Abstract
Background
Bioscaffolds and cells are two main components in the regeneration of damaged tissues via cell therapy. Umbilical cord stem cells are among the most well-known cell types for this purpose. The main objective of the present study was to evaluate the effect of the pretreatment of the foreskin acellular matrix (FAM) by monophosphoryl lipid A (MPLA) and Lactobacillus casei supernatant (LCS) on the attraction of human umbilical cord mesenchymal stem cells (hucMSC).
Methods and results
The expression of certain cell migration genes was studied using qRT-PCR. In addition to cell migration, transdifferentiation of these cells to the epidermal-like cells was evaluated via immunohistochemistry (IHC) and immunocytochemistry (ICC) of cytokeratin 19 (CK19). The hucMSC showed more tissue tropism in the presence of MPLA and LCS pretreated FAM compared to the untreated control group. We confirmed this result by scanning electron microscopy (SEM) analysis, glycosaminoglycan (GAG), collagen, and DNA content. Furthermore, IHC and ICC data demonstrated that both treatments increase the protein expression level of CK19.
Conclusion
Pretreatment of acellular bioscaffolds by MPLA or LCS can increase the migration rate of cells and also transdifferentiation of hucMSC to epidermal-like cells without growth factors. This strategy suggests a new approach in regenerative medicine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tissue engineering is an interdisciplinary field that combines engineering and life sciences towards the development of biological substitutes that restore, retain, or enhance tissue function [1]. Bioscaffolds and cells are two main components in this area which are widely used for tissue regeneration [2]. Bioscaffolds can be produced through the decellularization of different parts of animals or cadavers which some of them are commercially available [3]. This technique is used to remove cells from organs, with minimal disruption of their three-dimensional (3D) structure and their extracellular matrix (ECM) composition. Such scaffolds provide an adequate biological microenvironment for recellularization process [4, 5]. Decellularized ECMs are complex networks of several compounds of collagens, laminin, elastin, fibronectin, proteoglycans, and other glycoproteins. These complex networks are essential for promoting cell migration, proliferation, and differentiation [6, 7]. Biological scaffolds in combination with cells play a central role in regeneration medicine, so bioscaffolds should be highly porous to allow cell attachment and to provide a suitable environment for cell growth, proliferation, and differentiation [1, 8].
Because of their unique properties, Mesenchymal Stem Cells (MSCs) are used extensively in conjunction with scaffolds. Among different cellular sources of stem cells, the umbilical cord is considered an important source providing a large pool of material using non-invasive techniques. This source has several properties, including high cell number, low rate of Graft-Versus-Host-Disease (GVHD), the ability to differentiate into adipocytes, osteoblasts hepatocytes, and neuronal-like cells [9]. Since Effective recellularization of scaffolds is a key step for successful regeneration [10], the efficacy of stem cell migration toward a scaffold is necessary. Several genes have the main role in different steps of cell homing and migration. Genes such as Stromal Cell-Derived Factor-1 (SDF-1), C-X-C chemokine receptor type 4 (CXCR-4), Vascular Cell Adhesion Molecule-1(VCAM-1), Tissue inhibitor of metalloproteinases (TIMP), and Matrix metalloproteinase (MMP) [11]. Many research groups have focused on enhancing the rate of cell migration and recellularization using a variety of techniques [12]. For instance, genetically modified (MSCs) overexpressing chemokine receptors [13], local injection of chemoattractants [14], and incubation of stem cells in conditions similar to the initial niches [15]. All of them improve cell migration. Furthermore, there has been a great deal of effort by using a variety of soluble factors, including chemokines and growth factors. Among the studied factors, tumor necrosis factor-alpha (TNF-α) leads to a substantial increase in bone marrow MSC migration. Other growth factors such as insulin-like growth factor-1(IGF-1) and platelet-derived growth factor-AB (PDGF-AB) have been also suggested as the most effective ones [16]. On the other hand, one of the factors that can induce proinflammatory cytokines, including TNF-α, interleukin-1β(IL-1β), and gamma interferon(IFN-γ), is Monophosphoryl lipid A (MPLA) [17]. MPLA is a low-toxicity derivative of lipopolysaccharide (LPS) (100-fold less toxicity) which is commercially available as a ligand for toll-like receptor-4 (TLR-4) [18]. This derivate has useful immunostimulatory properties, which is used as a human vaccine adjuvant and prophylactic drug for a septic shock. Therefore, this substance may indirectly enhance cell migration by inducing proinflammatory cytokines, however, the impact of MPLA on the hucMSC function is yet to be understood.
Probiotics are a single strain or a combination of different organisms that are believed to improve the immune system, reduce inflammation, and accelerate the wound healing process following the accumulation of macrophages, lymphocytes, and polymorphonuclear in the injury site [19]. Such bacteria have recently received clinical attention for the prevention or treatment of Inflammatory bowel disease (IBD) [20]. However, further investigation is required to understand the molecular mechanism of this procedure.
Lactobacillus casei (L. casei) is one of the most popular probiotics, previous studies have indicated that the coculture of inflamed tissue withL.casei significantly reduces TNF-α release [21]. Furthermore, an in vivo study showed that it improves liver function, anticholesterolaemic properties, and protection of the gastrointestinal tract of rat models [22]. Previous research has also shown that L. casei supernatant can impact the characteristics of chemotactic and angiogenic cells in vitro. In addition, it can stimulate chemotaxis and proliferation of fibroblasts, endothelial cells, and inflammatory cells in vivo [23].
In our previous study we succeeded to optimize the human foreskin decellularization [24]. It proved that the obtained human foreskin acellular matrix (FAM), is a suitable scaffold with the potential of recellularization to deliver hucMSC to the injured site. In this study, we hypothesized that pretreatment of foreskin bioscaffold with MPLA and L. casei supernatant (LCS) have a positive impact on the migration of hucMSC towards them Is the pretreated scaffold more attractive for the cells? Furthermore, we evaluated the effect of these treatments on the transdifferentiation of MSCs into epidermal-like cells through cytokeratin 19 assay.
Materials and methods
Decellularization and FAM preparation
In the beginning, samples were taken from the foreskin of boys aged one month to four years who had been referred to the Imam-Ali Clinic, Shahre-Kord for circumcision. Under sterile conditions, foreskin samples were transferred to the laboratory. Briefly, samples were rinsed several times with distilled water and the outer fat layer was separated physically, then transferred in 5% sodium dodecyl sulfate (SDS). After 6 h, trypsin (0.05%) and EDTA (0.01%) (Life Technologies, USA) were used to digest the residual cells (6 h. 4 ° C). Hank’s Balanced Salt Solution (HBSS) (BIO-IDEA, Iran) was used to wash the samples. Finally, the specimens were digested by Triton x-100 (Bio Basic Inc. cas # 9002-93-1) for 1 h, then washed with HBSS and stored in 75% alcohol at -20˚C until use. Finally, Decellularization was confirmed by H&E, Hoechst Staining, and, SEM analysis.
Cell culture
After obtaining informed consent, the umbilical cord was taken and (hucMSCs) were isolated by the MEEM method as previously described [25]. Surface antigens including CD29, CD90, CD105, CD34, and CD45 were examined by flowcytometry. Furthermore, hucMSCs have been tested for multipotent differentiation capacity into adipogenic, osteogenic, and chondrogenic lineages as previously described [25]. Finally, isolated and characterized cells were cultured in Dulbecco’s modified Eagle’s medium-low glucose (DMEM, Gibco, Thermo Fisher Science, US) supplemented with 13% fetal bovine serum (FBS) (Gibco) and 1% of penicillin-streptomycin (Pen/Strep) (Gibco). Flasks were placed in a humidified incubator with 5% CO2 at 37˚C. Every 2 days, the medium was changed until the cells reached 90% confluency.
Lactobacillus casei supernatant (LCS) and MPLA preparation
The L.casei (ATCC 39,392) was purchased as a lyophilized powder from the Iranian Research Organization Science and Technology (IROST). Briefly, L.casei was cultured under microaerophilic conditions in the medium of MRS broth (Merck, Germany). The number of bacteria was approximately 2.5 × 108 colony-forming units after 5 days. The suspension was then centrifuged at 12,000 g at 4 ° C for 15 min. Finally, the supernatant was filtered by a 0.2 μm filter, and stored for further testing at − 20 ° C. The final concentration used was 3 µl of LCS per 1 ml medium. MPLA’s primary stock solution was prepared by solving 1 mg of MPLA powder (InvivoGen, USA) in 1000 µl dimethyl sulfoxide (DMSO) (Merck, Germany). Working aliquots were prepared in a final concentration of 2 µg/ ml.
Pretreatment of FAMs with LCS / MPLA to recellularization
For the pretreatment process, initially, the FAMs were soaked separately in the MPLA and LCS solutions for 24 h. Approximately 2 × 105 hucMSCs/ well were cultured in 6-well plates. When cells reached ∼ 90% confluency, two pieces of the treated FAMs were placed adjacent to the hucMSCs in each well. For the control group, untreated FAMs were used. Furthermore, at the same time, in the LCS pre-treated group, 2 µl of LCS per 1 ml of medium was used in the cell culture. and in the MPLA pre-treated group,2 µl of working solution of MPLA per 1 ml of the medium used in the cultured cells. Finally, the treated hucMSCs were carefully monitored for 4, 8, and 12 days (the medium was changed every 2 days and each time, the mentioned amount of treatments were added).
Resazurin assay
2 × 105 hucMSCs were seeded into a 6 well plate. After 24 h, pre-treated and untreated FAMs (3mm2) were placed next to the cells. The toxicity test was performed at 48 and 72 h. Then, scaffolds and medium were removed, 1 ml FBS-free medium containing 50 µl Resazurin (Kiazist. In. Tehran, Iran) was added and incubated for 4 h at 37˚C, and finally, the absorbance was read at 520–570 nm using Microplate Reader (Stat-Fax-2100, USA).
Histology, immunohistochemistry (IHC), and immunocytochemistry (ICC)
To assess the efficiency of the recellularization procedure, the recellularized FAMs were embedded in paraffin 10% and cut into 5 μm thickness sections to be prepared for histology and immunofluorescence analysis. Samples were stained with Hematoxylin and Eosin dye as routine protocols [26]. H&E staining results were examined under the light microscope (Olympus). The Hoechst 33,258 (Sigma-Aldrich Corp., MI, USA) (1:5000 dilution) was applied to the nucleic acid staining, this staining was also performed as usual protocol [27]. The results were assessed under a fluorescent microscope (Nikon-TS-100 F. Tokyo. Japan).
Furthermore, to assess the expression of cytokeratin-19 (CK-19) IHC technique was performed according to standard procedures by using a monoclonal mouse anti-human cytokeratin-19 antibody (ab220193) and secondary peroxidase-labeled goat anti-mouse IgG H&L (HRP) (ab205719). The chromogenic reaction was performed with a chromogenic solution of 3, 3′-diaminobenzidine (Dako, USA), resulting in the expected brown-colored signal. Finally, the positive percentage of CK-19 expression was determined by ImageJ software.
To compare the expression of cytokeratin-19 (CK-19) between recellularized tissue and treated cells, we also measured the variation of CK19 expression in cells. Cells were grown in 6 well plates and treated with MPLA and LCS as mentioned above. After 12 days, cells were rinsed with PBS and fixed in 10% formaldehyde. Immunostaining was performed using DAPI and specific fluorescent-labeled monoclonal mouse anti-human cytokeratin-19 antibody (ab220193). Photos were finally merged, and expression levels were evaluated in treated and control groups.
Scanning electron microscopy (SEM)
The pretreated and control recellularized scaffolds were evaluated using SEM at intervals of 4, 8, and 12 days. To prepare the samples, FAMs were incubated in glutaraldehyde 2.5% for 1 h at room temperature and then dehydrated by increasing concentrations of ethanol for 20 min, then froze at 20 °C for 40 min and finally transferred to the freeze dryer device for 3 h. Fixed samples coated with gold (Desk Sputter Coater-DSR1) and a scanning electron microscope (Philips XL 30, North Billerica, MA) was used to evaluate the scaffolds.
Glycosaminoglycan (GAG) and collagen content in recellularized FAMs
Recellularized scaffolds and acellular foreskin (control) were appropriate for the analysis of collagen and GAG content. All of the FAMs were washed in PBS and, 20 mg of lyophilized scaffolds were digested in a papain buffer overnight at 65 °C. The assessment of GAG was performed according to the kit instruction (KGAG96, Kiazist, Iran), and then assessed by Elisa Reader (Stat fax-2100, USA) at 510–560 nm. GAG content was evaluated with a standard curve using chondroitin6-sulfate in the kit.
The biochemical measurement of hydroxyproline is one of the most reliable and cost-effective methods for collagen assay [28]. After washing with PBS and lyophilization, 20 mg of FAMs were homogenized in 100 µL H2O plus 100 µL 12 M HCL and incubated for 4 h at 120˚C. Then, collagen assessment was performed based on the kit instructions (KHPA96, Kiazist, Iran). The results were assessed by a microplate reader at 540–560 nm as triplicate and plotting the standard curve.
DNA quantification in recellularized FAMs
DNA from recellularized and control scaffolds was isolated by the Geno PlusTM Mini extraction kit (GG2001, Viogene, Taipei, Taiwan) at each interval. The quality and quantity of total DNA were evaluated by spectroscopy at 260 nm using Nanodrop 2000 (Thermo Scientific). All samples were normalized to the dry weight of the FAMs.
RNA isolation, cDNA synthesis, and qRT‑PCR
For RNA extraction, after 12 days, total RNA was isolated from the hucMSCs (tree groups: MPLA/LCS treated and untreated-control), all experiments were done in triplicates and by using RNeasy Mini Kit (Qiagen, Hilden, Germany). On-column DNA digestion was included in this protocol to remove residual genomic DNA contaminants and finally measured by nanodrop 2000(Thermo Fisher Scientific, MA). Then, equal amounts of total isolated RNA (1 µg/ sample) were reverse transcribed using cDNA Synthesis Kit (RevertAidTM First Strand cDNA Synthesis Kit) and transferred into the qRT-PCR reaction. The mRNA level of MMP-2, VCAM-1, Integrin ß-1(ITGß-1), and, CXCR-4 genes were evaluated using SYBR® Green PCR Master Mix (Takara, Kusatsu, Japan) in a qRT-PCR thermocycler (Rotor gene 3000, Qiagen, Germany). Also, the GAPDH gene was used as an internal control. Primers used for qRT-PCR are as follow: forward 5’CGAACCCAAACAAAGGCAGA − 3’ and reverse 5’ ACAGGATTTTCGGAGCAGGA − 3’ for VCAM-1 gene, forward 5’ ACCACAGCCAACTACGATGA-3’ and reverse 5’GCTCCTGAATGCCCTTGATG-3’forMMP-2gene, forward ' TCCAACCTGATCCTGTGTC-3’ and reverse 5’TCGTTGTTCCCATTCACTG-3’ for ITG-ß1gene, forward5’ACCATCTACTCCATCATCTTC-3’ and reverse 5’ TGATGACAAAGAGGAGGTC-3’ for CXCR-4 gene, and forward 5’ GGCAAGTTCAATGGCACAGT-3’ and reverse 5’. TTGTGAAGACGCCAGTAGACTC-3’ for GAPDH gene. The 2 − ΔΔCt method was applied as a comparative method of quantification. All experiments were done in triplicate and expressed as means ± standard error of the mean.
Statistical analysis
Two separate experiments with triplicate samples were conducted for each group. qRTPCR.
results were statistically analyzed by GraphPad Prism software (version 5) (GraphPad Software, CA, USA). Comparisons between experimental and control groups were performed by one-way analysis of variance (ANOVA) and Tukey’s post hoc test.
Results
Characteristics of human-derived umbilical cord MSCs
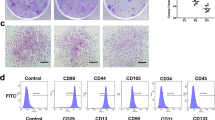
Isolated hucMSCs were adherent cells that showed fibroblast-like morphology. According to flow cytometry results, these cells were positive for CD105, CD90, and CD29 (adhesion and integrin markers), and negative for CD45 and CD34. Also, these cells showed the ability to differentiate into the adipogenic and osteogenic lineages (Fig. 1).
Characterization of hucMSCs. (a) Osteogenesis and (b) Adipogenesis of hucMSCs were detected by Alizarin red S staining and Oil red O staining respectively (200x). (c) hucMSCs are adherent cells with fibroblast-like morphology (40x magnification). (d): Flow cytometry results show that hucMSCs were uniformly negative for CD34, CD45, and positive for CD29, CD90, and CD105
Acellular human foreskin
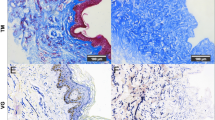
The H&E and Hoechst staining indicated that the foreskin was thoroughly acellularized without residual cells or nucleic acids. This result was also confirmed by SEM analysis. Three types of evaluations imply that decellularization is effective and acceptable (Fig. 2). Furthermore, the results of our previous study confirmed the successful decellularization process by DNA, GAG and proline quantification, mechanical properties, and histological staining (data have not shown/submitted).
Comparison between native and acellular foreskin. (a) SEM evaluation showed a high density of cells in native tissue, (1500x magnification) and (b) Acellular foreskin showed complete cell removal as well as regular maintenance of matrix and collagen structure with appropriate porosity(1000x magnification). Confirmation of foreskin decellularization through (c, d) Hoechst staining (100x magnification), and (e, f) H & E staining (200x magnification)
Cytotoxicity effects of pre-treated FAMs with MPLA and LCS on hucMSCs
Resazurin assay was used to evaluate the toxicity of pre-treated FAMs for hucMSCs. Comparison of the viability percentage of hUMSCs via resazurin assay in 48 h and 72 h indicated that both of our treated FAMs did not have a significant effect on cell viability after 48 h, viability percentage of hUMSCs remained by 82%, 101%, and 89.6% in untreated, MPLA and LCS treated groups respectively. In addition, compared to the untreated group, MPLA-treated FAMs increased the cell proliferation after 72 h significantly and viability percentage was 71% vs. 90% (Fig. 3).
(a): Comparing the viability of hucMSCs in the vicinity of FAMs. MPLA-treated FAMs showed better cell compatibility than the LCS-treated and untreated control. (P ≤ 0.03*). (b): hucMSCs tropism to the FAMs. In addition to increased proliferation, hucMSCs showed better tropism to MPLA-treated FAMs rather than LCS-treated and untreated control groups. Furthermore, the untreated control group showed less proliferation with some apoptotic phenotype on day 12. (40X magnification)
It is also noteworthy to mention that in the case of MPLA-treated scaffold, hucMSCs had better scaffold tropism with slightly increased proliferation with no apoptotic phenotype. Similar effects have been found for LCS –treated FAMs, but they showed lower tropism also apoptosis happened for some of the hucMSCs on day 12 in the untreated control group. Taken together, both treatments had a better effect than the untreated control group (Fig. 3b).
Recellularization confirmation
After putting pre-treated FAMs on the isolated hucMSCs, recellularization was assessed performing H&E and Hoechst staining at intervals of 4, 8, and 12 days. Significant cell distribution was observed in MPLA pre-treated FAMs on day 12 in comparison to LCS pretreated and control FAMs. In addition, the adherence of hucMSCs to the FAM surface was observed using SEM. All of the images showed that MPLA has the best effect and after that in the second place, LCS treatment leads to a better recellularization in comparison to the untreated control group (Fig. 4). It is worth noting that, we did not use seeding or any other technique in the recellularization step to deliver cells to the scaffolds and we just put the acellularized scaffold adjacent to the hucMSCs.
Recellularization of FAMs by hucMSCs on days 4, 8, and 12. (a) H&E staining in different days showed that MPLA-treated FAMs resulted in the most effective recellularization on the 12th day (200x magnification). (b) Fluorescent staining with Hoechst. The nuclei of the cells are bright spots, which are more fluorescent in the MPLA-treated FAM, on day 12 (100x magnification). (c) SEM images of recellularized MPLA treated-FAMs showed a significant increase in the number of migrated cells on day 12 compared to other groups
GAG, collagen, and DNA content
As shown in Fig. 5, the collagen and GAG content is well conserved in the recellularized FAMs after 12 days and even increased in MPLA-treated groups. Also, DNA content in recellularized FAMs has been increased in comparison to the primitive acellular foreskin, indicating the higher rate of recellularization in the MPLA-treated group.
Comparison of the GAG, collagen, and DNA contents of recellularized FAMs with primitive acellular foreskin. (a) GAG content increased in the MPLA-treated group after 12 days but it was not significantly increased in the LCS-treated group. (b) Collagen content increased in both treatments compared to the untreated control, particularly on the 12th day of MPLA treatment. (c) After 12 days, recellularized FAMs treated with MPLA had a considerable increase in DNA content. For the LCS-treated FAMs, This increase was seen but to a lesser extent. Data with (P ≤ 0.03*,P ≤ 0.002**, and P ≤ 0.001***) were statistically different according to one-way ANOVA and post hoc testing (Tukey’s procedure, data are presented as Mean ± SD)
Expression levels of the studied genes
Figure 7 shows the expression levels of the VCAM-1, MMP-2, ITG-ß1, and CXCR-4 genes in the hucMSCs adjacent to the treated and untreated scaffold. Our data demonstrated that the expression of the VCAM-1 gene increased in both treated groups compared to the untreated control group, but the highest expression was in the LCS-treated group on day 2 (fold changes 13.60 ± 1.3), then slowly decreased until day 8. Whereas the expression level of this gene increased gradually in the MPLA-treated group until day 12, which showed the highest expression (fold change 6.4 ± 1.7) on day 12 (Fig. 6a). The MMP-2 expression rate in the LCS-treated group showed the highest level on day 2 by 4.9 fold which decreased dramatically to 0.2 fold on day 8. In the MPLA-treated group, the expression rate of MMP-2 increased sharply to day 12 (fold changes 4.99 ± 0.2), also this increasing pattern was seen for ITG-ß1 gene in the MPLA-treated group. The maximum upregulation of the ITG-ß1 gene was 5.8 ± 0.7 fold in LCS- treated group after 8 days post-treatment (Fig. 6b and d). Both treated groups showed increased CXCR-4 expression on day 2 with a sharp decline by day 8 and then a slight increase by day 12 (Fig. 6c).
comparative analysis of differential expression of VCAM-1, MMP-2, ITG-ß1, and CXCR-4 genes in hucMSCs after being treated with MPLA and LCS for 12 days. After LCS treatment, VCAM-1 and CXCR-4 genes expression increased sharply on the second day and then gradually decreased, whereas treatment with MPLA led to a gradual increase in the expression levels of VCAM-1, MMP-2, and ITG-ß1 genes. GAPDH was used as the internal housekeeping gene to normalize data. (P ≤ 0.03*, P ≤ 0.002**, P ≤ 0.0002*** and, P ≤ 0.0001**** data are presented as Mean ± SD)
ICC and IHC results
The results of IHC analysis (Fig. 7) on recellular FAMs showed that the expression of cytokeratin 19 (CK19) protein increased in both treatments (MPLA and SLC) in comparison to the untreated control group; however, the expression of the CK19 became slightly higher on day 12. It is worth noting that mesenchymal stem cells can differentiate into epidermal cells and one of the early markers of epidermal cell lineage is cytokeratin19 (CK19) [29].
IHC analysis of recellular FAMs on day 8 and 12, treated by LCS and MPLA for CK19. As shown, the expression of CK19 increased in both treated FAMs at every two intervals. Quantitative results of IHC analysis for CK19 demonstrated significant differences between treated and untreated groups. (***) indicate statistically significant differences (p < 0.0001)
We also performed ICC analyses on treated cells (without scaffold) to determine whether the scaffold or our treatments are more effective on ck19 expression Fig. 8. The findings suggested that after treatment with LCS and MPLA, hucMSCs were induced to express cytokeratin 19 (CK19).
Immunocytochemistry images of cytokeratin 19, in hucMSCs treated by LSC and MPLA on day 12. K19 immunostaining of cells shown in green and negative control nuclei are stained blue with DAPI. Merged photos in each group showed that CK 19 expression increased significantly in both treated groups compared to the untreated control group
Discussion
MSCs have been used in regenerative medicine as innovative cells that showed great enthusiasm for the treatment of diseases [30]. In cell therapy, increasing the MSCs immigration capacity by exogenous factors is of important subject [16]. There are major challenges in the process of recellularization of bio-scaffolds, such as the growth and redistribution of cells within the scaffolds, and the establishment of appropriate cell density with the appropriate cellular phenotype in the scaffold microenvironment [31, 32]. Our study aimed to recruit MSCs to the extracellular matrix using an exogenous agent for migration. In this study, the effect of pre-treated acellular foreskin with MPLA and Lactobacillus casei supernatant with the aim of improved recellularization with hucMSCs was investigated. In decellularized tissues, the pore characteristics (shape, size, interconnection, alignment, collapse resistance) have been demonstrated to affect the scaffold’s reseeding capacity and permeability [33]. In our previous study, we reached an optimized method for suitable decellularization of the foreskin. Our FAMs had all the characteristics of an appropriate scaffold in terms of preserving ECM’s three-dimensional structure, pore characteristics, and, cell compatibility. Additionally, quantification results showed the significant removal of DNA in FAMs, enhancement of collagen content, and reduction of GAG amount [24].
Preidis et al. indicated that probiotics improved the enterocyte migration rate in the neonatal mouse intestine [34]. Probiotics were also found to modulate dendritic cell surface features and cytokine release in bone marrow-derived dendritic cells [35]. So in the present study we decided to evaluate the effect of the pretreatment of this scaffold with these chemoattractant, because it seems that scaffold can release these factors slowly and affect the recellularization potential.
On the other hand, MPLA is a detoxified derivative of LPS with immunostimulatory properties, which is a ligand for Toll-like receptors. The chemoattractive effects of MPLA have been demonstrated in recent studies, it appears that this reagent can be regarded as a stimulator of MSC migration [36]. Results of a research showed that MPLA can probably be considered as an inducing factor in cell migration, by increasing both VLA-4 and VCAM-1 expression [37]. Based on our knowledge, the majority of studies have evaluated this factor as adjuvant in dendritic cells for vaccines. So there is a lot of uncertainty about the impact of this factor on migration of MSCs, which needs more extensive studies.
Here the effects of MPLA as a synthetic reagent and LCS as a probiotic were compared. Our findings showed that both treatments made the scaffold more attractive to the cells. According to our histology results, MPLA had more prominent effects on the rate of recellularization rather than LCS pre-treatment. As shown in Fig. 4, we used H&E staining in the first step, then Hoechst staining and SEM analysis to see the results more clearly. In a study by Barreto et al. on the mouse placenta, H&E and SEM analysis showed that mouse embryonic fibroblasts grew on the placental scaffold and interacted with each other under 6 days [38]. In a separate study, the other members of our team compared the effect of MPLA and Lactobacillus acidophilus [39]. Their results were consistent with our findings which showed that both treatments increased the MSC migration, especially in the MPLA-treated group. They only followed the findings for 6 days, so we tried to extend the experiment to 12 days.
To quantify and support the descriptive histological findings, we also examined other parameters in the recellularized FAMs such as GAG, collagen, and DNA quantification. Our findings demonstrated that, after decellularization, GAG, collagen, and DNA content increased in pre-treated recellularized FAMs in comparison to the untreated control group. The most significant increase was seen in the collagen and DNA content of the MPLA-treated group after 12 days. It refers period observed for recellularization of the scaffold. Similar to our results, in other studies, DNA-isolation quantified cell density, as well as H&E staining and SEM analysis showed the rate of recellularization [3, 40]. This observation was further confirmed by GAG and collagen content measurement in recellularized FAMs. In comparison to the untreated control group, the treatment groups’ increased GAG and collagen levels demonstrated better recellularization, as seen in Fig. 5.
Inappropriate homing of MSCs and low-level expression of migratory genes can influence the scaffold’s therapeutic effects [41]. Here we evaluated the expression level of genes involved in different steps of migration and homing. Based on our results, MPLA-treated FAMs increased the expression of MMP-2 and VCAM-1 genes in the last intervals, unlike bacteria which had the earliest effects. Integrin β1 is a subunit of very late antigen 4 (VLA-4) that interacts with VCAM-1, this interaction is functionally involved in MSC homing [41]. Results of the other study demonstrated that the capability of MSC migration was increased after MMP-2 up-regulation [42]. It has also been found that MMP-2 knock-down significantly impaired the hMSC invasion [43]. (VCAM-1) also modulate cell adhesion and motility functioning in cell homing [44]. In the LCS-treated group, the expression levels of VCAM-1 decreased over time while it showed a significant increase in the MPLA-treated group.
In another study, we investigated the effect of pretreatment of acellular human amniotic membrane (HAM) with MPLA for fascia repair in rats. Our results showed that pretreatment of HAM with MPLA increased the tolerance of fascia rupture at higher pressures and attract more cells to the wound site, resulting in faster wound healing [45].
The SDF-1/CXCR4 axis controls the migration, growth, and differentiation of different cell types, including stem cells [46]. Our study showed the higher expression of CXCR-4 in treated groups on the second day which decreased significantly after 12 days but this reduction in MPLA- treated group was minor. On the other hand, our treats increased ITGß-1 gene expression after 12 days especially LCS treatment. In addition to examining the migration rate, we also examined the impact of our pre-treated scaffolds on the differentiation capacity of MSCs into epidermal-like cells. Previous studies showed transdifferentiation of Huc-MSCs into epidermal-Like cells can occur by mimicking the skin microenvironment [47]. This group, used a 3-dimensional (3D) microenvironment fabricated of collagen and chitosan to culture hucMSCs, after 14 days, western blotting and immunohistochemistry results showed that hucMSCs on the surface of the scaffold is positive for the cytokeratin 19 (epidermal marker). Also in our study, the results of immunohistochemistry showed that pre-treated (both MPLA and LCS) scaffolds have a positive impact on the transdifferentiation of hucMSCs into epidermal-Like cells. (Fig. 7)
Another main factors for effective in vitro proliferation and differentiation of MSCs are the conditions of culture. The expression of Cytokeratins in the absence of epidermal growth factors has not been reported before. Hence, we here, in addition to immunohistochemistry, also tested the treated cells by immunocytochemistry (without the presence of scaffolds), to study the effect of the micro-environment was greater or the effect of the treatments. Immunocytochemistry results of CK19 after 12 days of treatment showed that both MPLA and LCS significantly increase the differentiation of MSCs to the epidermal-like cells. (Fig. 8)
Conclusion
In conclusion, we showed that scaffold pretreatment with MPLA can be considered as an effective agent in scaffold recellularization. Application of probiotics such as L.Casei also benefits the migration capacity of hucMSCs towards scaffolds in the field of regenerative medicine.
Furthermore, IHC and ICC results showed that both MPLA and LCS may affect differentiation characteristics of MSCs to the epidermal-like cells. To confirm this hypothesis it’s necessary to test more skin markers. Finally, it appears that beside cell migration, other mechanisms are involved in the cell tropism towards the scaffolds. Maybe these treatments have some effects on FAM microstructure. Therefore, further investigations are required for discovering the exact molecular mechanisms in future such as whole-transcriptome RNAseq analysis, which is our next goal of study.
Abbreviations
- FAM:
-
Foreskin acellular matrix
- MPLA:
-
Monophosphoryl lipid A
- LCS:
-
Lactobacillus casei supernatant
- hucMSC:
-
Human umbilical cord mesenchymal stem cells
- IHC:
-
Immunohistochemistry
- ICC:
-
Immunocytochemistry
- CK19:
-
Cytokeratin 19
- SEM:
-
Scanning electron microscopy
- GAG:
-
Glycosaminoglycan
- ECM:
-
Extracellular matrix
- MSCs:
-
Mesenchymal Stem Cells
- GVHD:
-
Graft-Versus-Host-Disease
- SDF-1:
-
Stromal Cell-Derived Factor-1
- CXCR-4:
-
C-X-C chemokine receptor type 4
- VCAM-1:
-
Vascular Cell Adhesion Molecule-1
- TIMP:
-
Tissue inhibitor of metalloproteinases
- MMP:
-
Matrix metalloproteinase
- TNF-α:
-
Tumor necrosis factor-alpha
- IGF-1:
-
Insulin-like growth factor-1
- PDGF-AB:
-
Platelet-derived growth factor-AB
- IL-1β:
-
Interleukin-1β
- IFN-γ:
-
Gamma interferon
- LPS:
-
Lipopolysaccharide
- TLR-4:
-
Toll-like receptor-4
- IBD:
-
Inflammatory bowel disease
- SDS:
-
Sodium dodecyl sulfate
- HBSS:
-
Hank’s Balanced Salt Solution
- ITGß-1:
-
Integrin ß-1
- HAM:
-
Human amniotic membrane
References
Khazaei M, Rahmati S, Khazaei MR, Rezakhani L (2023) Accelerated wound healing with resveratrol-loaded decellularized pericardium in mice model. Cell Tissue Banking 1–9
Katari R, Peloso A, Orlando G (2015) Tissue engineering and regenerative medicine: semantic considerations for an evolving paradigm. Front Bioeng Biotechnol 2:57
Kim BS, Choi JS, Kim JD, Choi YC, Cho YW (2012) Recellularization of decellularized human adipose-tissue-derived extracellular matrix sheets with other human cell types. Cell Tissue Res 348(3):559–567
Fu R-H, Wang Y-C, Liu S-P, Shih T-R, Lin H-L, Chen Y-M, Sung J-H, Lu C-H, Wei J-R, Wang Z-W (2014) Decellularization and recellularization technologies in tissue engineering. Cell Transplant 23(4–5):621–630
Keane TJ, Swinehart IT, Badylak SF (2015) Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 84:25–34
Zhang Y, Zhang C, Li Y, Zhou L, Dan N, Min J, Chen Y, Wang Y (2023) Evolution of biomimetic ECM scaffolds from decellularized tissue matrix for tissue engineering: a comprehensive review. Int J Biol Macromol 125672
Midwood KS, Williams LV, Schwarzbauer JE (2004) Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol 36(6):1031–1037
Hutmacher DW (2001) Scaffold design and fabrication technologies for engineering tissues—state of the art and future perspectives. J Biomater Sci Polym Ed 12(1):107–124
Howard D, Buttery LD, Shakesheff KM, Roberts SJ (2008) Tissue engineering: strategies, stem cells and scaffolds. J Anat 213(1):66–72
Dong F, Caplan AI (2012) Cell transplantation as an initiator of endogenous stem cell-based tissue repair. Curr Opin Organ Transplant 17(6):670–674
De Becker A, Van Riet I (2016) Homing and migration of mesenchymal stromal cells: how to improve the efficacy of cell therapy? World J stem Cells 8(3):73
Bilodeau C, Goltsis O, Rogers IM, Post M (2019) Limitations of Recellularized Biological scaffolds for Human Transplantation. Journal of Tissue Engineering and Regenerative Medicine
Cheng Z, Ou L, Zhou X, Li F, Jia X, Zhang Y, Liu X, Li Y, Ward CA, Melo LG (2008) Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther 16(3):571–579
Purcell BP, Elser JA, Mu A, Margulies KB, Burdick JA (2012) Synergistic effects of SDF-1α chemokine and hyaluronic acid release from degradable hydrogels on directing bone marrow derived cell homing to the myocardium. Biomaterials 33(31):7849–7857
Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH (2003) Systemic delivery of bone marrow–derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation 108(7):863–868
Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, Charbord P, Domenech J (2007) The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells 25(7):1737–1745
Romero C-DM (2012) Mechanisms of Immuno-Modulation with the TLR4 agonist monophosphoryl lipid A in the treatment of Sepsis. The University of Texas Medical Branch Graduate School of Biomedical Sciences
Romero CD, Varma TK, Hobbs JB, Reyes A, Driver B, Sherwood ER (2011) The toll-like receptor 4 agonist monophosphoryl lipid a augments innate host resistance to systemic bacterial infection. Infect Immun 79(9):3576–3587
Devi S, Kumar P (2021) Use of probiotic bacteria and their bioactive compounds for wound care. Wound Healing Research: Curr Trends Future Dir 301–330
Sartor RB (2004) Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology 126(6):1620–1633
Haro C, Mónaco ME, Medina M (2018) Lactobacillus casei beneficially modulates immuno-coagulative response in an endotoxemia model. Blood Coagul Fibrinolysis 29(1):104–110
Oyetayo V, Adetuyi F, Akinyosoye F (2003) Safety and protective effect of Lactobacillus acidophilus and Lactobacillus casei used as probiotic agent in vivo. Afr J Biotechnol 2(11):448–452
Halper J, Leshin L, Lewis S, Li W (2003) Wound healing and angiogenic properties of supernatants from Lactobacillus cultures. Experimental Biology Med 228(11):1329–1337
Rahmati S, Jalili A, Dehkordi MB, Przedborski M (2022) An effective method for decellularization of human foreskin: implications for skin regeneration in small wounds. Cell J (Yakhteh) 24(9):506
Dehkordi MB, Madjd Z, Chaleshtori MH, Meshkani R, Nikfarjam L, Kajbafzadeh A-M (2016) A simple, rapid, and efficient method for isolating mesenchymal stem cells from the entire umbilical cord. Cell Transplant 25(7):1287–1297
Fischer AH, Jacobson KA, Rose J, Zeller R (2008) Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harbor Protocols 2008(5):pdb.prot4986
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques. Churchill Livingstone, London, England
Kajbafzadeh A-M, Masoumi A, Hosseini M, Borjian MA, Akbarzadeh A, Mohseni MJ (2014) Sheep colon acellular matrix: immunohistologic, biomechanical, scanning electron microscopic evaluation and collagen quantification. J Biosci Bioeng 117(2):236–241
Chun-mao H, Su-yi W, Ping-ping L, Hang-hui C (2007) Human bone marrow-derived mesenchymal stem cells differentiate into epidermal-like cells in vitro. Differentiation 75(4):292–298
Andreas K, Sittinger M, Ringe J (2014) Toward in situ tissue engineering: chemokine-guided stem cell recruitment. Trends Biotechnol 32(9):483–492
Huang Z, Godkin O, Schulze-Tanzil G (2017) The challenge in using mesenchymal stromal cells for recellularization of decellularized cartilage. Stem cell Reviews Rep 13(1):50–67
Syedain ZH, Bradee AR, Kren S, Taylor DA, Tranquillo RT (2013) Decellularized tissue-engineered heart valve leaflets with recellularization potential. Tissue Eng Part A 19(5–6):759–769
Rana D, Zreiqat H, Benkirane-Jessel N, Ramakrishna S, Ramalingam M (2017) Development of decellularized scaffolds for stem cell‐driven tissue engineering. J Tissue Eng Regen Med 11(4):942–965
Preidis GA, Saulnier DM, Blutt SE, Mistretta TA, Riehle KP, Major AM, Venable SF, Finegold MJ, Petrosino JF, Conner ME, Versalovic J (2012) Probiotics stimulate enterocyte migration and microbial diversity in the neonatal mouse intestine. Faseb J 26(5):1960–1969
Drakes M, Blanchard T, Czinn S (2004) Bacterial probiotic modulation of dendritic cells. Infect Immun 72(6):3299–3309
Kuai R, Sun X, Yuan W, Ochyl LJ, Xu Y, Hassani Najafabadi A, Scheetz L, Yu MZ, Balwani I, Schwendeman A, Moon JJ (2018) Dual TLR agonist nanodiscs as a strong adjuvant system for vaccines and immunotherapy. J Control Release 282:131–139
Shojaeian A, Mehri-Ghahfarrokhi A, Banitalebi-Dehkordi M (2019) Migration gene expression of human umbilical cord mesenchymal stem cells: a comparison between monophosphoryl lipid A and supernatant of Lactobacillus acidophilus. Int J Mol Cell Med 8(2):154
Barreto RS, Romagnolli P, Fratini P, Mess AM, Miglino MA (2019) Mouse placental scaffolds: a three-dimensional environment model for recellularization. J Tissue Eng 10:2041731419867962
Shojaeian A, Mehri-Ghahfarrokhi A, Banitalebi-Dehkordi M (2019) Increased in vitro migration of human umbilical cord mesenchymal stem cells toward acellular foreskin treated with bacterial derivatives of monophosphoryl lipid A or supernatant of Lactobacillus acidophilus. Hum Cell 1–13
Zhou P, Huang Y, Guo Y, Wang L, Ling C, Guo Q, Wang Y, Zhu S, Fan X, Zhu M (2016) Decellularization and recellularization of rat livers with hepatocytes and endothelial progenitor cells. Artif Organs 40(3):E25–E38
Rüster B, Göttig S, Ludwig RJ, Bistrian R, Müller S, Seifried E, Gille J, Henschler R (2006) Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood 108(12):3938–3944
Yu Q, Chen L, You Y, Zou C, Zhang Y, Liu Q, Cheng F (2011) Erythropoietin combined with granulocyte colony–stimulating factor enhances MMP-2 expression in mesenchymal stem cells and promotes cell migration. Mol Med Rep 4(1):31–36
Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P (2007) MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood 109(9):4055–4063
Gao F, Hu X, Xie X, Liu X, Wang J (2015) Heat shock protein 90 stimulates rat mesenchymal stem cell migration via PI3K/Akt and ERK1/2 pathways. Cell Biochem Biophys 71(1):481–489
Baghaee R, Rahmati S, Banitalebi M, Afzali L (2023) Comparison of human acellular amniotic membranes with acellular amniotic membranes pretreated with MPLA for repair of fascia in rats. Cell Tissue Banking 24(2):495–501. https://doi.org/10.1007/s10561-022-10049-x
Dar A, Kollet O, Lapidot T (2006) Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp Hematol 34(8):967–975
Chen D, Hao H, Tong C, Liu J, Dong L, Ti D, Hou Q, Liu H, Han W, Fu X (2015) Transdifferentiation of umbilical cord–derived mesenchymal stem cells into epidermal-like cells by the mimicking skin microenvironment. Int J Low Extrem Wounds 14(2):136–145
Funding
This project financially supported by Shahrekord University of Medical Sciences (No. 3653).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, and data collection were performed by Ali Jalili, Shima Rahmati, and Fereshteh Shojaei- Ghahrizjani. Data analysis was performed by Mohammad Amin Tabatabaiefar. The first draft of the manuscript was written by Shima rahmati and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval and consent to participate
This study was accepted under the management of the Ethics Committee of Shahrekord University of Medical Sciences and with the ethics code IR.SKUMS.REC.1397.85.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jalili, A., Shojaei-Ghahrizjani, F., Tabatabaiefar, M.A. et al. Decellularized skin pretreatment by monophosphoryl lipid A and lactobacillus casei supernatant accelerate skin recellularization. Mol Biol Rep 51, 675 (2024). https://doi.org/10.1007/s11033-024-09599-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09599-y