiology 48(3):150–159
Sundgren PC, Fan X, Weybright P et al (2006) Differentiation of recurrent brain tumor versus radiation injury using diffusion tensor imaging in patients with new contrast-enhancing lesions. Magn Reson Imaging 24(9):1131–1142
Zeng QS, Li CF, Liu H, Zhen JH, Feng DC (2007) Distinction between recurrent glioma and radiation injury using magnetic resonance spectroscopy in combination with diffusion-weighted imaging. Int J Radiat Oncol Biol Phys 68(1):151–158
Ando K, Ishikura R, Nagami Y et al (2004) Usefulness of Cho/Cr ratio in proton MR spectroscopy for differentiating residual/recurrent glioma from non-neoplastic lesions. Nihon Igaku Hoshasen Gakkai Zasshi 64(3):121–126
Chuang CF, Chan AA, Larson D et al (2007) Potential value of MR spectroscopic imaging for the radiosurgical management of patients with recurrent high-grade gliomas. Technol Cancer Res Treat 6(5):375–382
Lichy MP, Henze M, Plathow C, Bachert P, Kauczor HU, Schlemmer HP (2004) Metabolic imaging to follow stereotactic radiation of gliomas—the role of 1H MR spectroscopy in comparison to FDG-PET and IMT-SPECT. RoFo : Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 176(8):1114–1121
Matsusue E, Fink JR, Rockhill JK, Ogawa T, Maravilla KR (2010) Distinction between glioma progression and post-radiation change by combined physiologic MR imaging. Neuroradiology 52(4):297–306
Plotkin M, Gneveckow U, Meier-Hauff K et al (2006) 18F-FET PET for planning of thermotherapy using magnetic nanoparticles in recurrent glioblastoma. Int J Hyperth 22(4):319–325
Rabinov JD, Lee PL, Barker FG et al (2002) In vivo 3-T MR spectroscopy in the distinction of recurrent glioma versus radiation effects: initial experience. Radiology 225(3):871–879
Srinivasan R, Phillips JJ, Vandenberg SR et al (2010) Ex vivo MR spectroscopic measure differentiates tumor from treatment effects in GBM. Neuro Oncol 12(11):1152–1161
Traber F, Block W, Flacke S et al (2002) 1H-MR spectroscopy of brain tumors in the course of radiation therapy: use of fast spectroscopic imaging and single-voxel spectroscopy for diagnosing recurrence. RoFo : Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 174(1):33–42
Wald LL, Nelson SJ, Day MR et al (1997) Serial proton magnetic resonance spectroscopy imaging of glioblastoma multiforme after brachytherapy. J Neurosurg 87(4):525–534
Weybright P, Sundgren PC, Maly P et al (2005) Differentiation between brain tumor recurrence and radiation injury using MR spectroscopy. AJR Am J Roentgenol 185(6):1471–1476
Zeng QS, Li CF, Zhang K, Liu H, Kang XS, Zhen JH (2007) Multivoxel 3D proton MR spectroscopy in the distinction of recurrent glioma from radiation injury. J Neurooncol 84(1):63–69
Lichy MP, Bachert P, Henze M, Lichy CM, Debus J, Schlemmer HP (2004) Monitoring individual response to brain-tumour chemotherapy: proton MR spectroscopy in a patient with recurrent glioma after stereotactic radiotherapy. Neuroradiology 46(2):126–129
Plotkin M, Eisenacher J, Bruhn H et al (2004) 123I-IMT SPECT and 1H MR-spectroscopy at 3.0 T in the differential diagnosis of recurrent or residual gliomas: a comparative study. J Neurooncol 70(1):49–58
Petrirena GJ, Goldman S, Delattre JY (2011) Advances in PET imaging of brain tumors: a referring physician’s perspective. Curr Opin Oncol 23(6):617–623
Enslow MS, Zollinger LV, Morton KA et al (2012) Comparison of 18F-fluorodeoxyglucose and 18F-fluorothymidine PET in differentiating radiation necrosis from recurrent glioma. Clin Nucl Med 37(9):854–861
Potzi C, Becherer A, Marosi C et al (2007) [11C] methionine and [18F] fluorodeoxyglucose PET in the follow-up of glioblastoma multiforme. J Neurooncol 84(3):305–314
Tripathi M, Sharma R, Varshney R et al (2012) Comparison of F-18 FDG and C-11 methionine PET/CT for the evaluation of recurrent primary brain tumors. Clin Nucl Med 37(2):158–163
Chin HW, Fruin AH, Bertoni JM et al (1991) Application of positron emission tomography to neurological oncology. Neb Med J 76(3):70–73
Galldiks N, Kracht LW, Burghaus L et al (2010) Patient-tailored, imaging-guided, long-term temozolomide chemotherapy in patients with glioblastoma. Mol Imaging 9(1):40–46
Eary JF, Mankoff DA, Spence AM et al (1999) 2-[C-11]thymidine imaging of malignant brain tumors. Cancer Res 59(3):615–621
Schnell O, Krebs B, Carlsen J et al (2009) Imaging of integrin alpha(v)beta(3) expression in patients with malignant glioma by [18F] galacto-RGD positron emission tomography. Neuro Oncol 11(6):861–870
Ishizu K, Sadato N, Yonekura Y et al (1994) Enhanced detection of brain tumors by [18F]fluorodeoxyglucose PET with glucose loading. J Comput Assist Tomogr 18(1):12–15
Yamamoto Y, Wong TZ, Turkington TG, Hawk TC, Reardon DA, Coleman RE (2006) 3’-Deoxy-3’-[F-18]fluorothymidine positron emission tomography in patients with recurrent glioblastoma multiforme: comparison with Gd-DTPA enhanced magnetic resonance imaging. Mol Imaging Biol 8(6):340–347
Vos MJ, Tony BN, Hoekstra OS, Postma TJ, Heimans JJ, Hooft L (2007) Systematic review of the diagnostic accuracy of 201Tl single photon emission computed tomography in the detection of recurrent glioma. Nucl Med Commun 28(6):431–439
Carvalho PA, Schwartz RB, Alexander E 3rd et al (1992) Detection of recurrent gliomas with quantitative thallium-201/technetium-99m HMPAO single-photon emission computerized tomography. J Neurosurg 77(4):565–570
Kline JL, Noto RB, Glantz M (1996) Single-photon emission CT in the evaluation of recurrent brain tumor in patients treated with gamma knife radiosurgery or conventional radiation therapy. AJNR Am J Neuroradiol 17(9):1681–1686
Lorberboym M, Baram J, Feibel M, Hercbergs A, Lieberman L (1995) A prospective evaluation of thallium-201 single photon emission computerized tomography for brain tumor burden. Int J Radiat Oncol Biol Phys 32(1):249–254
Schwartz RB, Carvalho PA, Alexander E 3rd, Loeffler JS, Folkerth R, Holman BL (1991) Radiation necrosis vs high-grade recurrent glioma: differentiation by using dual-isotope SPECT with 201TI and 99mTc-HMPAO. AJNR Am J Neuroradiol 12(6):1187–1192
Schwartz RB, Holman BL, Polak JF et al (1998) Dual-isotope single-photon emission computerized tomography scanning in patients with glioblastoma multiforme: association with patient survival and histopathological characteristics of tumor after high-dose radiotherapy. J Neurosurg 89(1):60–68
Slizofski WJ, Krishna L, Katsetos CD et al (1994) Thallium imaging for brain tumors with results measured by a semiquantitative index and correlated with histopathology. Cancer 74(12):3190–3197
Sonoda Y, Kumabe T, Takahashi T, Shirane R, Yoshimoto T (1998) Clinical usefulness of 11C-MET PET and 201T1 SPECT for differentiation of recurrent glioma from radiation necrosis. Neurol Med Chir 38(6):342–347 (discussion 347–348)
Vos MJ, Berkhof J, Hoekstra OS et al (2012) MRI and thallium-201 SPECT in the prediction of survival in glioma. Neuroradiology 54(6):539–546
Kosuda S, Fujii H, Aoki S et al (1994) Prediction of survival in patients with suspected recurrent cerebral tumors by quantitative thallium-201 single photon emission computed tomography. Int J Radiat Oncol Biol Phys 30(5):1201–1206
Namba H, Togawa T, Yui N et al (1996) The effect of steroid on thallium-201 uptake by malignant gliomas. Eur J Nucl Med 23(8):991–992
Vertosick FT Jr, Selker RG, Grossman SJ, Joyce JM (1994) Correlation of thallium-201 single photon emission computed tomography and survival after treatment failure in patients with glioblastoma multiforme. Neurosurgery 34(3):396–401
Galban CJ, Chenevert TL, Meyer CR et al (2011) Prospective analysis of parametric response map-derived MRI biomarkers: identification of early and distinct glioma response patterns not predicted by standard radiographic assessment. Clin Cancer Res Off J Am Assoc Cancer Res 17(14):4751–4760
Sanghera P, Rampling R, Haylock B et al (2012) The concepts, diagnosis and management of early imaging changes after therapy for glioblastomas. Clin Oncol (Royal College of Radiologists (Great Britain)) 24(3):216–227
Acknowledgments
We would like to acknowledge the AANS/CNS Joint Guidelines Committee for their review, comments and suggestions, the contributions of Laura Mitchell, CNS Guidelines Manager for organizational assistance, Maxine Brown for searching for and retrieving literature and Amy Allison for reference library consultations. We would also like to acknowledge the following individual JGC members for their contributions throughout the review process: Sepideh Amin-Hanjani, MD, FAANS, FACS, FAHA, Martina Stippler, MD, Alexander Khalessi, MD, Isabelle Germano, MD, Sean D. Christie, MD, FRCS (C), Gregory J. Zipfel, MD, Zachary Litvack, MD, MCR, Ann Marie Flannery, MD, Patricia B Raksin, MD, Joshua M. Rosenow, MD, FACS, Steven Casha, MD, PhD, Julie G. Pilitsis, MD, PhD, Gabriel Zada, MD, Adair Prall, Krystal Tomei, MD, Gregory W Hawryluk, MD.
Conflict of interest (COI)
Task Force members report potential COIs prior to beginning work on the guideline and at the time of publication. COI disclosures are reviewed by the Task Force Chair and taken into consideration when determining writing assignments. Resolution of potential COIs included Task Force members were assigned to chapters that did not involve or in any way relate to the potential COIs disclosed.
Disclaimer of liability
The information in these guidelines reflects the current state of knowledge at the time of completion. The presentations are designed to provide an accurate review of the subject matter covered. These guidelines are disseminated with the understanding that the recommendations by the authors and consultants who have collaborated in their development are not meant to replace the individualized care and treatment advice from a patient’s physician(s). If medical advice or assistance is required, the services of a physician should be sought. The proposals contained in these guidelines may not be suitable for use in all circumstances. The choice to implement any particular recommendation contained in these guidelines must be made by a managing physician in light of the situation in each particular patient and on the basis of existing resources.
Funding source
These guidelines were funded exclusively by the CNS and Tumor Section of the American Association of Neurological Surgeons and the Congress of Neurological Surgeons whom received no funding from outside commercial sources to support the development of this document unless otherwise stated in this section.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ryken, T.C., Aygun, N., Morris, J. et al. The role of imaging in the management of progressive glioblastoma. J Neurooncol 118, 435–460 (2014). https://doi.org/10.1007/s11060-013-1330-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-013-1330-0