Abstract
Pepper is mostly produced in greenhouses and fields in spring up to the end of summer. The reproductive stage coincides with high temperature of summer, which causes flowers to drop, leading to reduction in the yield, Se as a beneficial element can improved some stress indices. Control randomized design experiment was conducted to investigate the effect(s) of Se on heat stresses of pepper in control environment. Se in three concentrations of SeCl2 (4 (Se1), 6 (Se2) and 8 (Se3) mg L−1) was used at 35 ± 2 °C for 4 h a day, matching the high afternoon temperature. Growth, photosynthesis traits (Photosynthesis rate, transpiration and stomatal conductance), flower dropping and antioxidant changes were all measured. Results showed that Se1 decreased deleterious effects of heat stresses on vegetative traits (fresh and dry weight of fruit). Including dry weight of shoot, fresh and dry weight of root, and reproductive growth, such as Fresh weight and dry weight of fruit, flowers and fruit number. Photosynthesis rate, fruit antioxidant and phenol improved with the application of Se to heat stresses. POD and SOD activity increased, and MDA content decreased with Se application at the high temperature. Se also improved the P and S uptake. Generally, using 4 and 6 mg L−1 of Se could improve growth and physiological and phytochemical parameters of pepper and decrease the flower dropping at high temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In higher plants, selenium (Se) is known to be a beneficial nutrient, not essential though [1, 2]. This element can be in inorganic forms, that is to say, selenate (SeO42−), selenite (SeO32−), selenide (Se2−) and elemental Se, and in organic forms, namely SeCys and SeMet [3,4,5]. A number of studies have shown that low doses of Se can improve photosynthetic apparatus, while the high doses of this element could disrupt photosynthesis and photosynthetic apparatus. The same results were found concerning the use of plant and its effects on photosynthesis [6]. The beneficial effects of Se on plants have been reported from different points of view. It was reported that Se improved photosynthesis in rice seedlings [7]. Se could also increase growth of plants, such as tobacco, lettuce and potato [8,9,10], increase the yield in Cucerbita pepo, and enhance nutritive values of potatoes [11].
Moreover, Se increased the tolerance of plants against the detrimental effects of abiotic stresses, including the heat of heavy metals [12] and drought [13]. Se could delay senescence [14]. All of the mentioned effects of Se on decreasing stresses can be because of a decrease in ROS levels and an increase in antioxidant activities [13]. Plants pretreated with 100 µM selenium showed a signification increase in plant height, leaf area, dry weight, and weight of 100 seed [15,16,17,18].
Although pepper is a warm seasonal plant, it is sensitive to heat stresses. Pepper receives heat stresses in greenhouses and fields, especially during August in which the temperature reaches 40 °C. This is also the time when flower anthesis and fruit induction mostly occur. Thus, high temperature may cause flowers to drop, resulting in a decrease in reproduction and yield [19]. The effectiveness of Se on decreasing deleterious effects of heat stresses on cucumbers has been reported [12]. It was shown that Se increased photosynthetic traits, chlorophyll content, and yield of cucumbers and decreased stresses by increasing antioxidant levels and scavenging reactive oxygen species (ROS). It was observed that cucumber could better initiate flowering upon the application of heat stresses in the presence of Se [12]. Djanaguiraman et al. [6], studied the effects of nano-Se on reproductive growth of sorghum. All plants were foliar sprayed with sodium selenate (75 mg L−1) at 63 days after sowing, and high temperature stress (40/30 °C) was imposed from 65 days after sowing through maturity. Higher pollen germination and greater seed yield were seen in high temperature when nano-Se was applied.
Moreover, the application of heat stresses to pepper decreased its yield, seed production, and vegetative and reproductive growth [19]. Heat stresses increased proline, carbohydrates and chlorophyll degradation of pepper transplants [20, 21].
Considering previous studies, Se was supposed to have the ability of decreasing deleterious effects of heat stresses on pepper and controlling flower dropping [22, 23]. This research was done to study the probable effects of Se on growth, antioxidant, nutrition, and photosynthetic traits of pepper.
Materials and methods
This experiment was conducted in an environmentally controlled greenhouse with an average day/night temperature of 25/17 °C at the Department of Horticulture, Isfahan University of Technology (18°7′23″N latitude; 51°53′2″E longitude), Iran. The experimental was conducted as a control randomized design (CRD). Treatments included SeCl2 (4 (Se1), 6 (Se2) and 8 (Se3) mg L−1), applied in nutrient solution [17, 18]. The composition of nutrient solution (in mg L−1) was as follows: N = 116, P = 21, K = 82, Ca = 125, Mg = 21, S = 28, Fe = 6.8, Mn = 1.97, Zn = 0.25, B = 0.70, Cu = 0.07 and Mo = 0.05, provided by KH2PO4, NH4NO3, Fe EDTA, CuSO4, MnSO4, ZnSO4, Ca(NO3)2, Na2MO4 and H3BO3 [24, 25]. The seedlings of Caspicum annum L. Dutch Simins 301 were transplanted into a 5-L container filled with perlite. When the plants had 4–5 leaves, they were transplanted into hydroponic nutrient solution. On the first week of the experiment, seedlings were exposed to a half-nutrient solution concentration and then to full-nutrient solution and irrigated; on the 7th day after transplanting, treatments were applied to growth containers. Plants grew at sub-optimal temperature, i.e. 35 ± 2 °C, for 4 h a day (H) and then at optimum temperature, i.e. 25 ± 2 °C, in vegetation chambers (EYELA LTI-1000 SD) under a 14-h photoperiod with a photosynthetic photon-flux density of 270 µmol m−2 s−1, at 75% relative humidity. A number of plants, as the control group, were keep at 25 ± 2 °C in all growth periods. Plants were grown under this condition for 3 months more [26].
Parameter was measured
Chlorophyll content was measured using a chlorophyll meter (SPAD-502, Minolta Corp., Ramsey, NJ, USA) [27]. Photosynthetic parameters were measured on fully-expanded leaves of second youngest nodes by means of an infrared gas analyzer NE, (USA), between 11:00 h and 13:00 h, at light saturation intensity.
In order to determine the total phenol content, samples were mixed with 4- and 5-mL Folin–Ciocalteu and aqueous Na2CO3 separately. The phenols were determined by a spectrophotometer at 765 nm as gallic acid equivalents per gram (mg GAE g−1 DW) [28].
Antioxidant activity of pepper leaves was determined according to Yu et al. [29]. 3 mg of the sample was dissolved into 5 mL of methanol stock; 1.4 mL of this solution was blended with 0.6 mL of DPPH solution. After 30 min, the absorbance of the solution was recorded at 515 nm by the spectrophotometer (V-530, JASCO, Japan) against methanol as a blank. 0.2 mM of DPPH solution in methanol was used as a stock of DPPH for the determination of free radical scavenging activity of samples. The antiradical activity was calculated by the following equation:
where Asample and Acontrol were the absorbance of the sample and control.
SOD determination was done using 50 mM of potassium phosphate buffer (pH 6.1) (the same buffer was used for CAT and MDA), 1% guaiacol (w/v), 0.4% H2O2 (v/v) and 1 mL of enzyme extract, all of which were mixed. The enzyme activity was calculated at 470 nm as the µM of guaiacol oxidized min−1 (g fresh weight)−1 at 25 ± 2 °C [30,31,32].
CAT activity was measured according to Samantary [33] and Haghighi et al. [34] by monitoring the reaction mixture containing 50 mM of phosphate buffer (pH 7.0), 10 mM of H2O2 and enzyme aliquot. The decomposition of H2O2 was followed at 240 nm.
MDA was assayed using a modified version of the method described by Dhindsa et al. [35] and Haghighi et al. [36]. The mixture of extracted supernatant and thiobarbituric acid (0.6%) was centrifuged and the absorbance of the supernatant was measured at 532, 600, and 450 nm. The MDA concentration was calculated according to the following formula [37]:
Total nitrogen in the leaf samples was determined based on the method proposed by Kjeldahl [37]. The concentration of nutrients (K, S and Se) was measured in leaves, shoots and flowers with X-ray fluorescence (XRF) (Unisantis XMF104, Germany). The concentration of phosphorus was estimated based on the method of vanadomolybdo phosphoric acid colorimetric at 460 nm [38, 39].
The number of open and abscised flowers was counted at the end of the experiment. The shoot length was measured with a ruler. Plants were harvested and washed using tap water. Shoots were excised from the roots using a steel blade, and then oven-dried at 70 °C for 2 days to constant weight. The fresh weight and dry weight of shoots, roots, and fruits were measured.
Data were analyzed using Statistix 8 (Tallahassee FL, USA). All data were analyzed using one-way ANOVA and the means were compared for verifying their significance by the least significant difference (LSD) test at P < 0.05.
Results and discussion
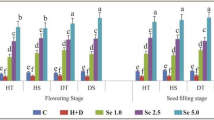
All growth parameters, including fresh and dry weight of shoots and roots, flowers and fruit number, decreased at high temperature compared with the control. Flower Dropping was the highest upon heat stresses; Se application generally decreased flower dropping at all levels, even lower than the control (Table 1). Fresh weight of shoots increased in H × Se1 and decreased in Se2 and Se3 with the application of heat stresses. There were no significant differences between Se concentrations in dry weight of shoots, fresh and dry weight of roots and flowers and fruit number upon stresses. Fresh and dry weight of fruit and shoot length increased with the application of Se and reached the highest in Se3 under stresses (Table 1).
According to our results, Se1 decreased deleterious effects of heat stresses on pepper in terms of vegetative traits, such as dry weight of shoots, and fresh and dry weight of roots. On the other hand, reproductive growth, such as fresh weight and dry weight of fruit, flowers and fruit number, was the highest in both Se1 and Se3 (Table 1). Results of this research showed that vegetative growth (fresh and dry weight of shoots and roots) increased with the application of Se upon heat stresses and even outperformed the control. Finally, it seems that, on one hand, Se was more effective in vegetative growth at low concentrations upon heat stresses, on the other hand, its high concentrations were more effective in fruit growth. Selenium in both high and low concentrations was effective in both flower and fruit induction, indicating higher number of fruits and flowers. Hence, it is recommended to investigate the application of various concentrations of selenium in vegetative and reproductive stages in future studies. A decrease in the growth of cucumbers [12] and tomatoes [25] was reported upon the application of heat stresses.
All photosynthetic traits like chlorophyll index, photosynthesis, stomata conductance, transpiration, and internal CO2 of stomata decreased with increasing temperature. Chlorophyll index, photosynthesis and internal CO2 of stomata increased by Se in high temperature although there was not any significant difference between various levels of Se application. Transpiration was the highest in the control and the lowest in H × Se2. Internal CO2 of stomata increased in H × Se3 (Table 2). The effect of Se on expression of genes implied in antioxidant activity and defense responses [15, 16]. The protective effect of Se against inappropriate senescence could be due to its reported ability to reduce respiratory intensity and ethylene production in different plant species [16]. The inhibitor of ethylene production Co2+ prevents cell death and cytoplasmic shrinkage. Feng et al. [17] reported that, the restoration of photosynthesis in stressed plants after Se application may be closely related to the decreased ROS levels, reactivation of antioxidants, restored structure of the damaged chloroplasts and enhanced production of other vital metabolites (such as GSH and SH-like substances) [18].
The effects of Se on photosynthesis traits at high temperature were greater than other parameters. Selenium increased photosynthesis by 22% in comparison with heat stresses without Se application. Increasing photosynthesis may be because of the effect of Se on the enzyme activity of photosynthesis, rather than stomata apparatus. A decrease observed in chlorophyll content, photosynthesis, stomatal conductance, and transpiration rate was the same as what was reported by Balal et al. [12] concerning cucumbers under heat stresses.
Se did not affect stomatal conductance and intercellular CO2 concentrations significantly. Se promoted the net photosynthetic rate in tomato leaves under non-saline conditions [40]. The same results were observed in pepper under heat stresses.
Leaf phenol and antioxidant activity did not change between treatments. Fruit antioxidant and phenol increased with H × Se in all Se levels, specially Se1 (Table 3).
Nutraceutical values of pepper increased with Se in terms of phenol and antioxidant capacity at high temperature.
MDA content increased upon heat stresses and decreased with Se application. POD activity decreased upon heat stresses and increased in Se1 and Se2. SOD activity increased with Se at all levels. Protein content increased in Se2 and Se3 (Table 4).
Heat stresses, as shown by different researchers, destroy plasma membrane and increase. MDA content; Se can prevent the destruction of plasma membrane effectively [41]. Concerning SOD and POD, SOD was more affected by Se as it increased almost twice (51.49%) compared with POD (15.83%). Enzymatic antioxidant (SOD and POD) was affected by lower concentrations of Se, while proteins of pepper leaves were influenced by the high Se concentrations.
An increase in several enzyme antioxidants was reported in wheat [42], sorghum [6], lettuce [43], and ryegrass [44] upon the application of Se. Reduction of MDA was found in ryegrass with Se application [44]. Our results confirmed [12] those revealed about cucumbers where Se improved photosynthesis, growth, and productivity by increasing the antioxidant upon heat stresses. With increasing antioxidant capacity and SOD and POD activity, photosynthesis and growth of pepper also increased.
N and K concentrations decreased upon heat stresses and were not affected by Se levels in leaves. N concentrations in fruits and N and K uptake did not significantly change in roots.
P concentrations decreased with Se application in all parts of pepper. P was greatly reduced in leaves, fruits, and roots upon the application of Se, respectively.
The highest amount of Se was observed in leaves and fruits of Se2 and Se3 in and in roots of Se3. In whole plants, the highest contribution of Se belonged to leaves (mean 53.92% of the total), fruits (mean 25.37% of the total), and roots (mean 20.69% of the total), respectively at all Se levels.
S concentrations decreased when Se was applied, especially at Se2 and Se3, at all leaves of S uptake and decreased in the same way in leaves, fruits, and roots (Table 5). S decreased upon the application of Se.
The amounts of N, P, K and S were almost the same as those reported by Fontes et al. [45]. According to their results, the differences observed in nutrient concentrations could be due to the differences seen in the cultivars, nutrient solution and cultivation systems and substrates. It should be highlighted that the focus of this study was not on the amount of elements, rather on changes occurred under the application of Se and upon heat stresses.
P concentrations decreased with Se application in leaves. A decrease seen in P was almost in leaves, fruits and roots respectively. In the same way, S absorption decreased in leaves, fruits, and roots. Se translocation to shoot tissues depended on the rate of xylem loading, affected by transpiration [46].
Moreover, our results did not support this finding because Se was higher in leaves when decreasing transpiration rate. Our results mostly confirmed active transporting instead of passive one via transpiration [47]. It maybe the role of transporters presented in root cell membrane, such as phosphate transporters [48]; sulfate transporters and channels were more effective in pepper [49, 50].
Besides that, our results concerning P and S uptake confirmed that the uptake of P decreased in pepper in the presence of Se, which might be due to the selectivity nature of these transporters for Se uptake instead of P and S when excessive Se was applied [43, 51].
Conclusion
Selenium application increased the nutritional values of pepper with increasing antioxidant content and Se concentrations in pepper fruits. On the other hand, Se reduced deleterious effects of heat stresses in pepper. There were no significant differences between Se concentrations used in this experiment although Se1 and Se2 performed better in a number of parameters. The effects of Se on the antioxidant enzyme and growth, especially reproductive growth and flower number, were more effective. It seems that the effects of Se on photosynthesis traits and phenol content was less than other parameter was measured. The positive effects of Se on S and P absorption were observed, especially in leaves. Se did not affect the absorption of other elements. It is recommended to apply Se at high temperature for preventing flower dropping though further studies are needed to be done on Se at various soil substrates in different growth stages.
Abbreviations
- POD:
-
Peroxidase
- SOD:
-
Superoxide dismutase
- MDA:
-
Malondialdehyde
- ROS:
-
Reactive oxygen species
- CAT:
-
Catalase
- Se:
-
Selenium
- XRF:
-
X-ray fluorescence
References
Hartikainen H (2005) Biogeochemistry of selenium and its impact on food chain quality and human health. J Trace Elem Med Biol 18:309–331
Pilon-Smits EA, Quinn CF, Tapken W, Malagoli M, Schiavon M (2009) Physiological functions of beneficial elements. Curr Opin Plant Biol 12(3):267–274
Sors TG, Ellis DR, Na GN, Lahner B, Lee S, Leustek T, Pickering IJ, Salt DE (2005) Analysis of sulfurand selenium assimilation in Astragalus plants with varying capacities to accumulate selenium. Plant J 42:785–797
Bodnar M, Konieczka P, Namiesnik J (2012) The properties, functions, and use of selenium compounds in living organisms. J Environ Sci Health Part C 30:225–252
Wu Z, Banuelos GS, Lin ZQ, Liu Y, Yuan L, Yin X, Li M (2015) Biofortification and phytoremediation of selenium in China. Front Plant Sci 6:136
Djanaguiraman M, Prasad PV, Seppanen M (2010) Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol Biochem 48:999–1007
Wang YD, Wang X, Wong YS (2012) Proteomics analysis reveals multiple regulatory mechanisms in response to selenium in rice. J Proteomics 75:1849–1866
Whanger PD (2004) Selenium and its relationship to cancer: an update. Br J Nutr 91:11–28
Zayed A, Lytle CM, Terry N (1998) Accumulation and volatilization of different chemical species of selenium by plants. Planta 206:284–292
Germ M, Kreft I, Osvald J (2005) Influence of UV-B exclusion and selenium treatment on photochemical efficiency of photosystem II, yield and respiratory potential in pumpkins (Cucurbita pepo L.). Plant physiol Biochem 43(5):445–448
Turakainen M, Hartikainen H, Ekholm P, Seppänen MM (2006) Distribution of selenium in different biochemical fractions and raw darkening degree of potato (Solanum tuberosum L.) tuber supplemented with selenate. J Agric Food Chem 54:8617–8622
Balal RM, Shahid MA, Javaid MM, Iqbal Z, Anjum MA, Garcia-Sanchez F, Mattson NS (2016) The role of selenium in amelioration of heat-induced oxidative damage in cucumber under high temperature stress. Acta Physiol Plant 38(6):158
Yao X, Chu J, Wang G (2009) Effects of selenium on wheat seedlings under drought stress. Biol Trace Elem Res 130:283–290
Xue TL, Hartikainen H, Piironen V (2001) Antioxidative and growth-promoting effect of selenium on senescing lettuce. Plant Soil 237:55–61
Gupta M, Gupta S (2017) An overview of selenium uptake, metabolism, and toxicity in plants. Front Plant Sci 7:2074–2085
Puccinelli M, Malorgio F, Pezzarossa B (2017) Selenium enrichment of horticultural crops. Molecules 22:933
Feng R, Wei C, Tu S (2013) The roles of selenium in protecting plants against abiotic stresses. Environ Exp Bot 87:58–68
Pennanen A, Tailin XUE, Hartikainen H (2002) Protective role of selenium in plant subjected to severe UV irradiation stress. J Appl Bot 76:66–76
Thuy TL, Kenji M (2015) Effect of high temperature on fruit productivity and seed-set of sweet pepper (Capsicum annuum L.) in the field condition. J Agric Sci Technol 515:516–521
Javanmardi J, Rahemi M, Nasirzadeh M (2014) Responses of tomato and pepper transplants to high-temperature conditioning. Int J Veg Sci 20(4):374–391
Haghighi M, Heidarian S, Teixeira da Silva Jaime A (2012) The effect of titanium amendment in N-withholding nutrient solution on physiological and photosynthesis attributes and micronutrient uptake of tomato. Biol Trace Elem Res 150:381–390
Haghighi M, Sheibanirad A, Pessarakli M (2016) Effects of selenium as a beneficial element on growth and photosynthetic attributes of greenhouse cucumber. J Plant Nutr 39(10):1493–1498
Yang H, Wu F, Cheng J (2011) Reduced chilling injury in cucumber by nitric oxide and the antioxidant response. Food chem 127(3):1237–1242
Ghasemnezhad M, Sherafati M, Payvast GA (2011) Variation in phenolic compounds, ascorbic acid and antioxidant activity of five coloured bell pepper (Capsicum annum L.) fruits at two different harvest times. J Funct foods 3(1):44–49
Marin A, Rubio JS, Martinez V, Gil MI (2009) Antioxidant compounds in green and red peppers as affected by irrigation frequency, salinity and nutrient solution composition. J Sci Food Agric 89(8):1352–1359
Haghighi M, Abolghasemi R, Teixeira da Silva JA (2014) Low and high temperature stress affect the growth characteristics of tomato in hydroponic culture with Se and nano-se amendment. Sci Hortic 178:231–240
Siwek P, Libik A, Zawiska I (2012) The effect of biodegradable nonwovens in butter head lettuce cultivation for early harvest. Folia Hort 24(2):161–166
Kahkonen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS (1999) Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem 47(10):3954–3962
Yu L, Haley S, Perret J, Harris M, Wison J, Qian M (2002) Free radical scavenging properties of wheat extracts. J Agric Food Chem 50:1619–1624
Agarwal S (2007) Increased antioxidant activity in Cassia seedlings under UV-B radiation. Biol Plant 51(1):157–160
Demiral T, Turkan I (2005) Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ Exp Bot 53(3):247–257
Haghighi M, Nikhbakht A, Ping Xia Y, Pessarakli M (2014) Influence of humic acid in diluted nutrient solution on growth nutrient efficiency and postharvest attributes of gerbera. Commun Soil Sci Plant Anal 45(2):177–188
Samantary S (2002) Biochemical responses of Cr-tolerant and Cr-sensitive mung bean cultivars grown on varying levels of chromium. Chemosphere 47:1065–1072
Haghighi M, Kafi M, Pessarakli M, Sheibanirad A, Sharifinia MR (2016) Using kale (Brassica oleracea var. acephala) as a phyto remediation plant species for lead (pb) and cadmium (cd) removal in saline soils. J Plant Nutr 10(39):1460–1471
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Haghighi M, Fang P, Pessarakli M (2015) Effects of ammonium nitrate and monosodium glutamate in waste water on the growth, antioxidant activity, and nitrogen assimilation of lettuce (Lactuca sativa L.). J Plant Nutr 38:2217–2229
Estan MT, Martinez-Rodriguez MM, Perez-Alfocea F, Flowers TJ, Bolarin MC (2004) Grafting raises the salt tolerance of tomato through limiting the transport of sodium and chloride to the shoot. J Exp Bot 56(412):703–712
Mittal V, Singh O, Nayyar H, Kaur J, Tewari R (2008) Stimulatory effect of phosphate-solubilizing fungal strains (Aspergillus awamori and Penicillium citrinum) on the yield of chickpea (Cicer arietinum L. cv. GPF2). Soil Biol Biochem 40(3):718–727
Govindaraj M, Selvi B, Rajarathinam S, Sumathi P (2011) Genetic variability and heritability of grain yield components and grain mineral concentration in India’s pearl millet (Pennisetum glaucum (L) R. Br.) accessions. Afr J Food Agric Nutr Dev 11(3):4758e4771
Diao M, Ma L, Wang J, Cui J, Fu A, Liu HY (2014) Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system. J Plant Growth Regul 33(3):671–682
Savicka M, Skute N (2010) Effects of high temperature on malondialdehyde content, superoxide production and growth changes in wheat seedlings (Triticum aestivum L.). Ekologija 56(1):26–33
Nowak J, kaklewski K, Ligocki M (2004) Influence of selenium on oxidoreductive enzymes activity in soil and in plants. Soil Biol Biochem 36:1553–1558
Rios JJ, Rosales MA, Blasco B, Cervilla LM, Romero L, Ruiz JM (2008) Biofortification of Se and induction of the antioxidant capacity in lettuce plants. Sci Hortic 116:248–255
Cartes P, Gianfreda L, Mora ML (2005) Uptake of selenium and its antioxidant activity in ryegrass when applied as selenate and selenite forms. Plant Soil 276:359–367
Fontes PCR, Silva DJH (2005) Cultura do tomate. In: Fontes PCR (ed) Olericultura teoria e pratica. Suprema, Vicosa, pp 457–476
Renkema H, Koopmans A, Kersbergen L, Kikkert J, Hale B, Berkelaar E (2012) The effect of transpiration on selenium uptake and mobility in durum wheat and spring canola. Plant Soil 354:239–250
Li HF, McGrath SP, Zhao FJ (2008) Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol 178:92–102
Kikkert J, Berkelaar E (2013) Plant uptake and translocation of in organic and organic forms of selenium. Arch Environ ContamToxicol 65:458–465
Feist LJ, Parker DR (2001) Ecotypic variation in selenium accumulation among populations of Stanleya pinnata. New Phytol 149:61–69
Zhang Y, Pan G, Chen J, Hu Q (2003) Uptake and transport of selenite and selenate by soybean seedlings of two genotypes. Plant Soil 253:437–443
White PJ, Bowen HC, Parmaguru P, Fritz M, Spracklen WP (2004) Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J Exp Bot 55:1927–1937
Acknowledgements
Each named author has substantially contributed to conducting the underlying research and drafting this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Additionally, to the best of our knowledge, the named authors have no conflict of interest, financial or otherwise.
Rights and permissions
About this article
Cite this article
Haghighi, M., Ramezani, M.R. & Rajaii, N. Improving oxidative damage, photosynthesis traits, growth and flower dropping of pepper under high temperature stress by selenium. Mol Biol Rep 46, 497–503 (2019). https://doi.org/10.1007/s11033-018-4502-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-018-4502-3