Abstract
Skin substitutes are one of the main treatments for skin loss, and a skin substitute that is readily available would be the best treatment option. However, most cell-based skin substitutes require long production times, and therefore, patients endure long waiting times. The proteins secreted from the cells and tissues play vital roles in promoting wound healing. Thus, we aimed to develop an acellular three-dimensional (3D) skin patch with dermal fibroblast conditioned medium (DFCM) and collagen hydrogel for immediate treatment of skin loss. Fibroblasts from human skin samples were cultured using serum-free keratinocyte-specific media (KM1 or KM2) and serum-free fibroblast-specific medium (FM) to obtain DFCM-KM1, DFCM-KM2, and DFCM-FM, respectively. The acellular 3D skin patch was soft, semi-solid, and translucent. Collagen mixed with DFCM-KM1 and DFCM-KM2 showed higher protein release compared to collagen plus DFCM-FM. In vitro and in vivo testing revealed that DFCM and collagen hydrogel did not induce an immune response. The implantation of the 3D skin patch with or without DFCM on the dorsum of BALB/c mice demonstrated a significantly faster healing rate compared to the no-treatment group 7 days after implantation, and all groups had complete re-epithelialization at day 17. Histological analysis confirmed the structure and integrity of the regenerated skin, with positive expression of cytokeratin 14 and type I collagen in the epidermal and dermal layer, respectively. These findings highlight the possibility of using fibroblast secretory factors together with collagen hydrogel in an acellular 3D skin patch that can be used allogeneically for immediate treatment of full-thickness skin loss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Conventional tissue replacement or autologous split or full-thickness skin graft (SG) is the standard treatment for skin loss. However, the use of SG is restricted in the case of extensive skin loss due to limited donor skin [1]. Moreover, it results in scar tissue formation and affects mobility due to graft contracture, which requires multiple corrective surgeries [2, 3]. This affects a patient’s quality of life, primarily because of physical limitations and physical pain [2, 4]. Although the use of allogenic SG is recommended when skin source is limited, it increases the risk of graft rejection and viral transmission [4]. Currently, alternative treatment strategies are being investigated to improve the healing of injured skin to yield physiological functionality and cosmetic appearance to the native skin. With the advancement of tissue engineering techniques, skin substitutes have been developed by integrating cells, biomaterials, or biochemical factors and are used as functional substitutes to replace lost or damaged tissues in the case of the chronic wound [1].
Reconstruction using skin substitutes was introduced in 1975 [5, 6]. Many skin substitutes are available commercially and are primarily used for treating major skin loss due to chronic wounds [7]. They differ in terms of the presence or absence of living cells, being autologous (from the patient), allogeneic (from another person) or xenogeneic (from an animal); use of biomaterials, either biological or synthetic; and physical stimulation [8]. The commercially available tissue-engineered skin substitutes can be divided into four groups [9]: (a) epidermal substitutes (e.g. Epicel™; Epidex™), (b) dermal substitutes (e.g. Dermagraft™, Alloderm™), (c) bilayer substitutes (e.g. Apligraf™; Integra™) and (d) amniotic membrane (e.g. EpiFix®; AmnioFix). Each has their respective advantages and disadvantages and are selected based on the patient’s clinical condition. Some skin substitutes act as a temporary wound dressing, such as Biobrane™ and TranCyte™, which need to be removed upon healing [10]. Some are used to enhance the healing of chronic wounds, as the wound is deeper and involves a larger body surface area, or for smaller, non-healing wounds that are due to underlying factors such as ageing, medication, and chronic wound ulceration [11, 12]. Skin substitute transplantation delivers cells for skin regeneration, and the cells secrete factors that enhance wound healing [13].
Fibroblasts are recognised as cells that are important to skin regeneration and wound healing through implantation [14]. However, the overall production of autologous cell-based skin substitutes requires a longer time to produce the desired number of cells. Thus, it results in longer patient waiting time and enhances the cost of wound management and production, rendering some of these products more expensive compared to conventional wound dressing, and also presents a higher risk for contamination and patient mortality [13, 15]. Allogenic cells are used as an alternative skin substitute, but are subject to ethical and safety issues, in that the cells can induce immune rejection [16]. Besides that, skin substitutes with living cells require stringent shipping and storage temperatures due to their short shelf-life, limiting their distribution worldwide [10, 15, 17]. Therefore, there is a necessity for producing an off-the-shelf skin substitute to aid skin regeneration that can be applied immediately to patients with life-threatening skin injury.
In normal skin wound healing, the secretion of mediators is important and takes place through activation or inhibition of the respective signalling pathways, and these secretory factors are essential for wound healing. Preclinical studies have shown that single-layer keratinocytes, single-layer fibroblasts, and bilayer skin (MyDerm™) have healing potential [17,18,19]. Moreover, these skin substitutes secrete essential factors for wound healing, which are also believed to contribute to healing through paracrine signalling [20]. Mediators such as growth factors, cytokines, chemokines, and matrix molecules play a role in wound healing. For chronic wounds, the level of some growth factors and cytokines (e.g. epidermal growth factor (EGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), tumour necrosis factor (TNF), interleukin-1 (IL-1)) is low, requiring growth factor therapy to increase metabolic demand and promote healing [21, 22]. However, replacing one growth factor alone does not alleviate a chronic wound, as the complexity of healing requires many factors to regulate wound healing properly [21, 23]. Therefore, researchers have explored the application of potential healing mediators with many wound healing models for therapies in the future [21, 24, 25].
Previously, we successfully identified the secretory proteins of human dermal fibroblast conditioned medium (DFCM) containing abundant healing mediators. DFCM supplementation in an in vitro keratinocyte culture and scratch assay increased keratinocyte attachment, proliferation, and migration [26, 27]. However, the efficiency of in vivo wound healing with DFCM is unclear. Therefore, we evaluated the safety and potential benefit of DFCM in wound healing in vivo in the present study. Ovine collagen hydrogel was fortified with DFCM to form a three-dimensional (3D) acellular skin patch (ASP) for delivering DFCM and to be used for immediate implantation for full-thickness skin loss. We successfully fabricated an ASP using collagen hydrogel with DFCM, and evaluated the release profile of the DFCM. The immunogenic properties of the construct were evaluated in vitro and in vivo. The ASP healing efficiency in treating full-thickness wounds was evaluated using an animal model.
Materials and methods
This study was approved by the Universiti Kebangsaan Malaysia Research Ethics Committee (UKMREC) with the approval code UKM FPR.4/244/FF-2015-204 and the UKM Animal Ethics approval code PP/TEC/2015/SHIPLU/20-MAY/675-MAY-2015-DEC-2016.
Cell isolation and culture
Redundant skin tissue samples from abdominoplasty or face-lift surgery were obtained from three consenting healthy patients (n = 3) and processed as described previously [17]. Briefly, the samples were cleaned and minced, digested with 0.6% type I collagenase (Worthington, NJ, USA) for 4–6 h in a 37 °C incubator shaker and disassociated using 0.05% trypsin–EDTA (Gibco, Waltham, MA, USA) for 8–10 min. The digested cells were resuspended in co-culture medium [equivalent mixture of keratinocyte growth medium, i.e. EpiLife® (Gibco) and fibroblast growth medium, i.e. F-12:Dulbecco’s modified Eagle’s medium (1:1; FD; Sigma, St. Louis, MO, USA) supplemented with 10% foetal bovine serum (FBS; Gibco)] and 1% antibiotic-antimycotic (Gibco). The cells were seeded into 6-well culture plates (Greiner Bio-One, Monroe, NC, USA) at 37 °C in a 5% CO2 incubator. The waste medium was replaced every 2–3 days. The fibroblasts were removed using 0.05% trypsin–EDTA (Gibco) when the cells were 70–80% confluent, and sub-cultured in a T75 flask (Nunc, Rochester, NY, USA) using FD + 10% FBS until passage 3 (P3).
Preparation and collection of DFCM
P3 fibroblasts were used to prepare the DFCM. Once the fibroblasts reached 80–100% confluence, the waste culture medium was removed, and the cells were washed twice with Dulbecco’s phosphate-buffered saline (DPBS) to remove the excess medium. Then, fresh serum-free keratinocyte-specific medium with growth supplement (EpiLife®; Gibco; referred to here as KM1), defined keratinocyte serum-free medium with supplement (DKSFM; Gibco; referred to here as KM2) or fibroblast-specific culture medium (serum-free F-12: Dulbecco’s Modified Eagle medium (DMEM); Sigma; referred to here as FM) was added to the fibroblasts separately. The culture medium used for the DFCM preparation was free from antibiotic-antimycotic. The cells were incubated at 37 °C in a 5% CO2 incubator for 72 h, and the waste media was collected and designated DFCM-KM1, DFCM-KM2, and DFCM-FM, respectively.
Protein filtration and concentration
The conditioned media were filtered using a 3-kDa Amicon Ultra-4 centrifugal filter (Merck Millipore, Germany), which was centrifuged at 4000×g at 25 °C for 40 min to concentrate the proteins. Molecules larger than 3-kDa, which were retained at the surface of the membrane, were collected and dialysed using a mini dialysis kit (GE, UK) with 1-kDa cut-off and stored at − 80 °C prior to use. The protein concentrations of the DFCM were determined using the bicinchoninic acid (BCA) assay (Sigma), and the absorbance at 562 nm was measured using a spectrophotometer (Bio-Tek, Winooski, VT, USA). The protein quantity was estimated by comparing the readings with those of the protein standards (Sigma).
Fabrication of the ASP
Type I collagen from ovine tendon was prepared in-house according to the protocol described by Fauzi et al. [28] to produce collagen hydrogel. The pure collagen gel was neutralised by dropwise addition of 1 M sodium hydroxide (NaOH, Sigma-Aldrich) until the solution pH reached 7.0. Then, the collagen mixture was centrifuged at 4000 rpm at 4 °C for 2 min to remove air bubbles. The neutralised collagen solution was then mixed with DFCM (DFCM-KM1 and DFCM-KM2, 200 and 400 μg/mL; DFCM-FM, 400 and 800 μg/mL), and incubated at 37 °C to initiate gelation into the 3D construct.
ASP release profile
ASP were prepared with the DFCM in Transwell cell culture inserts (Greiner bio-one, Austria) and incubated in 0.0015% type I collagenase (2 U/mL) (Worthington) at 37 °C for 24 h according to the protocol described by Sakamoto et al. [29]. The protein release to the collagenase was collected for the first 30 min and at subsequent 2-h intervals for 24 h and measured using the BCA assay.
PBMC assay of DFCM in vitro immune response
Human peripheral blood mononuclear cells (PBMC) from healthy donors were collected in heparinised vacutainers (BD, CA, USA) and separated by centrifugation at 400×g for 30 min using a Ficoll-Paque density gradient. The PBMC were stained with carboxyfluorescein succinimidyl ester (CFSE) as per the manufacturer’s instructions (BioLegend, CA, USA). The cells were resuspended and cultured in 12-well plates at a seeding density of 1 × 106 cells per well and cultured using normal RPMI 1640 culture medium (Invitrogen, Waltham, MA, USA) containing 10% FBS (Invitrogen) as control or the mitogen phytohemagglutinin (PHA, 1 μg/mL) as a positive control; the test groups were supplemented with DFCM (DFCM-KM1 and DFCM-KM2, 400 μg/mL; DFCM-FM, 800 μg/mL). The PBMC proliferative response was evaluated by assessing the incorporation of tritiated thymidine (3H-TdR, 0.037 MBq/well [0.5 μCi/well]; PerkinElmer, Boston, MA, USA), which was added to the cell culture plates and incubated at 37 °C for 96 h. The 3H-TdR was incorporated into the DNA of proliferating cells and measured using liquid scintillation spectroscopy on a beta counter (MicroBeta® TriLux; PerkinElmer) after the addition of scintillation fluid (OptiPhase Supermix Cocktail; Perkin Elmer); readouts were in count per minute (CPM).
ASP in vivo immune response
Sensitisation was performed using the guinea pig maximisation test (GPMT) using Dunkin Hartley albino guinea pigs weighing 500–750 g. The guinea pigs were acclimatised for 7 days to the laboratory environment prior to experimentation. There were five samples: the positive and negative controls and the three experimental groups comprising collagen hydrogel loaded with DFCM-KM1 (400 μg/mL), DFCM-KM2 (400 μg/mL) or DFCM-FM (800 μg/mL) (i.e. Col + DFCM-KM1 400, Col + DFCM-KM2 400 or Col + DFCM-FM 800, respectively). Briefly, the five samples were each injected intracutaneously with 0.1 mL Freund’s complete adjuvant (FCA; Sigma) into the back of the guinea pigs’ scapula (six sites/guinea pig, n = 6 per sample) (Fig. 1). The positive control received 0.1% (w/w) 2,4-dinitrochlorobenzene (DNCB, Sigma), which causes an allergic reaction and stimulates the immune response; the negative control received DFCM-free collagen hydrogel, i.e. collagen-only. After 7-day intracutaneous sensitisation, 10% (w/w) sodium lauryl sulphate (SLS; Sigma) ointment, an inflammatory agent, was applied topically to the sensitised site, and removed after 24 h. Finally, 0.2 mL collagen hydrogel with DFCM was patched on the site for 24 h to induce sensitisation.
After 14 days, a patch was applied to the skin on the bilateral flank of the guinea pigs for 24 h to induce inflammation (Fig. 1). The skin reaction (erythema and swelling) was observed at 24 h and 48 h after patch removal and scored according to the criteria proposed by Magnusson and Kligman [30] (Table 1). Table 2 summarises the GPMT test procedure. After 48 h, skin biopsy of the guinea pigs was stained with haematoxylin and eosin (H&E) to evaluate the skin structure. The tissue samples were fixed with 10% buffered formalin, followed by sample processing, paraffin-blocking, sectioning into 5-μm thick slices using a microtome (Leica, Germany), dewaxed with xylene and alcohol series (100%, 95%, and 70%), and stained with H&E. The stained sections were evaluated under a light microscope (Olympus, San Jose, CA, USA), and the skin thickness was measured using ImageJ software (National Institute of Health, USA).
In vivo model for rapid treatment of full-thickness skin wound
Two-month-old BALB/c mice weighing 30–35 g were provided with food (Altromin #1324 Diet, Germany) and autoclaved water ad libitum. The mice were acclimatised for 1 week before the experiment was started and kept in controlled conditions. The mice were anesthetised intramuscularly using an anaesthetic cocktail consisting of ketamine (Ketamav 50, 5.6 mg/kg; Mavlab, Australia), xylazil-100 (5.6 mg/kg; Troy Laboratories, Australia) and Zoletil (Zoletil 20, 2.8 mg/kg; Virbac Laboratories, France) with a dilution ratio of 1:4 water for injection (WFI). A full-thickness skin excision wound with 1-cm diameter was made on the dorsum of the mice using surgical scissors. The ASP (collagen hydrogel supplemented with 200 and 400 μg/mL DFCM-KM1 and DFCM-KM2 or 400 and 800 μg/mL DFCM-FM) were implanted onto the wound area, and the peripheral region was sutured to avoid construct movement. The non-treated group (NT, treated with 10 μL normal saline) and treated group (collagen-only) were used as controls (n = 6 per sample). All wounds were covered with sterile wound dressing (Leukomed, Germany) and secured with Hypafix adhesive tape (BSN Medical, Germany). The wound dressing was changed weekly; wound healing efficiency was analysed by capturing wound closure images at day 0, 7, 14 and 17 using a digital camera (Sony, Japan) and analysing them with ImageJ software.
Histological analysis of regenerated skin
Skin biopsies were excised from the wound centre and periphery at 7 and 17 days after implantation. The spleen and thymus were collected at day 17 from the euthanised normal mice. The spleen and thymus are the two organs of the immune system, and control the production and maturation of defence cells such as lymphocytes. Increased lymphocyte proliferation and accumulation in the spleen and thymus indicate an antigen- or mitogen-induced immune response [31]. All samples were fixed with 10% buffered formalin (Sigma), processed, embedded in paraffin blocks, and sectioned at 5-μm thickness using a microtome (Leica) for histological staining with H&E (IHC World, Ellicott City, MD, USA), Masson’s trichrome (MS; Sigma) and picrosirius red (PR; Polysciences, Warrington, PA, USA) to evaluate the skin structure and collagen production.
Immunohistochemical analysis of regenerated skin
Immunohistochemical (IHC) staining was performed to evaluate the structure of the epidermis and dermis layers and to characterise the maturity of the regenerated skin. Cytokeratin 14 (CK14) and type I collagen expression was used to evaluate the maturity of the epidermal and dermal layer, respectively. The tissue sections (5 μm) were deparaffinised, hydrated with deionised water and treated with antigen retrieval citrate buffer (pH 9.0, Sigma) at 98 °C for 20 min. Then, the sections were incubated with 10% goat serum at 37 °C to block nonspecific binding. Next, the sections were incubated with specific primary antibodies (rabbit anti-human CK14 and rabbit anti-human collagen type I; Abcam, UK) at 4 °C overnight. Later, the sections were incubated with secondary antibodies (goat anti-rabbit immunoglobulin G [IgG], Abcam) at 37 °C for 1 h and counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen) for 20 min. The expression of specific antibodies was observed using confocal laser scanning microscopy (Nikon A1R-A1, Nikon, Japan).
Statistical analysis
The quantitative results are reported as the mean ± standard error of the mean (SEM). GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, USA) was used for the statistical analysis; results were analysed using two-way analysis of variance (ANOVA). The difference between groups was significant if p < 0.05.
Results
Protein release from ASP in vitro
Figure 2a shows the gross morphology of the ASP, which was soft, semi-solid and translucent. Figure 2b shows the protein released from the ASP after 24-h incubation with type I collagenase. Most of the ASP was fully digested after 8-h incubation. The graph of the proteins released by all ASP showed the same pattern, i.e. the protein concentrations increased with incubation time. The concentration of the released proteins in all DFCM groups was normalised by deducting the DFCM-free collagen concentration. The collagen mixed with DFCM-KM1 and DFCM-KM2 had higher protein release than the collagen mixed with DFCM-FM.
In vitro and in vivo immunogenicity
The PBMC assay showed that DFCM did not increase PBMC proliferation, as there were no significant differences between DFCM and the negative control, normal RPMI 1640 culture medium (p > 0.05) (Fig. 3a). In contrast, PBMC proliferation was higher in the PHA-supplemented medium, i.e. the positive control (p < 0.0001). This confirms that DFCM does not induce the immune response during in vitro culture. To confirm this, further analysis was performed using an in vivo model.
In vitro and in vivo immunogenicity of DFCM. a) PBMC proliferation assay with PHA as the positive control. The DFCM had no effect on PBMC proliferation, indicating no immune response. Asterisk indicates a significantly higher difference compared to other groups. b) Evaluation of in vivo immune response via GPMT of DFCM mixed with collagen hydrogel. The grading scale was according to the Magnusson and Kligman scale (indicated with an arrow) and histological observation of the skin samples using H&E staining. No sensitisation effect of DFCM and collagen was observed on the guinea pig skin. c) Quantitative evaluation of the epidermal thickness of the control and test groups. No significant difference was observed in the test groups as compared to the negative control group, indicating that DFCM and collagen do not induce an immune response. *Significantly higher difference compared to other groups. Scale bar = 100 μm. PC positive control, NC negative control, E epidermis layer, D dermis layer, C crust
The GPMT showed no erythema or swelling after induction and challenge with DFCM (grade 0) as compared to the positive control (grade 3) (Fig. 3b). The negative control (only collagen hydrogel) also elicited no reaction (grade 0), which confirms that neither the DFCM nor ovine tendon-derived collagen induce an immune response in vivo. Moreover, histological analysis through H&E staining showed epidermal thickening with crust or scab formation and weakening of the dermal–epidermal junction in the positive control group. In contrast, normal skin structure histology was observed in the negative control and test groups (Fig. 3b). The epidermal thickness was significantly higher in the positive control (436.7 ± 55.8 μm) than the negative control (34.7 ± 13.8 μm) and test groups (Col + DFCM-KM1: 48.8 ± 10.3 μm, Col + DFCM-KM2: 43.2 ± 6.8 μm, Col + DFCM-FM: 28.5 ± 3.6 μm) (Fig. 3c). The results demonstrate that DFCM supplementation of collagen does not stimulate an immune response in vivo, making it safe to use in allogenic applications.
Gross appearance of regenerated skin
The gross appearance and wound closure were observed at days 7, 14 and 17 (Fig. 4), and the wound size (area) was calculated. The wounds in all mice healed with time. The hair surrounding the wound grew as early as day 7, indicating a favourable healing process. At macroscopic level, all groups exhibited dry and full epithelialisation at day 17. Minimal signs of skin contraction were observed in all groups, with no obvious difference in terms of time taken for wound closure. Quantitative analysis showed that the wound area of all mice treated with the ASP, with or without DFCM, was significantly reduced by more than 50% at day 7 as compared to the initial wound (day 0), and was significantly smaller than that of the NT group (Fig. 5). This indicates that DFCM-supplemented collagen hydrogel induces a faster healing rate. Besides, ASP plus DFCM-KM1 400 also showed significantly faster healing than the collagen-only group. However, no difference was observed between the DFCM groups. At days 14 and 17, the percentage of wound area of the animals treated with or without DFCM was slightly lower compared to the control group, but there was no significant difference between all groups for both days.
Percentage of wound size reduction in all mice in the test and NT groups. All test groups had faster healing rates than the NT group at day 7. *The wound area was significantly higher in the NT group than in the other groups at day 7; **the wound area in the DFCM-KM1 400 group was significantly lower than that of the collagen-only group at day 7 (p < 0.05)
Histological analysis of regenerated skin
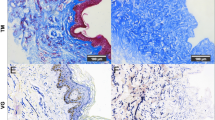
The regenerated skin was evaluated histologically through H&E, MS and PR staining. The normal bilayer skin structure had discrete, keratinised epidermis and highly cellularised dermis. Figure 6 shows the histological difference between the normal and newly synthesised skin. The normal skin structure had a thin epidermal layer with matured collagen matrix in the dermis layer and hair follicles, whereas the newly synthesised skin had a thickened epidermal layer with loose dermal matrix and granulation tissue.
The NT group had no clear epidermis layer at day 7 compared to the test groups, which showed an intact, thickening epidermis layer, known as hyperplasia (Fig. 7), indicating that healing was in progress. Complete re-epithelialisation was observed at day 17 in all groups, with the formation of a keratin layer on top of the epidermis, indicating maturation of the epidermal layer. The epidermal thickness of test groups Col + DFCM-KM1 200, Col + DFCM-KM1 400 and Col + DFCM-KM2 200 (51.3 ± 9.9 μm, 75.5 ± 28.8 μm and 78.9 ± 46.6 μm, respectively) was comparable to the native skin of the mice (40.1 ± 6.3 μm), indicating maturation of the regenerated epithelial layer (Fig. 8). Moreover, the epidermal thickness of test group Col + DFCM-KM1 200 was significantly lower than that of the NT (108.8 ± 25.0 μm) and DFCM-FM groups (95.8 ± 24.6 μm; 101.2 ± 32.0 μm), revealing that Col + DFCM-KM1 200 facilitates better healing in vivo.
The epidermal thickness of regenerated skin of the test groups and NT group (n = 6). **Epidermal thickness in the native skin is significantly lower than that in the NT, DFCM-KM2 400 and both DFCM-FM groups; # shows that the epidermal thickness of the DFCM-KM1 200 group was significantly lower than that of the NT and both DFCM-FM groups
The histology analysis also showed that the newly regenerated dermis had more fibroblasts and granulation tissue. The results were confirmed by MS and PR staining, which yielded positive staining as early as day 7 and more prominent staining at day 17. This indicates the production and organisation of collagen matrix in the newly regenerated dermis (Figs. 9 and 10).
IHC analysis of regenerated skin
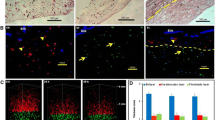
Immunohistochemistry was performed to characterise the quality of the regenerated skin by detecting the expression of specific markers in the epithelial and dermal layers. The newly synthesised epidermal layer was stained with anti-CK14 antibody that stains proliferative basal keratinocytes. All groups showed positive expression of CK14 in the epidermis at day 17, including the NT group (but not at day 7) (Fig. 11), indicating the completion of re-epithelialisation and epidermis maturation at day 17. The newly formed dermis was stained for type I collagen, which was present in all groups at days 7 and 17 (Fig. 12), as demonstrated by the MS and PR staining.
Histopathology analysis
Histopathology of the mouse thymus and spleen was performed to evaluate the innate immune response, based on necrosis, the distribution of thymocytes (immature T cells) or lymphocytes, and the presence of multinucleated giant cells. The gross histology of the spleen (Fig. 13) showed normal to mild lymphocyte proliferation in all test groups as compared to the positive control. There were fewer or no multinucleated giant cells in all test groups. The thymus histology showed normal thymocyte distribution, indicating that supplementation of DFCM and collagen does not stimulate the systemic immune response (Fig. 14). Although the mice treated with DFCM-KM2 400 and DFCM-FM 800 had slightly increased lymphocyte proliferation in the thymus and spleen, the increment was not as severe as that of the positive control. The mice appeared healthy, and no mice died during the experiment. Rejection of the ASP was also not observed.
Histological cross section of the spleen at 17 days after implantation (H&E staining). The positive control showed severe lymphocyte proliferation; arrows indicate multinucleated giant cells. Other test groups showed normal to mild lymphocyte proliferation and fewer or no multinucleated giant cells as compared to the positive control. Scale bar = 100 μm
Histological cross section of the thymus at 17 days after implantation (H&E staining). The positive control showed severe lymphocyte proliferation; arrows indicate multinucleated giant cells. Other test groups showed normal to mild lymphocyte proliferation and fewer or no multinucleated giant cells as compared to the positive control. Scale bar = 100 μm
Discussion
The limitation of SG, the conventional treatment of skin loss, has triggered the use of skin substitutes for wound healing. Recently, many skin substitutes have been used clinically to cure and repair large and deep wound injury [32]. The best skin substitute should have no antigenicity, have tissue compatibility, and lack toxicity and disease transmission [7]. Moreover, skin substitutes confer (a) protection by establishing a mechanical barrier against microorganisms to reduce bacterial infection and to impede the loss of vapour, water, proteins and electrolytes; (b) procrastination by providing wound cover after early wound debridement until permanent wound closure by skin grafts; (c) promotion by delivering dermal matrix components, cytokines and growth factors to the wound bed, which promote and enhance wound healing; and (d) provision of new structures, such as dermal collagen or cultured cells, that are incorporated into the wound during wound healing to restore skin functions [33, 34]. The high demand for skin substitutes has encouraged researchers and surgeons to collaborate in developing skin substitutes or wound-care products to promote healing.
Previous studies have demonstrated that proteins secreted from fibroblast culture, i.e. DFCM, contain wound-healing mediators that promote wound healing in vitro [20, 27, 35]; thus, DFCM has the potential to be used as a supplement for treating skin injuries. In the present study, the wound healing potential of DFCM was evaluated in an in vivo model by constructing ASP, in which the proteins secreted were fortified on collagen hydrogel. Type I collagen is a major extracellular matrix (ECM) component of the skin; thus, it forms a suitable architecture for regenerating new skin. Besides, collagen is degradable, making it an ideal skin substitute to protect the wound and enable the formation of newly regenerated skin [32].
The hydrogel structure was chosen based on its properties, as it has a soft, tissue-like texture that acts as a carrier that encapsulates the proteins in DFCM and allows cell migration and the diffusion of nutrients and cellular waste [36]. Incubation with DFCM-fortified ASP in vitro, with collagenase simulating the in vivo environment, demonstrated efficient degradation of constructs that sustained the release of the proteins. Other studies have also confirmed the ability of the hydrogel to be degraded by incubation in collagenase, which is present in the form of biological fluids and tissues of the human body, such that it releases 20–70% of the encapsulated biomaterials [37,38,39]. The collagen hydrogel degradability facilitated the sustained release of the encapsulated DFCM proteins to the wounded area, thus stimulating healing.
The DFCM-fortified ASP was implanted in the dorsum of BALB/c mice to determine the efficiency of DFCM in regenerating a full-thickness skin wound. The implantation of the ASP, with or without DFCM, significantly increased the healing rate as compared to the NT group at 7 days after implantation, where mice treated with DFCM-KM1 400 had a significantly faster healing rate than those treated with collagen only. This was due to the sustained release of collagen and DFCM, which helped enhance healing at an early stage. At day 7, histological analysis confirmed the integrity and maturity of the graft, demonstrating two distinct layers, i.e. the epidermis and dermis, with epithelial hyperplasia in the centre and more stratified layers near the wound. A fully stratified epithelium was observed at day 17, indicating that the graft had successfully integrated with the native tissue at the wound edge. IHC analysis also showed positive expression of CK14, a basal epithelial cell marker [40]. Based on the results, the wound treated with DFCM-KM1 showed better healing, with more epithelial layers than the other groups.
Wound re-epithelialisation and keratinocyte migration rely on dermal regeneration [41]. Our results showed positive MS and PR staining, indicating the production and organisation of collagen matrix in the newly regenerated dermis. IHC also showed positive expression of type I collagen in all groups, which was more prominent at day 17. Collagen types I, III, V and VI are mostly found during wound healing, playing a significant role during the remodelling phase in wound healing [42]. The DFCM-fortified collagen hydrogel enhanced wound healing efficiency and the maturation of regenerated skin at day 17, which closely resembled the native tissue. This indicates that the DFCM-fortified collagen hydrogel prepares the wound bed for better wound healing. The DFCM contains the ECM protein class and the biological process leads to keratinocyte and fibroblast migration from the wound edge to produce ECM and close the wound, forming newly regenerated skin. The migration of the surrounding cells is also regulated by cell–cell contact, which involves the release of soluble factors, cell–scaffold interaction, and 3D environment conditions to expedite wound healing [43].
In the clinical setting, the focus is to produce a safe, effective skin substitute that promotes wound healing and repairs skin defects, and that has no immunogenicity or immune rejection after implantation [44]. In the present study, the in vitro PBMC assay involving only DFCM was performed. PBMC are blood cells such as lymphocytes or monocytes that are important for the immune system to fight infection and respond to intruders. In a normal human body, PBMC levels will increase when the body notices or detects microbial invasion, thus activating the body’s defence system [45]. Our results show that exposure to DFCM does not increase PBMC proliferation; in fact, the PBMC levels in the test groups were the same as that of the negative control. Meanwhile, the GPMT involving only collagen hydrogel and DFCM-fortified collagen hydrogel confirmed that the ovine tendon-derived collagen and the DFCM do not induce an immune response in vivo. The ovine collagen used in this study consists of type I collagen, which elicits a low immunogenic response [46,47,48]. This result indicates that DFCM does not induce an immune response in vitro and in vivo. Therefore, the combination of collagen hydrogel and DFCM is safe for clinical application.
Moreover, histological analysis of the lymphoid organs (thymus and spleen) of BALB/c mice implanted with ASP showed normal lymphocyte distribution, fewer or no multinucleated giant cells, and normal thymocyte distribution. The thymus and spleen are the immune cell production sites, where changes in immune cell level are detected based on necrosis, lymphocyte or thymocyte (immature T cells) distribution and the presence of multinucleated giant cells, which facilitate the immune response and are good indicators of immune modulation [31]. Macrophages and T lymphocytes activate and destroy anything recognised as non-self substances or pathogens. This confirms that the implantation of ASP with DFCM-fortified collagen hydrogel does not stimulate a systemic immune response. Thus, the present ASP is safe and effective for use without any symptoms of immune response or rejection.
Acellular skin substitutes are beneficial for wound healing because they are readily available and transplantable immediately after injury, which results in lower risk of infection and morbidity. Consequently, patient suffering, duration of hospital stay and the overall cost of treatment are reduced [10, 49, 50]. Commercially available acellular skin substitutes, namely Biobrane® and Integra®, are produced using biosynthetic materials such as nylon mesh or silicon membrane incorporated with natural biomaterial such as porcine or bovine collagen using chemical bonds. These substitutes are usually used as temporary coverage for superficial or partial-thickness burns and wounds, and promote wound healing and reduce pain [51]. Other acellular skin substitutes such as Alloderm® and DermACELL™ are derived from the acellular matrix of cadaveric or donated human skin tissues that provide ECM to the wounded area [52, 53]. In contrast, the ASP we tested here was developed as a permanent skin coverage focused on delivering fibroblast-secreted wound healing mediators as well as providing the ECM architecture to accelerate healing.
The application of ASP was beneficial, as the DFCM-fortified collagen hydrogel was enriched with ECM and wound-healing mediators, thus promoting wound healing by enhancing native cell migration to regenerate new skin tissues. Also, this ASP contains DFCM and collagen hydrogel, which can be produced in bulk and be readily available for implantation. The evaluation and histological analysis showed that the collagen hydrogel with or without DFCM is superior to NT, in which there were no symptoms of graft rejection. Furthermore, the use of DFCM-KM1-fortified collagen hydrogel showed more promising outcomes than only collagen. However, no difference was observed between the DFCM groups, suggesting that DFCM-fortified collagen hydrogel can be used as a skin substitute for immediate treatment of full-thickness skin loss.
Most in vivo wound healing studies use conditioned medium, usually in the form of liquid injected subcutaneously along the margin of the wound sites [54, 55], whereas our intention was to cover the whole wound area using DFCM-fortified collagen hydrogel as a 3D skin patch. This 3D tissue model is useful for mimicking the in vivo cell physiology to establish cell, protein and material interactions. However, to simulate actual skin loss, a large animal model with bigger wound size would better facilitate evaluation of the effectiveness of conditioned medium in promoting wound healing. In addition, tumorigenicity testing should be performed as a preclinical safety test to ensure that the DFCM and collagen do not induce tumours.
Conclusion
The fabrication of a DFCM-fortified collagen hydrogel via an in vitro 3D model shows that this construct is degradable, where it releases proteins to the wound area. In vitro and in vivo testing revealed that this construct does not induce an immune response, has low or no immunogenicity and is safe for use for clinical application in humans. The findings demonstrate that implantation of the ASP, containing DFCM-fortified collagen hydrogel, repairs full-thickness wounds in an animal model without signs of rejection. Histological analysis confirmed the structure, integrity and maturation of the regenerated skin. Therefore, this ASP is a potential off-the-shelf product to be applied for immediate treatment of skin injuries.
References
Halim AS, Khoo TL, Mohd Yussof SJ. Biologic and synthetic skin substitutes: an overview. Indian J Plast Surg. 2010;43(Suppl):S23–8. https://doi.org/10.4103/0970-0358.70712.
Harrison CA, MacNeil S. The mechanism of skin graft contraction: an update on current research and potential future therapies. Burns. 2008;34(2):153–63. https://doi.org/10.1016/j.burns.2007.08.011.
Schneider JC, Holavanahalli R, Helm P, Goldstein R, Kowalske K. Contractures in burn injury: defining the problem. J Burn Care Res. 2006;27(4):508–14. https://doi.org/10.1097/01.bcr.0000225994.75744.9d.
Ojeh N, Akgül B, Tomic-Canic M, Philpott M, Navsaria H. In vitro skin models to study epithelial regeneration from the hair follicle. PLoS One. 2017;12(3):e0174389. https://doi.org/10.1371/journal.pone.0174389.
Coulomb B, Friteau L, Baruch J, Guilbaud J, Chretien-Marquet B, Glicenstein J, et al. Advantage of the presence of living dermal fibroblasts within in vitro reconstructed skin for grafting in humans. Plast Reconstr Surg. 1998;101(7):1891–903.
Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6(3):331–43.
Snyder DL, Sullivan N, Schoelles KM. AHRQ Technology Assessments. Skin substitutes for treating chronic wounds. Rockville (MD): Agency for Healthcare Research and Quality; 2012.
Berthiaume F, Maguire TJ, Yarmush ML. Tissue engineering and regenerative medicine: history, progress, and challenges. Annu Rev Chem Biomol Eng. 2011;2:403–30.
Jones I, Currie L, Martin R. A guide to biological skin substitutes. Br J Plast Surg. 2002;55(3):185–93. https://doi.org/10.1054/bjps.2002.3800.
Varkey M, Ding J, Tredget EE. Advances in skin substitutes—potential of tissue engineered skin for facilitating anti-fibrotic healing. J Funct Biomater. 2015;6(3):547–63. https://doi.org/10.3390/jfb6030547.
Auger FA, Lacroix D, Germain L. Skin substitutes and wound healing. Skin Pharmacol Physiol. 2009;22(2):94–102. https://doi.org/10.1159/000178868.
Usui ML, Mansbridge JN, Carter WG, Fujita M, Olerud JE. Keratinocyte migration, proliferation, and differentiation in chronic ulcers from patients with diabetes and normal wounds. J Histochem Cytochem. 2008;56(7):687–96. https://doi.org/10.1369/jhc.2008.951194.
Spiekstra SW, Breetveld M, Rustemeyer T, Scheper RJ, Gibbs S. Wound-healing factors secreted by epidermal keratinocytes and dermal fibroblasts in skin substitutes. Wound Repair Regen. 2007;15(5):708–17.
Hur W, Lee HY, Min HS, Wufuer M, Lee C, Hur JA, et al. Regeneration of full-thickness skin defects by differentiated adipose-derived stem cells into fibroblast-like cells by fibroblast-conditioned medium. Stem Cell Res Ther. 2017;8:92. https://doi.org/10.1186/s13287-017-0520-7.
Debels H, Hamdi M, Abberton K, Morrison W. Dermal matrices and bioengineered skin substitutes: a critical review of current options. Plast Reconstr Surg Glob Open. 2015;3(1):e284. https://doi.org/10.1097/GOX.0000000000000219.
Collawn SS, Mobley JA, Banerjee NS, Chow LT. Conditioned media from adipose-derived stromal cells accelerates Healing in 3-dimensional skin cultures. Ann Plast Surg. 2016;76(4):446–52. https://doi.org/10.1097/sap.0000000000000754.
Seet WT, Maarof M, Anuar KK, Chua K-H, Irfan AWA, Ng MH, et al. Shelf-life evaluation of bilayered human skin equivalent, MyDerm™. PLoS One. 2012;7(8):e40978.
Mazlyzam AL, Aminuddin BS, Fuzina NH, Norhayati MM, Fauziah O, Isa MR, et al. Reconstruction of living bilayer human skin equivalent utilizing human fibrin as a scaffold. Burns. 2007;33(3):355–63. https://doi.org/10.1016/j.burns.2006.08.022.
Idrus RBH Rameli MAbP, Low KC, Law JX, Chua KH, Latiff MbA, Saim Ab. Full-thickness skin wound healing using autologous keratinocytes and dermal fibroblasts with fibrin: bilayered versus single-layered substitute. Adv Skin Wound Care. 2013.
Maarof M, Law JX, Chowdhury SR, Khairoji KA, Saim AB, Idrus RB. Secretion of wound healing mediators by single and bi-layer skin substitutes. Cytotechnology. 2016;68:1873–84. https://doi.org/10.1007/s10616-015-9940-3.
Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34(3):599–610. https://doi.org/10.1007/s12325-017-0478-y.
Braund R, Hook S, Medlicott NJ. The role of topical growth factors in chronic wounds. Curr Drug Deliv. 2007;4(3):195–204.
Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585–601. https://doi.org/10.1111/j.1524-475X.2008.00410.x.
Wu L, Xia YP, Roth SI, Gruskin E, Mustoe TA. Transforming growth factor-beta1 fails to stimulate wound healing and impairs its signal transduction in an aged ischemic ulcer model: importance of oxygen and age. Am J Pathol. 1999;154(1):301–9.
Emmerson E, Campbell L, Davies FC, Ross NL, Ashcroft GS, Krust A, et al. Insulin-like growth factor-1 promotes wound healing in estrogen-deprived mice: new insights into cutaneous IGF-1R/ERalpha cross talk. J Invest Dermatol. 2012;132(12):2838–48. https://doi.org/10.1038/jid.2012.228.
Manira MCS, Rosliza A, Yi Ling A, Abidah A, Vittarino J, Nurul ‘Izzah AG, et al. Concentration dependent effect of dermal fibroblast conditioned medium on in vitro wound healing properties of keratinocytes. Regenerative Research. 2014;3(2):3.
Chowdhury SR, Aminuddin BS, Ruszymah BH. Effect of supplementation of dermal fibroblasts conditioned medium on expansion of keratinocytes through enhancing attachment. Indian J Exp Biol. 2012;50(5):332–9.
Fauzi MB, Lokanathan Y, Aminuddin BS, Ruszymah BH, Chowdhury SR. Ovine tendon collagen: extraction, characterisation and fabrication of thin films for tissue engineering applications. Mater Sci Eng C Mater Biol Appl. 2016;68:163–71. https://doi.org/10.1016/j.msec.2016.05.109.
Sakamoto M, Morimoto N, Ogino S, Jinno C, Taira T, Suzuki S. Efficacy of gelatin gel sheets in sustaining the release of basic fibroblast growth factor for murine skin defects. J Surg Res. 2016;201(2):378–87. https://doi.org/10.1016/j.jss.2015.11.045.
Magnusson B, Kligman AM. The identification of contact allergens by animal assay. The guinea pig maximization test. J Invest Dermatol. 1969;52(3):268–76.
Thejaswi K, Amarnath M, Srinivas G, Jerald MK, Raj TA, Singh S. Immune modulatory responses of mesenchymal stem cells from different sources in cultures and in vivo. Cell & Tissue Transplantation & Therapy. 2012;4(3374-CTTT-Immune-Modulatory-Responses-of-Mesenchymal-Stem-Cells-from-Different-S.pdf):1–13. https://doi.org/10.4137/CTTT.S9812.
Wu Z, Fan L, Xu B, Lin Y, Zhang P, Wei X. Use of decellularized scaffolds combined with hyaluronic acid and basic fibroblast growth factor for skin tissue engineering. Tissue Eng A. 2015;21(1–2):390–402. https://doi.org/10.1089/ten.TEA.2013.0260.
Shakespeare PG. The role of skin substitutes in the treatment of burn injuries. Clin Dermatol. 2005;23(4):413–8.
Manira M, Anuar KK, Seet WT, Irfan AWA, Ng MH, Chua KH, et al. Comparison of the effects between animal-derived trypsin and recombinant trypsin on human skin cells proliferation, gene and protein expression. Cell Tissue Bank. 2013:1–9.
Maarof M, Lokanathan Y, Ruszymah HI, Saim A, Chowdhury SR. Proteomic analysis of human dermal fibroblast conditioned medium (DFCM). Protein J. 2018;37(6):589–607. https://doi.org/10.1007/s10930-018-9800-z.
Zhu J, Marchant RE. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devices. 2011;8(5):607–26. https://doi.org/10.1586/erd.11.27.
McBane JE, Vulesevic B, Padavan DT, McEwan KA, Korbutt GS, Suuronen EJ. Evaluation of a collagen-chitosan hydrogel for potential use as a pro-angiogenic site for islet transplantation. PLoS One. 2013;8(10):e77538. https://doi.org/10.1371/journal.pone.0077538.
Vulpe R, Popa M, Picton L, Balan V, Dulong V, Butnaru M, et al. Crosslinked hydrogels based on biological macromolecules with potential use in skin tissue engineering. Int J Biol Macromol. 2016;84:174–81. https://doi.org/10.1016/j.ijbiomac.2015.12.019.
Choi J, Park H, Kim T, Jeong Y, Oh MH, Hyeon T, et al. Engineered collagen hydrogels for the sustained release of biomolecules and imaging agents: promoting the growth of human gingival cells. Int J Nanomedicine. 2014;9:5189–201. https://doi.org/10.2147/ijn.s71304.
Aoki S, Takezawa T, Uchihashi K, Sugihara H, Toda S. Non-skin mesenchymal cell types support epidermal regeneration in a mesenchymal stem cell or myofibroblast phenotype-independent manner. Pathol Int. 2009;59(6):368–75. https://doi.org/10.1111/j.1440-1827.2009.02379.x.
Parenteau-Bareil R, Gauvin R, Cliche S, Gariepy C, Germain L, Berthod F. Comparative study of bovine, porcine and avian collagens for the production of a tissue engineered dermis. Acta Biomater. 2011;7(10):3757–65. https://doi.org/10.1016/j.actbio.2011.06.020.
Zhao J, Hu L, Gong N, Tang Q, Du L, Chen L. The effects of macrophage-stimulating protein on the migration, proliferation, and collagen synthesis of skin fibroblasts in vitro and in vivo. Tissue Eng A. 2015;21(5–6):982–91.
Liu Y, Ma L, Gao C. Facile fabrication of the glutaraldehyde cross-linked collagen/chitosan porous scaffold for skin tissue engineering. Mater Sci Eng C. 2012;32(8):2361–6. https://doi.org/10.1016/j.msec.2012.07.008.
Shevchenko RV, James SL, James SE. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J R Soc Interface. 2010;7(43):229–58. https://doi.org/10.1098/rsif.2009.0403.
Pourahmad J, Salimi A. Isolated human peripheral blood mononuclear cell (PBMC), a cost effective tool for predicting immunosuppressive effects of drugs and xenobiotics. Iran J Pharm Res. 2015;14(4):979.
Busra FM, Lokanathan Y, Nadzir MM, Saim A, Idrus RBH, Chowdhury SR. Attachment, proliferation, and morphological properties of human dermal fibroblasts on ovine tendon collagen scaffolds: a comparative study. Malays J Med Sci. 2017;24(2):33–43. https://doi.org/10.21315/mjms2017.24.2.5.
Busra FM, Chowdhury SR, Saim AB, Idrus RB. Genotoxicity and cytotoxicity of ovine collagen on human dermal fibroblasts. Saudi Med J. 2011;32(12):1311–2.
Yamamoto A, Mishima S, Maruyama N, Sumita M. Quantitative evaluation of cell attachment to glass, polystyrene, and fibronectin- or collagen-coated polystyrene by measurement of cell adhesive shear force and cell detachment energy. J Biomed Mater Res. 2000;50(2):114–24.
Vyas KS, Vasconez HC. Wound healing: biologics, skin substitutes, Biomembranes and Scaffolds. Healthcare (Basel). 2014;2(3):356–400. https://doi.org/10.3390/healthcare2030356.
Boyce ST, Lalley AL. Tissue engineering of skin and regenerative medicine for wound care. Burns Trauma. 2018;6(1):4. https://doi.org/10.1186/s41038-017-0103-y.
Alrubaiy L, Al-Rubaiy KK. Skin substitutes: a brief review of types and clinical applications. Oman Med J. 2009;24(1):4–6. https://doi.org/10.5001/omj.2009.2.
Park JY, Lee TG, Kim JY, Lee MC, Chung YK, Lee WJ. Acellular dermal matrix to treat full thickness skin defects: follow-up subjective and objective skin quality assessments. Arch Craniofac Surg. 2014;15(1):14–21. https://doi.org/10.7181/acfs.2014.15.1.14.
Moore MA, Samsell B, Wallis G, Triplett S, Chen S, Jones AL, et al. Decellularization of human dermis using non-denaturing anionic detergent and endonuclease: a review. Cell Tissue Bank. 2015;16(2):249–59. https://doi.org/10.1007/s10561-014-9467-4.
Payushina OV, Butorina NN, Sheveleva ON, Domaratskaya EI. Effect of mesenchymal stromal cells and conditioned media on mealing of skin wound. Bull Exp Biol Med. 2018;165(4):572–5. https://doi.org/10.1007/s10517-018-4215-6
Sun J, Zhang Y, Song X, Zhu J, Zhu Q. The healing effects of conditioned medium derived from mesenchymal stem cells on radiation-induced skin wounds in rats. Cell Transplant. 2018;963689718807410. https://doi.org/10.1177/0963689718807410
Acknowledgements
We are thankful to our colleague Dr. Shinsmon Jose, who provided expertise that greatly assisted part of the research. Some parts of this work were performed at the UKM Bioserasi Laboratory.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Research Grants
This study was funded by the Science Fund (02–01-02-SF0964), UKM fundamental fund (FF-2015-204) and the Tissue Engineering Centre, UKM Medical Centre.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the responsible committee on human experimentation (UKMREC), with approval code UKM FPR.4/244/FF-2015-204, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all patients included in the study. All institutional and national guidelines for the care and use of laboratory animals were followed with approval code PP/TEC/2015/SHIPLU/20-MAY/675-MAY-2015-DEC-2016.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maarof, M., Mh Busra, M.F., Lokanathan, Y. et al. Safety and efficacy of dermal fibroblast conditioned medium (DFCM) fortified collagen hydrogel as acellular 3D skin patch. Drug Deliv. and Transl. Res. 9, 144–161 (2019). https://doi.org/10.1007/s13346-018-00612-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-018-00612-z