Abstract
Introduction
This paper aims to study the value of MRI and Thallium 201 (201Tl) single-photon emission computed tomography (SPECT) in the prediction of overall survival (OS) in glioma patients treated with temozolomide (TMZ) and to evaluate timing of radiological follow-up.
Methods
We included patients treated with TMZ chemoradiotherapy for newly diagnosed glioblastoma multiforme (GBM) and with TMZ for recurrent glioma. MRIs and 201Tl SPECTs were obtained at regular intervals. The value of both imaging modalities in predicting OS was examined using Cox regression analyses.
Results
Altogether, 138 MRIs and 113 201Tl SPECTs in 46 patients were performed. Both imaging modalities were strongly related to OS (P ≤ 0.02). In newly diagnosed GBM patients, the last follow-up MRI (i.e., after six adjuvant TMZ courses) and SPECT (i.e., after three adjuvant TMZ courses) were the strongest predictors of OS (P = 0.01). In recurrent glioma patients, baseline measurements appeared to be the most predictive of OS (P < 0.01). The addition of one imaging modality to the other did not contribute to the prediction of OS.
Conclusions
Both MRI and 201Tl SPECT are valuable in the prediction of OS. It is adequate to restrict to one of both modalities in the radiological follow-up during treatment. In the primary GBM setting, MRI after six adjuvant TMZ courses contributes significantly to the prediction of survival. In the recurrent glioma setting, baseline MRI appears to be a powerful predictor of survival, whereas follow-up MRIs during TMZ seem to be of little additional value.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In glioma patients, follow-up during and after treatment is usually performed with clinical and imaging assessments, and therapeutic efficacy is evaluated with standard criteria for response and progression. In 1990, Macdonald et al. recommended criteria for response assessment in high-grade glioma, based on two-dimensional measurement of CT or MRI contrast-enhancing tumor area while considering the use of corticosteroids and changes in neurologic functioning [1]. These criteria enabled the comparison of response rates between high-grade glioma clinical trials and have been widely used since their introduction. However, it is increasingly apparent that the Macdonald criteria have a number of limitations, such as difficulties in measuring nonenhancing or multifocal tumors and interobserver variability [2]. Furthermore, contrast enhancement in itself is not tumor specific and may be influenced by changes in corticosteroid doses [3, 4], postsurgical changes, ischemia, and seizure activity. Changes in contrast enhancement can also represent post-radiotherapy abnormalities [5–9], and pseudoprogression or pseudoresponse [10–16]. Recently, a proposal for updated response criteria in high-grade glioma was outlined by the RANO Working Group, including serial MRI evaluation of tumor contrast enhancement as well as the nonenhancing (T2/FLAIR) component [17].
Other brain imaging techniques, like single-photon emission computed tomography (SPECT) and positron emission tomography (PET) are supposed to be more tumor specific than conventional MRI techniques. Thallium 201 (201Tl) SPECT is useful in the differentiation of tumor recurrence and radiation necrosis [18–21] and in the evaluation of glioma treatment response [22–26].
Nonetheless, the relation between radiological abnormalities and patient survival is not always clear, and one might argue what the benefit is of stringent imaging follow-up in glioma patients. For example, recent anti-angiogenic studies with bevacizumab in combination with irinotecan demonstrated an increased tumor response on (conventional) MRI in patients with high tumor vascular endothelial growth factor expression, which was not associated with survival benefit, thus not reflecting true tumor response (pseudoresponse) [13, 14, 16].
The aim of the current analysis was to study the value of MRI and 201Tl SPECT in the prediction of overall survival in glioma patients treated with TMZ. Furthermore, the prognostic value of multiple, sequential imaging assessments was evaluated, in order to optimize timing of radiological follow-up during treatment.
Methods
Patients
Two studies (study A and B) were performed in this prospective trial, which was approved by the local medical ethics committee. Patients were asked to participate by their treating physician and informed consent was obtained.
In study A, we included patients treated with radiotherapy in combination with concomitant and six cycles adjuvant TMZ for newly diagnosed GBM. The treatment evaluation of these patients consisted of MRI and 201Tl SPECT postoperatively, i.e., prior to the concomitant phase (baseline), and prior to the adjuvant phase. Thereafter, additional MRI was performed after three and six courses and 201Tl SPECT after three adjuvant TMZ courses (flowchart interventions imaging, see Table 1).
Study B, comprised patients treated with TMZ for recurrent glioma. The treatment evaluation of these patients consisted of MRI and 201Tl SPECT prior to chemotherapy (baseline), and additional MRI after three, six, and nine courses, and 201Tl SPECT after three and nine TMZ courses (Table 1).
The difference in scanning frequency between MRI and 201Tl SPECT was related to the applied 201Tl SPECT radiation dose.
Imaging
MRI scans were performed on a 1.5-T MRI scanner (Siemens Sonata, Siemens Medical Systems, Erlangen, Germany). Imaging protocol included axial T2- and T1-weighted spin-echo images before and after gadolinium administration. Tumor size was defined as the product of the two largest perpendicular transverse enhancing tumor diameters measured on a post-contrast T1-weighted image.
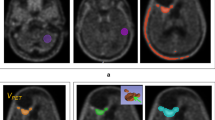
SPECT was started 30 min after intravenous injection of 150 MBq 201Tl-chloride, using a dual-head gamma camera (ECAM, Siemens, Chicago, IL). Projection data were acquired with a 64 × 64 matrix, 60 s per projection. Images were reconstructed with a Hanning filter (cut-off frequency, 0.56 cycles/cm), without attenuation correction. Previously validated maximal tumor intensity was defined as the ratio of the mean tumor counts in the axial slice with the maximal tumor activity and the mean activity in the contralateral supratentorial cerebral hemisphere, and expressed as tumor–nontumor ratio (TNT) (Fig. 1) [25, 27]. Based on this definition, the maximal intensity of normal brain tissue is one, and accordingly, in case no tumor activity is detected by 201Tl SPECT, TNT is one.
Manual region of interest (ROI) definition technique: focally enhanced uptake in a left-sided glioma, showing the axial slice with the maximal tumor uptake. Typical manually drawn ROI displayed around the tumor (plus sign), the contralateral hemisphere (A), its contralateral mirror (B) and the scalp (C)
Analysis
Both MRIs and 201Tl SPECTs were evaluated prospectively, by one examiner (MJV and OSH, respectively) who was blinded to the alternative method and clinical outcome. In the analyses, absolute tumor measurements (MRI tumor size and 201Tl SPECT maximal tumor intensity) as well as relative changes in tumor measurement were used.
Age, tumor histology, and Karnofsky performance score (KPS) were included as clinical variables, of which the latter was obtained repeatedly at the different follow-up time-points. Overall survival (OS) was used as outcome variable, and defined as the interval from baseline imaging to death.
Statistical analysis
The value of MRI and 201Tl SPECT, in combination with clinical variables, in predicting OS was examined by Cox regression analysis with time-dependent covariates. This type of analysis is a variant of the common Cox regression analysis where the value of covariate MRI or 201Tl SPECT is updated when a new imaging result becomes available [28]. The effects of the regression coefficients were evaluated using a Wald test (two-tailed test, P < 0.05). We examined the effects of MRI tumor size and 201Tl SPECT maximal tumor intensity on OS separately, and in combination with each of the clinical variables. In these analyses, all MRI and 201Tl SPECT scans were used. In case one of both was missing, the preceding scan result was carried forward (analysis I). We also calculated cut-off values for MRI tumor size (in cm2) and 201Tl SPECT maximal tumor intensity (in TNT) in the prediction of OS by maximizing the log-likelihood of a Cox model with regard to the cut-off value. Ninety-five percent confidence intervals (95% CI) for the cut-off values were obtained by the profile likelihood method.
In subsequent analyses (analysis II), we assessed whether renewing MRIs and 201Tl SPECTs during the course of treatment would contribute significantly to the prediction of OS. We started with a regression model with only the baseline MRI or 201Tl SPECT measurement as a covariate for the whole time window regardless whether the MRI or 201Tl SPECT had been updated during follow-up. We estimated the effect of renewing the scan at the second time-point on OS by extending the regression model with the renewed MRI or 201Tl SPECT measurement as a covariate. The time window of this covariate is from the second time-point onwards. The effects of renewing the scans at the third and fourth time-points were estimated in an analogous way.
The effect of renewing MRI or 201Tl SPECT at a certain time-point on OS is equivalent to the effect of the absolute change in MRI or 201Tl SPECT (when comparing that time-point with the preceding time-point). We repeated analysis II studying the effect of relative change in MRI tumor size or 201Tl SPECT maximal tumor intensity (instead of absolute change) on OS.
The descriptive analyses were performed in SPSS 15.0 (SPSS Inc, Chicago, IL), and the Cox regression analyses were performed in STATA 10 (StataCorp LP, College Station, TX).
Results
Patient characteristics
From September 2004 to October 2008, 46 patients treated with TMZ in our institution were included: 24 patients for study A and 22 patients for study B. Patient characteristics and outcomes are shown in Table 2.
In study A, tumor histology was GBM, except for one patient with an astrocytoma (grade II), who had undergone surgery 1 year before, and started with TMZ chemoradiotherapy under the suspicion of tumor progression and dedifferentiation to a GBM. All GBM patients were operated on recently, and underwent standard focal fractionated radiotherapy (60 Gy) in combination with concomitant TMZ chemotherapy (75 mg/m2 daily during radiotherapy). After a 4-week interval, adjuvant TMZ treatment was given on a standard schedule (150–200 mg/m2 daily for 5 days, repeated every 28 days; six courses). All patients were chemonaive. Two patients did not receive adjuvant TMZ as a result of persistent myelosuppression. Eighty-one MRI and 65 201Tl SPECT scans were performed. The median interval between baseline imaging and start of chemoradiotherapy was 6 days. The median OS was 15 months, and the median (clinical) progression-free survival (PFS) was 9 months. Ten out of 24 patients had not died as of October 2008, two of which had already shown tumor progression. Survival data of these patients were censored at this date.
In study B, initial tumor histologies included GBM (n = 8), astrocytoma, (n = 6), anaplastic astrocytoma (n = 3), oligodendroglioma (n = 2), anaplastic oligodendroglioma (n = 2), and oligoastrocytoma (n = 1). Because of the heterogeneity of histology in this relatively small patient group, histology was dichotomised (GBM versus non-GBM). All patients had previously undergone craniotomy and 20 out of 22 irradiation. The median interval between initial glioma therapy and start of TMZ chemotherapy for recurrent disease was 33 (range, 4–187) months. Pathological verification of recurrent disease was obtained in 13 of 22 patients (seven GBMs versus six non-GBMs). TMZ was given on a standard schedule (150–200 mg/m2 daily for 5 days, repeated every 28 days). Fifty-seven MRIs and 48 201Tl SPECTs were performed. The median interval between baseline imaging and start of chemotherapy was 7 days. The median OS and PFS were 10 and 5 months, respectively. Six out of 22 patients had neither shown tumor progression nor had died as of October 2008. Survival data of these patients were censored at this date.
Study A: newly diagnosed GBM
Table 3 shows the results of regression analyses in which we examined the effects of MRI and 201Tl SPECT on OS in newly diagnosed GBM patients.
Overall, both MRI tumor size and 201Tl SPECT maximal tumor intensity correlated negatively with OS (P ≤ 0.02; corresponding hazard ratio (HR), 2.1 and 2.3, respectively; analysis I). The effect of MRI on OS remained significant when age or KPS was added to the model (P ≤ 0.01; results not tabulated). Cox regression analyses with both MRI and 201Tl SPECT as predictors demonstrated that the addition of one imaging modality to the other did not contribute to the prediction of OS. Cut-off values for MRI and 201Tl SPECT imaging modalities were 16 (95% CI, 10–21) and 2.5 (95% CI, 2.1–2.9), respectively.
Evaluating the effect of renewing MRIs and 201Tl SPECTs during the course of treatment (analysis II), we found that MRI at baseline was not significantly related to OS. Renewing MRI at time-point 3 and 4 contributed significantly to the prediction of OS (P = 0.04 and 0.01, respectively), whereas renewing the scan at time-point 2 had no significant effect on OS. In contrast to MRI, 201Tl SPECT at baseline was related to OS (P = 0.03). Renewing the 201Tl SPECT scan at time-point 3 also contributed significantly to the prediction of OS (P = 0.01). In the analyses with relative change as a covariate, only the change in MRI tumor size from time-point 3 to time-point 4 had a significant effect on OS (P ≤ 0.01; HR, 2.3). For 201Tl SPECT, the relative change from time-point 2 to time-point 3 had a significant effect on OS (P ≤ 0.01; HR, 512).
Figure 2 demonstrates SPECT images of two patients with respectively high (TNT 3.1, Fig. 2a) and low (TNT 1.4, Fig. 2b) 201Tl uptake, with corresponding T1-weighted post-gadolinium MR images representing the two largest perpendicular transverse enhancing tumor diameters (in Fig. 2a only the enhancing area with residual tumor postoperatively was calculated).
SPECT images of two patients with respectively high (TNT, 3.1) (a) and low (TNT, 1.4) (b) 201Tl uptake in right frontal, respectively, left parietotemporal localized tumor, with corresponding T1-weighted post-gadolinium MR images representing the two largest perpendicular transverse enhancing tumor diameters (in (a), only the enhancing area with residual tumor postoperatively was calculated)
Study B: recurrent glioma
Table 4 shows the results of regression analyses in which we examined the effects of MRI and 201Tl SPECT on OS of recurrent glioma patients.
Overall, both MRI tumor size and 201Tl SPECT maximal tumor intensity correlated negatively with OS (P < 0.01; HR, 1.5 and 2.8, respectively; analysis I). The effect of MRI on OS remained significant when age, KPS or tumor histology were added to the model (P ≤ 0.01; results not tabulated). Cox regression analyses with both MRI and 201Tl SPECT as predictors demonstrated that the addition of one imaging modality to the other did not contribute to the prediction of OS. Cut-off values for MRI and SPECT imaging modalities were 7 (95% CI, 1–10) and 1.7 (95% CI, 1.0–2.0), respectively.
Evaluating the effect of renewing MRIs and 201Tl SPECTs during the course of treatment (analysis II), results for MRI and 201Tl SPECT were largely comparable, though essentially different from the results of study A. Both for MRI and 201Tl SPECT, tumor measurements at baseline were related to OS (P < 0.01; HR, 2.2 and 5.0, respectively), whereas subsequent MRIs and 201Tl SPECTs during the course of treatment (time-points 2–4) did not contribute to the prediction of OS.
In the analyses with relative change as a covariate, the change in MRI tumor size from time-point 1 (baseline) to time-point 2 contributed significantly to the prediction of OS (P < 0.01; HR, 1.3). Relative 201Tl SPECT changes did not have a significant effect on OS.
Discussion
In the current study, we found that both MRI and 201Tl SPECT are valuable in the prediction of survival in glioma patients treated with TMZ. Furthermore, we found that the addition of one imaging modality to the other does not contribute to the prediction of survival.
In newly diagnosed GBM patients, the predictive capacity of both modalities appeared to be highest at the last follow-up time-point, i.e., after six adjuvant TMZ courses in case of MRI and after three adjuvant courses in case of 201Tl SPECT. MRI tumor size at baseline and post-concomitantly had no significant prognostic value for survival. We hypothesize that early after chemoradiotherapy the predictive capacity of MRI is influenced by pseudoprogression. In our cohort, 22% of patients met the definition of pseudoprogression, which is in line with data from literature [11, 12, 15]. Our imaging sample was too small to study the value of 201Tl SPECT in this setting.
In recurrent glioma patients, baseline MRI and 201Tl SPECT appeared to be the most predictive of OS, although the relative change in MRI tumor size from baseline to beyond three TMZ courses had a significant effect on OS, which is in line with our previous analyses [25, 26].
Data on the prognostic value of conventional contrast-enhanced MRI in the prediction of survival in glioma patients are somewhat conflicting [25, 26, 29–32]. Baseline MRI tumor size was not significantly related to survival in our newly diagnosed GBM patients. In literature, however, there is some evidence that more extensive surgical resection is associated with longer survival in glioma patients [33]. For 201Tl SPECT, similar data are scarce, yet 201Tl SPECT maximal tumor intensity was found to be a powerful predictor of survival in recurrent glioma patients [25, 26], which is supported by the current results.
To overcome limitations of conventional imaging techniques in the follow-up of glioma patients, the value of several SPECT tracers and other functional brain imaging techniques, such as PET, and advanced MRI techniques, such as perfusion imaging, dynamic contrast-enhanced MRI, diffusion imaging, and magnetic resonance spectroscopy, is being explored. Technetium-99m methoxyisobutylisonitrile SPECT, technetium-99m hexamethylpropylene amine oxime SPECT, and C-11-methionine PET seem to be useful in the differentiation of tumor recurrence and radiation necrosis and in the early detection of response to chemotherapy in glioma patients [18, 34, 35]. Perfusion MRI provides functional information on the regional cerebral blood volume, reflecting the degree of microvascular proliferation in tumor tissue, and has been used for grading, differentiation of tumor recurrence versus treatment-induced changes, and prediction of survival in glioma patients [36–41]. Apparent diffusion coefficient histogram analysis from diffusion-weighted MRI may predict response to anti-angiogenic therapy in patients with recurrent high-grade gliomas, and may be more sensitive than treatment assessment based solely on RANO criteria [42, 43]. Potential advantages of MR spectroscopy in glioma therapy include refinement of preoperative differential diagnosis, biopsy-site selection and distinction of progressive tumor from treatment effects [41, 44, 45].
Regarding the prognostic value of clinical variables, the results of the current study are largely in accordance with our previous analyses and with other glioma studies [25, 46]. In recurrent glioma patients, both KPS and histology were strongly related to OS. However, in multivariate analyses with MRI and 201Tl SPECT, the effect of the clinical variables on OS decreased, underlining the strength of MRI and 201Tl SPECT in the prediction of outcome. In patients treated with combined chemoradiotherapy for newly diagnosed GBM, the prognostic value of the clinical variables was poor, probably as a result of patient selection bias.
Our prospective study has some limitations. At first, our study sample is relatively small. Second, pathology in our recurrent glioma patients is varied. Therefore, our data should be substantiated in a larger cohort of glioma patients.
Regarding the question how glioma patients should be monitored radiologically during treatment, it seems adequate to restrict to one of both imaging modalities. Since MRI is also useful in the initiation and planning of potential additional treatment modalities (e.g., re-operation/-irradiation in case of tumor progression), in combination with the more invasive character of 201Tl SPECT, it seems logical to prefer MRI.
Regarding the timing of imaging in the course of treatment, a distinction can be made between newly diagnosed GBM and recurrent glioma patients. Our survival analyses indicate that, in patients treated with TMZ chemoradiotherapy for newly diagnosed GBM, MRI after six adjuvant TMZ courses contributes significantly to the prediction of survival. In patients treated with TMZ for recurrent glioma, baseline MRI appears to be a powerful predictor of survival, whereas follow-up MRIs during chemotherapy seem to be of little additional value.
The question as to whether stringent imaging follow-up in glioma patients is superior to follow-up without routine imaging in a clinically stable patient remains to be answered. In every day clinical neuro-oncology practice, the therapeutic dilemma how to deal with an asymptomatic glioma patient with new radiological abnormalities during follow-up appears. This dispute can only be answered in a large, prospective trial in which several modes of follow-up assessment are compared: clinical follow-up versus follow-up with clinical and routine imaging.
References
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Vos MJ, Uitdehaag BMJ, Barkhof F et al (2003) Interobserver variability in the radiological assessment of response to chemotherapy in glioma. Neurology 60:826–830
Cairncross JG, Macdonald DR, Pexman JH, Ives FJ (1988) Steroid-induced CT changes in patients with recurrent malignant glioma. Neurology 38:724–726
Watling CJ, Lee DH, Macdonald DR, Cairncross JG (1994) Corticosteroid-induced magnetic resonance imaging changes in patients with recurrent malignant glioma. J Clin Oncol 12:1886–1889
Cairncross JG, Pexman JH, Rathbone MP, DelMaestro RF (1985) Postoperative contrast enhancement in patients with brain tumor. Ann Neurol 17:570–572
Henegar MM, Moran CJ, Silbergeld DL (1996) Early postoperative magnetic resonance imaging following nonneoplastic cortical resection. J Neurosurg 84:174–179
Ulmer S, Braga TA, Barker FG et al (2006) Clinical and radiographic features of peritumoral infarction following resection of glioblastoma. Neurology 67:1668–1670
Finn MA, Blumenthal DT, Salzman KL, Jensen RL (2007) Transient postictal MRI changes in patients with brain tumors may mimic disease progression. Surg Neurol 67:246–250
Kumar AJ, Leeds NE, Fuller GN et al (2000) Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 217:377–384
Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ (2008) Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9:453–461
Taal W, Brandsma D, de Bruin HG et al (2008) Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer 113:405–410
Brandes AA, Franceschi E, Tosoni A et al (2008) MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 26:2192–2197
Desjardins A, Reardon DA, Herndon JE et al (2008) Bevacizumab plus irinotecan in recurrent WHO grade 3 malignant gliomas. Clin Cancer Res 14:7068–7073
Sathornsumetee S, Cao Y, Marcello JE et al (2008) Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol 26:271–278
van den Bent MJ, Vogelmaum MA, Wen PY, Macdonald DR, Chang SM (2009) End point assessment in gliomas: novel treatments limit usefulness of classical Macdonald's criteria. J Clin Oncol 27:2905–2908
Vredenburgh JJ, Desjardins A, Herndon JE et al (2007) Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25:4722–4729
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Schwartz RB, Carvalho PA, Alexander E III et al (1991) Radiation necrosis vs high-grade recurrent glioma: differentiation by using dual-isotope SPECT with 201TI and 99mTc-HMPAO. AJNR Am J Neuroradiol 12:1187–1192
Kosuda S, Fujii H, Aoki S et al (1993) Reassessment of quantitative thallium-201 brain SPECT for miscellaneous brain tumors. Ann Nucl Med 7:257–263
Lorberboym M, Mandell LR, Mosesson RE et al (1997) The role of thallium-201 uptake and retention in intracranial tumors after radiotherapy. J Nucl Med 38:223–226
Vos MJ, Tony BN, Hoekstra OS et al (2007) Systematic review of the diagnostic accuracy of 201Tl single photon emission computed tomography in the detection of recurrent glioma. Nucl Med Commun 28:431–439
Roesdi MF, Postma TJ, Hoekstra OS et al (1998) Thallium-201 SPECT as response parameter for PCV chemotherapy in recurrent glioma. J Neurooncol 40:251–255
Kallen K, Geijer B, Malmstrom P et al (2000) Quantitative 201Tl SPET imaging in the follow-up of treatment for brain tumour: a sensitive tool for the early identification of response to chemotherapy? Nucl Med Commun 21:259–267
Vallejos V, Balana C, Fraile M et al (2002) Use of 201Tl SPECT imaging to assess the response to therapy in patients with high grade gliomas. J Neurooncol 59:81–90
Vos MJ, Hoekstra OS, Barkhof F et al (2003) Thallium-201 single-photon emission computed tomography as an early predictor of outcome in recurrent glioma. J Clin Oncol 21:3559–3565
Vos MJ, Berkhof J, Postma TJ et al (2006) Thallium-201 SPECT: the optimal prediction of response in glioma therapy. Eur J Nucl Med Mol Imaging 33:222–227
Tony B, Vos MJ, Berkhof J et al (2005) Interobserver variability in the semi-quantitative assessment of 201Tl SPECT in cerebral gliomas. Nucl Med Commun 26:45–48
Klein JP, Moeschberger ML (1997) Survival analysis: techniques for censored and truncated data. New York, Springer, pp 271–282
Hamstra DA, Galban CJ, Meyer CR et al (2008) Functional diffusion map as an early imaging biomarker for high-grade glioma: correlation with conventional radiologic response and overall survival. J Clin Oncol 26:3387–3394
Lev MH, Ozsunar Y, Henson JW et al (2004) Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas. AJNR Am J Neuroradiol 25:214–221
Simon JM, Cornu P, Boisserie G et al (2002) Brachytherapy of glioblastoma recurring in previously irradiated territory: predictive value of tumor volume. Int J Radiat Oncol Biol Phys 53:67–74
Grant R, Walker M, Hadley D et al (2002) Imaging response to chemotherapy with RMP-7 and carboplatin in malignant glioma: size matters but speed does not. J Neurooncol 57:241–245
Sanai N, Berger MS (2008) Glioma extent of resection and its impact on patient outcome. Neurosurgery 62:753–766
Bleichner-Perez S, Le Jeune F, Dubois F et al (2007) 99mTc-MIBI brain SPECT as an indicator of the chemotherapy response of recurrent, primary brain tumors. Nucl Med Commun 28:888–894
Herholtz K, Kracht LW, Heiss WD (2003) Monitoring the effect of chemotherapy in a mixed glioma by C-11-methionine PET. J Neuroimaging 13:269–271
Hu LS, Baxter LC, Smith KA et al (2009) Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol 30:552–558
Oh J, Henry RG, Pirzkall A et al (2004) Survival analysis in patients with glioblastoma multiforme: predictive value of choline-to-N-acetylaspartate index, apparent diffusion coefficient, and relative cerebral blood volume. J Magn Reson Imaging 19:546–554
Leimgruber A, Ostermann S, Yeon EJ et al (2006) Perfusion and diffusion MRI of glioblastoma progression in a four-year prospective temozolomide clinical trial. Int J Radiat Oncol Biol Phys 64:869–875
Law M, Oh S, Babb JS et al (2006) Low-grade gliomas: dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging—prediction of patient clinical response. Radiology 238:658–667
Cao Y, Tsien CI, Nagesh V et al (2006) Survival prediction in high-grade gliomas by MRI perfusion before and during early stage of RT [corrected]. Int J Radiat Oncol Biol Phys 64:876–885
Matsusue E, Fink JR, Rockhill JK et al (2010) Distinction between glioma progression and post-radiation change by combined physiologic MR imaging. Neuroradiology 52:297–306
Nowosielski M, Recheis W, Goebel G et al (2011) ADC histograms predict response to anti-angiogenic therapy in patients with recurrent high-grade glioma. Neuroradiology 53:291–302
Pope WB, Kim HJ, Huo J et al (2009) Recuurent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Neuroradiology 252:182–189
Rock JP, Scarpace L, Hearshen D et al (2004) Associations among magnetic resonance spectroscopy, apparent diffusion coefficients, and image-guided histopathology with special attention to radiation necrosis. Neurosurgery 54:1111–1117
Xu M, See SJ, Ng WH et al (2005) Comparison of magnetic resonance spectroscopy and perfusion-weighted imaging in presurgical grading of oligodendroglial tumors. Neurosurgery 56:919–926
Wong ET, Hess KR, Gleason MJ et al (1999) Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 17:2572–2578
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vos, M.J., Berkhof, J., Hoekstra, O.S. et al. MRI and thallium-201 SPECT in the prediction of survival in glioma. Neuroradiology 54, 539–546 (2012). https://doi.org/10.1007/s00234-011-0908-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-011-0908-5