Abstract
Selenium (Se) plays a role in human health: It is an essential trace element but can be toxic if too much is consumed. The aim of this study was to determine which species of Se are most rapidly taken up and translocated to above-ground plant tissues. Specifically, we wished to determine if organic forms of Se in an exposure solution can contribute to the amount of Se found in shoot tissue. Durum wheat (Triticum turgidum) and spring canola (Brassica napus) were grown hydroponically, and young seedlings were exposed to 0.5 or 5.0 μM Se as selenate, selenite, seleno-methionione, or seleno-cystine for ≤300 min. Canola accumulated more Se than wheat, although the difference depended on Se speciation of the exposure solution. Organic forms of Se were taken up at a greater rate than inorganic forms. When exposed to 5.0 μM Se, the rate of uptake of selenite was 1.5- (canola) or 5-fold (wheat) greater than the rate of uptake of selenate, whereas seleno-methionine was taken up 40- (canola) or 100-fold (wheat) faster and seleno-cystine 2- (wheat) to 20-fold (canola) faster. Plants exposed to seleno-methionine had the highest shoot concentrations of Se even though selenate was more mobile once taken up; in plants exposed to selenate >50 % of accumulated Se was translocated to shoot tissue. Because organic forms of Se (especially seleno-methionine) can be readily taken up and translocated to above-ground tissues of wheat and canola, these Se species should be considered when attempting to predict Se accumulation in above-ground plant tissues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Selenium (Se), a metalloid with similarities to sulphur (S), is widely distributed in the environment. In soils, Se is present naturally as a result of soil-weathering processes. Anthropogenic activities, such as mining and refining of sulphide ores and refining and burning of fossil fuels (especially coal, which contains 1–5 mg kg−1 Se), result in release of Se to the atmosphere and its subsequent deposition to soils (Haygarth 1994; Barceloux 1999; Sors et al. 2005).
Although Se is not considered essential for plants, it can be readily accumulated from soil depending on the concentration and speciation of Se in soil. Evidence suggests that Se offers protection to some plants against certain biotic or abiotic stressors leading some to label it a beneficial element (Hanson et al. 2004). Se is essential for mammals; along with vitamin E, it plays a role in protecting membranes from damage caused by oxidative stress (Barceloux 1999; Birringer et al. 2002). In livestock, white muscle disease has been linked to Se deficiency, and in humans, Keshan’s disease (a congestive cardiomyopathy) can result from a low Se diet (Fordyce 2005; Navarro-Alarcon and Cabrera-Vique 2008).
For mammals, diet is a major source of Se, and consumption varies among individuals because of differences in the amount and speciation of Se in soils and in the ability of different plant species to accumulate Se. Se has a fairly narrow range between being deficient or toxic to humans, and for optimal health, Se should be consumed in doses that are neither too low (<40 to 55 μg day−1) nor too high (>400 μg day−1) (Gissel-Nielsen et al. 1984; Navarro-Alarcon and Cabrera-Vique 2008).
A number of factors influence accumulation of Se by plants, including the concentration and speciation of Se in soil and soil properties, such as pH, redox potential, and the concentration of competing ions in soil (Terry et al. 2000; Zhao et al. 2005). In addition, important are plant factors (such as the activity of membrane transporters or mechanisms responsible for translocation to shoots) that affect the rate of uptake or translocation of the element within the plant as well as external environmental factors (such as humidity) that influence rates of transpiration, which may in turn effect the rate translocation of the element (Terry et al. 2000; Renkema et al. 2012).
Selenate (SeO 2-4 ), thought to be the most prevalent form of bioavailable Se in agricultural soils (Terry et al. 2000; Missana et al. 2009; Sors et al. 2005), is more water soluble and bioavailable than selenite (SeO 2-3 ) (Oliver 1997; Barceloux 1999; Gawalko et al. 2002; Wu 2004). In one study, soil was spiked with 75Se-labelled selenite, and when Se speciation was measured 2 days later, close to 80 % of the Se in soil solution was found to be either selenate or selenite, with selenate being most prevalent (van Dorst and Peterson 1984). Likewise, when sampling surface waters of the Canadian Prairies, Hu et al. (2009) found that 86 % of dissolved Se was selenate. Less is known about organic forms of Se, although organic forms of Se, including seleno-glutathione and seleno-methionine, have been detected in soils (van Dorst and Peterson 1984; Abrams et al. 1990). Organic forms can make up a substantial fraction of soil Se. In seven California soils located on the west side of the San Joaquin Valley, organic forms of Se made up approximately 50 % of the Se extracted with an alkaline pyrophosphate solution (0.1 M NaOH and 0.1 M Na4P2O7) (Abrams et al. 1990).
Because of the chemical similarity of Se and S, the biochemistry of these two elements are similar. Selenate is transported across the root cell membrane through sulphate permeases and channels (Feist and Parker 2001; Zhang et al. 2003), whereas selenite is transported by way of phosphate transport mechanisms (Li et al. 2008) and other ion channels as a result of root metabolism (Arvy 1993; Zhang et al. 2003). Selenate and sulphate have been shown to compete for plant uptake (Arvy 1993; Bailey et al. 1995; Kopsell and Randle 1997; Mikkelsen and Wan 1990; Renkema et al. 2012). Once taken up, Se can then compete with S for incorporation into S-containing proteins, thus resulting in toxicity (Oliver 1997; Terry et al. 2000; Ellis and Salt 2003; Findley 2005; Ríos et al. 2008; Huang et al. 2008). Likewise, selenite and phosphate compete for plant uptake as well (Hopper and Parker 1999; Li et al. 2008). Wheat seedlings actively take up seleno-methionine from solution; the addition of 2,4-dinitrophenol (a metabolic inhibitor) to a solution containing seleno-methionine was found to decrease the uptake of seleno-methionine (Abrams et al. 1990).
Although it is important to study Se uptake by plants, it is also important to consider translocation within plants because it is often the above-ground parts of plants that are consumed by humans or by livestock. Like uptake, translocation of Se is influenced by Se speciation. Several studies have shown that once accumulated, selenate is more mobile within plant tissue than selenite (Renkema et al. 2012; Li et al. 2008; Zayed et al. 1998). Although there have been a number of studies measuring uptake and translocation of inorganic forms of Se, there have been relatively few that considered organic forms of Se, especially seleno-cystine, and these studies do not often directly compare both uptake and translocation of organic and inorganic forms of Se.
The overall aim of this study was to determine the influence of Se speciation on whole plant uptake and on root-to-shoot translocation of Se. More specifically, our objectives were to compare uptake and translocation of organic forms of Se (seleno-methionine and seleno-cystine) with that of inorganic forms of Se (selenate and selenite). The null hypothesis were as follows: (1) uptake of Se by wheat and canola are not influenced by Se speciation; and (2) translocation of Se to shoots of wheat and canola are not influenced by Se speciation.
Materials and Methods
Plant Culture and Se Exposure
Caryopses of durum wheat (Triticum turgidum L. cv ‘Kyle’) and seeds of spring canola (Brassica napus L. var ‘Hyola 401’) were germinated on moist filter paper in Petri dishes. These are important agronomically important species with different patterns of Se accumulation. After 2–3 days, seven germinated seeds were transferred to 500-mL high-density polyethylene (HDPE) containers wrapped in tinfoil and covered with nylon mesh in contact with modified ¾-strength Hoagland’s nutrient solution (Fe3+ was supplied as 26.8 μM Fe-HEDTA, and the MnCl2 concentration was decreased by half) (Hoagland and Arnon 1950). Unless otherwise noted, all chemicals were obtained from the Fisher Scientific Company (Fisher Scientific Company, Ottawa, ON). The pH of the solution was maintained at 6.0 ± 0.2 with the addition of dilute HNO3 or KOH as required. Water lost through transpiration was replaced as needed. Once established, plants were thinned to five plants per pot for wheat and four plants per pot for canola. Plants were grown and exposed to Se on a laboratory bench under fluorescent growth lights. Growing conditions were temperature 22–23 °C with a 16:8-h light-to-dark cycle and 250 μmol m−2 s−1 photosynthetically active radiation.
Twelve days after seeding, plants were transferred to acid-washed 500-mL HDPE containers and exposed to solutions containing 3.0 mM Ca(NO3)2, 1.5 mM MgSO4, and 4.0 mM KNO3 at a pH 6.0. In addition, these exposure solutions contained 0.50 or 5.0 μM of one of four Se species: selenate (as K2SeO4), selenite (as Na2SeO3), seleno-methionine, or seleno-cystine (Fig. 1) (Sigma-Aldrich Canada Co., Oakville, ON). At pH 6.0, most selenite was expected to be protonated to biselenite (HSeO -3 ), although the more common term “selenite” will be used throughout this article. Exposure solutions were sampled before exposure and Se measured to verify that Se concentrations were close to their nominal values: Actual concentrations were 0.49 or 4.60 μM (selenate), 0.42 or 4.17 μM (selenite), 0.47 or 4.76 μM (seleno-methionine), and 0.49 or 4.45 μM (seleno-cystine). Plants were harvested after 0, 50, 100, 150, or 300 min of exposure to each Se species and concentration. At each harvest, plants were removed from solution, and the roots were rinsed with type I (18.2 MΩ cm) water. The roots and shoots were separated, placed in paper envelopes, and dried at 45 °C for 48 h.
Se Analysis of Exposure Solutions and Tissue Digests
Exposure solutions were sampled before exposure and kept refrigerated at 4 °C in acid-washed HDPE vials until analysis. After drying, plant tissues were weighed and placed in acid-washed Teflon digestion vessels with 1.5 mL trace metal-grade HNO3 (Topper and Kotuby-Amacher 1990). Open digestion at room temperature for 5 h was followed by closed digestion overnight at 110 °C. After digestion, samples were diluted to 5 mL with type I water, placed in acid-washed HDPE vials, and kept refrigerated at 4 °C until analysis.
Selenium concentrations in the exposure solutions and plant digests were measured using graphite furnace atomic absorption spectroscopy (model SpectrAA-220 atomic absorption spectrometer with a GTA-110 graphite tube atomizer attachment; Varian, Australia) calibrated with a 1000 ± 3 μg mL−1 Se solution (High Purity Standards, Charleston, SC) diluted to 50 μg L−1 and matrix-matched for acidity. Quality control was ensured with inductively coupled plasma mass spectrometry analytical mixture 3 (containing aluminum [Al], arsenic, beryllium, cadmium, chromium, cobalt, copper, iron [Fe], lead, manganese, mercury, nickel, Se, vanadium and zinc; High Purity Standards) diluted to 50 μg L−1 Se and analysed along with experimental samples. Duplicate samples of spinach leaves (National Institute of Standards and Technology [NIST] Standard Reference Material No. 1570a, United States Department of Commerce, NIST, Gaithersberg, MD) were digested with each run. One of each pair of standards was spiked with a known amount of Se; recovery of Se from the spiked NIST sample was typically 85–90 %.
Data Analysis
The experiment was a complete factorial design with a total of 80 experimental units; there were two plant species (wheat and canola), four different Se species (selenate, selenite, seleno-methionine, and seleno-cystine), two nominal Se concentrations (0.5 and 5.0 μM), and five durations of exposure (0, 50, 100, 150, and 300 min). Variation in the total amount of Se taken up by roots (μg whole plant Se g−1 root dry weight [dw]) or shoot tissue (μg shoot Se g−1 shoot dw) or the translocation factor (TF [μg shoot Se μg−1 root Se]) was attributed to the appropriate factors (plant species, Se species, nominal Se dose, and duration of exposure) using SAS PROC GLM (SAS, Cary, NC, USA). Dependent variables were log-transformed to achieve normality. In each case, a full model containing main effects and all possible two- and three-way interactions was tested, and nonsignificant (p > 0.05) two and three-way interactions were dropped from the model, one at a time, so that the final model contained only main effects and significant interactions (p < 0.05).
Results
Uptake of Se
For each combination of species, nominal Se dose, and Se species, whole plant uptake of Se (μg whole plant Se g−1 root dw) was plotted against the duration of exposure (Fig. 2), and rates of Se accumulation (μg Se g−1 root dw min−1) were determined from the slopes of these plots (Table 1).
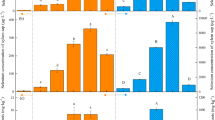
Whole plant uptake of Se increased with exposure to greater concentrations of Se in the exposure solution (p = 0.0073) and longer durations of exposure (p < 0.0001) (Fig. 2; Table 1). Wheat and canola differed in Se uptake, but the magnitude of the difference depended on the Se species to which plants were exposed (p = 0.0008) (Fig. 2; Table 1). On average, canola took up Se at a rate that was approximately three times faster than by wheat. The greatest difference resulted from exposure to selenate: Se uptake by canola was 15-fold faster than by wheat when exposed to 5.0 μM Se as selenate. Exposure to seleno-cystine resulted in the smallest difference: Se uptake by canola was twice as fast compared with wheat (Table 1). Uptake of Se also depended on the form of Se to which plants were exposed (p < 0.0001) (Fig. 2; Table 1). Exposure to selenite resulted in a rate of uptake that was 1.5- to 5-fold faster than Se uptake by plants exposed to selenate. Exposure to the organic forms (especially seleno-methionine) resulted in much greater rates of Se uptake. Compared with selenate, rates of uptake of seleno-methionine were approximately 40-fold greater for canola and approximately 100-fold greater for wheat. Rates of uptake of seleno-cystine were approximately twofold (wheat) to 20-fold (canola) greater than rates of accumulation of selenate.
Mobility of Se Within Wheat and Canola
Differences in shoot Se content was observed after a time delay because Se first had to be accumulated by roots before it could be translocated to shoots. No difference in background shoot Se concentrations were seen until 100–150 min after exposure began, but an increase in shoot Se was especially evident after 300 min of exposure (Fig. 3; Table 2).
Plants had greater concentrations of Se in shoots when exposed to greater concentrations of Se (p < 0.0001) for longer durations of exposure (p = 0.0002). Canola shoots had greater concentrations of Se than wheat shoots (p < 0.0001): The difference was 2- to 3-fold when exposed to 5.0 μM selenite, selenate, or seleno-cystine but approximately 17-fold when exposed to seleno-methionine. The Se species to which plants were exposed was also significant (p = 0.0022), but its influence was plant species specific (p < 0.0001). Shoot tissue had the highest concentration of Se when plants were exposed to seleno-methionine (14- to 70-fold greater Se concentrations than in wheat or canola shoots exposed to selenate), followed by selenate and seleno-cystine. Exposure to selenite resulted in the lowest concentration of shoot Se.
The mobility of Se within plants was evaluated by considering the TF (Table 2). Se was most mobile in plants exposed to selenate: Plants generally had a TF >1, meaning that >50 % of the Se in these plants was found in shoots (Table 2). Se was also mobile in plants exposed to seleno-methionine, which resulted in TFs of 0.25–0.75 after 300 min of exposure. Selenite or seleno-cystine was least mobile within plants: In this case TF values were generally <0.1 after 300 min of exposure, meaning that <10 % of accumulated Se was found in shoot tissue.
Discussion
Uptake of Se
In this study, canola accumulated Se approximately three times faster than wheat. Differences in Se accumulation by different plant species have been reported elsewhere. Ximénez-Embún et al. (2004) observed that sunflower (Helianthus annuus) accumulated lower concentrations of Se than lupin (Lupinus albus) or mustard (Brassica juncea). In older seedlings, Renkema et al. (2012) reported that canola (B. napus) accumulated 2- to 10-fold as much Se as wheat (T. turgidum) when exposed to selenite or selenate, respectively. Because selenate and selenite are known to be accumulated by way of sulphate and phosphate transporters, respectively (Sors et al. 2005; Li et al. 2008), differences in the activity of these transporters in different plant species may account for differences in uptake of Se. Seleno-methionine is actively taken up (Abrams et al. 1990), and in algae, uptake of seleno-methionine has been shown to be inhibited by methionine (Sandholm et al. 1973), thus suggesting that amino acid transporters may be able to take up this organic form of Se. Differences in the activity of these amino acid transporters among plant species may account for differences in seleno-methionine uptake. Differences in root morphology may also play a role. Uptake of Cd2+ and Tl+ appear concentrated near root tips (Piñeros et al. 1998; Harskamp et al. 2010), so increased Se accumulation by canola compared with wheat may also be due to more root branching.
Se speciation had a significant impact on the rate of Se uptake. In this study, the rate of uptake of selenite was slightly greater than that of selenate. Li et al. (2008) observed similar rates of uptake for these inorganic species. In hydroponic studies, uptake of selenate has been shown to be sensitive to sulphate levels (Renkema et al. 2012; Zayed et al. 1998), and in this study, selenate uptake may have been relatively low due to the presence of 1.5 mM sulphate in the exposure solution, which was greater than the 1.0 mM reported by Zayed et al. (1998).
In 12-day-old seedlings, the rate of uptake of seleno-cystine, especially seleno-methionine, were much greater than the rate of uptake of the inorganic species, suggesting that transporters capable of accumulating seleno-methionine or seleno-cystine are more active than transporters capable of taking up selenate or selenite. Compared with inorganic forms of Se, less is known about uptake of organic forms of Se, although uptake of intact amino acids by plants has been shown (Sauheitl et al. 2009; Wallenda and Read 1999). Thus it is not surprising that seleno-amino acids can also be taken up.
Although both Se dose and speciation, as well as the level of sulphate in the exposure solution, influenced Se accumulation by Ruppia maritima, a rooted aquatic macrophyte, the highest Se bioconcentration factor of plants exposed to seleno-methionine was 22,000 compared with approximately 1000 for those plants exposed to inorganic forms of Se (Bailey et al. 1995). Selenite, seleno-methionine, and seleno-cystine were taken up by two-grooved milkvetch (Astragalus bisculatus), an Se accumulator, and western wheatgrass (Pascopyrum smithii), a nonaccumulator species (Williams and Mayland 1992). The Se concentration in tissues of milkvetch was greater than in those of wheatgrass for all three Se species tested. Similar to the results seen in this study, exposure to seleno-methionine resulted in greater Se accumulation than exposure to seleno-cystine, although it is difficult to make comparisons to selenite because different Se concentrations were employed. After exposure to 20 μM Se for 1 week, (Zayed et al. 1998) showed that wheat exposed to selenate took up the most Se, followed by wheat exposed to seleno-methionine, whereas exposure to selenite resulted in the least Se accumulation.
Mobility of Se Within Wheat and Canola
In this study, the Se concentration in canola shoots was 3–17 times greater than in wheat shoots. Renkema et al. (2012) reported similar differences in shoot concentrations of 28-day-old wheat and canola seedlings exposed to selenite or selenate. Se speciation had a large impact on the amount of Se accumulated by shoot tissue. The observed differences in shoot Se were due in part to differences in rates of root uptake of Se because greater concentrations of Se in the root provide a larger pool of Se that has the potential to be translocated to the above-ground tissue; however, efficiently Se is translocated once it has been taken up by roots. Differences in shoot Se were also due to differences in the relative mobility of different Se species within the plant as evidenced by the differences in the TF values of plants exposed to different Se species for 150 or 300 min. Of the four Se species considered in this experiment, seleno-methionine, is the Se species most easily taken up and accumulated by shoot tissue followed by selenate. Although not the most mobile when comparing TF values, the rapid uptake of seleno-methionine by root tissue resulted in a substantial pool available to be moved to shoots. Yet, of all Se species, selenate was the most mobile within plants: Once it is taken up by roots, a large proportion is quickly translocated to shoots.
Others have reported that selenate is more mobile than selenite within plants (Li et al. 2008; Renkema et al. 2012; Zayed et al. 1998). The ability of an ion or molecule to be translocated to shoot tissue depends on the rate of xylem loading and, in some cases, the rate of transpiration (Renkema et al. 2012). Alternatively, selenite, more than other Se species, has been found to remain within root tissue (de Souza et al. 1998, 2000; Terry et al. 2000).
In our study, shoot concentrations of Se exposed to seleno-methionine (compared with selenate) were >10 times greater in wheat and 70 times greater in canola. In contrast, Zayed et al. (1998), who also measured shoot tissue concentration of Se in different plants exposed to seleno-methionine, found that compared with selenate, shoot Se concentrations of Se exposed to seleno-methionine were similar in mustard, lower in broccoli and rice, and greater in sugar beet. Similar to our results, Zayed et al. (1998) found that shoot-to-root Se ratios were greater in plants exposed to selenate than to selenite or to seleno-methionine. Zayed et al. (1998) grew and exposed plants in half-strength Hoagland’s solution (which would contain 1.0 mM sulphate), whereas in this study, the exposure solution contained 1.5 mM sulphate; the greater sulphate levels would be expected to result in comparatively lower rates of selenate uptake. Amino acids can be translocated to shoot tissue: 0.25–1 μM cysteine and as high as 3.6 μM methionine were found in the xylem sap of Picea abies (Köstner et al. 1998), so it is not surprising that seleno-methionine or seleno-cystine could be similarly transported.
Conclusion
Twelve-day old wheat and canola seedlings can readily take up and translocate organic forms of Se, including seleno-methionine and seleno-cystine, from hydroponic solution. Compared with selenate, seleno-methionine was taken up quickly (≤100-fold faster than selenate). In soils, Se species that are dissolved in soil solution can be taken up by roots, but they can also adsorb to binding sites within soil. Hydroponic experiments can help determine the nature of root uptake from solution, but they can not evaluate relationships between soil solution and soil-binding sites. In soils, selenite is known to be less mobile and less bioavailable than selenate (McNeal and Balistrieri 1989; Wang and Chen 2003), but bioavailability is dependent on soil properties, such as clay, organic matter, and Fe and Al oxide content (Johnsson 1991; Neal 1990; Nakamaru et al. 2005). Less is known about the fate of organic forms of Se in soil as well as to what extent they are found in soil solution. Our results suggest, however, that once in soil solution, concentrations of seleno-methionine as low as 1 % that of selenate may be important given how readily it is taken up and transported to above-ground tissues. If seleno-methionine or other organic forms of Se are present in soil from decaying organic matter, they may be an important pool of biavailable Se and should be measured in agricultural soils and included in models to predict accumulation of Se by crop species.
Abbreviations
- HDPE:
-
High density polyethylene
- TF:
-
Translocation factor
References
Abrams MM, Shennan C, Zasoski RJ, Burau RG (1990) Selenomethionine uptake by wheat seedlings. Agron J 82:1127–1130
Arvy MP (1993) Selenate and selenite uptake and translocation in bean plants (Phaseolus vulgaris). J Exp Bot 44:1083–1087
Bailey FC, Knight AW, Ogle RS, Klaine SJ (1995) Effect of sulfate level on selenium uptake by Ruppia maritima. Chemosphere 30:579–591
Barceloux DG (1999) Selenium. Clin Toxicol 37:145–172
Birringer M, Pilawa S, Flohe L (2002) Trends in selenium biochemistry. Nat Prod Rep 19:693–718
de Souza MP, Pilon-Smits EAH, Lytle CM, Hwang S, Tai J, Honma TSU et al (1998) Rate-limiting steps in selenium assimilation and volatilization by Indian mustard. Plant Physiol 117:1487–1494
Ellis DR, Salt DE (2003) Plants, selenium and human health. Curr Opin Plant Biol 6:273–279
Feist LJ, Parker DR (2001) Ecotypic variation in selenium accumulation among populations of Stanleya pinnata. New Phytol 149:61–69
Findley JW (2005) Selenium accumulation in plant foods. Nutr Rev 63:196–202
Fordyce F (2005) Selenium toxicity and deficiency in the environment. In: Selinus O (ed) Essentials of medical geology-impacts of the natural environment on public health. Elsevier, Amsterdam, pp 373–415
Gawalko EJ, Garrett RG, Nowicki TW (2002) Cadmium, copper, iron, manganese, selenium, and zinc in Canadian spring wheat. Commun Soil Sci Plant Anal 33:3121–3133
Gissel-Nielsen G, Gupta UC, Lamand M, Westermarck T (1984) Selenium in soils and plants and its importance to livestock and human nutrition. Adv Agron 37:397–436
Hanson B, Lindblom SD, Loeffler ML, Pilon-Smits EAH (2004) Selenium protects plants from phloem-feeding aphids due to both deterrence and toxicity. New Phytol 162:655–662
Harskamp JG, O’Donnell MJ, Berkelaar E (2010) Determining the fluxes of Tl+ and K+ at the root surface of wheat and canola using Tl(I) and K ion-selective microelectrodes. Plant Soil 335:299–310
Haygarth PM (1994) Global importance and global cycling of selenium. In: Frankenberger WT, Benson S (eds) Selenium in the environment. Marcel Dekker, New York, pp 1–27
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. California AES Bulletin 347. Agricultural Productions, University of California, Berkeley
Hopper JL, Parker DR (1999) Plant availability of selenite and selenate as influenced by the competing ions phosphate and sulphate. Plant Soil 210:199–207
Hu X, Wang F, Hanson ML (2009) Selenium concentration, speciation and behavior in surface waters of the Canadian prairies. Sci Total Environ 407:5869–5876
Huang Y, Hu Y, Liu Y (2008) Interactions between sulfur and selenium uptake by corn in solution culture. J Plant Nutr 31:43–54
Johnsson L (1991) Selenium uptake by plants as a function of soil type, organic matter content and pH. Plant Soil 133:57–64
Kopsell DA, Randle WM (1997) Selenate concentration affects selenium and sulfur uptake and accumulation by ‘Granex 33’ onions. J Am Soc Hortic Sci 122:721–726
Köstner B, Schup R, Schulze E-D, Rennenberg H (1998) Organic and inorganic sulfur transport in the xylem sap and the sulfur budget of Picea abies trees. Tree Physiol 18:1–9
Li H, McGrath SP, Zhao F (2008) Selenium uptake, translocation, and speciation in wheat supplied with selenate or selenite. New Phytol 178:92–102
McNeal JM, Balistrieri LS (1989) Geochemistry and occurrence of selenium: an overview. In: Jacobs LW (ed) Selenium in agriculture and the environment. SSSA Special Publication, Madison, pp 1–13
Mikkelsen RL, Wan HF (1990) The effect of selenium on sulfur uptake by barley and rice. Plant Soil 121:151–153
Missana T, Alonso U, García-Gutiérrez M (2009) Experimental study and modeling of selenite sorption onto illite and smectite clays. J Colloid Interface Sci 334:132–138
Nakamaru YM, Tagami K, Uchida S (2005) Depletion of selenium in soil solution due to its enhanced sorption in the rhizosphere of soybean. Plant Soil 278:293–301
Navarro-Alarcon M, Cabrera-Vique C (2008) Selenium in food and the human body: a review. Sci Total Environ 400:115–141
Neal RH (1990) Selenium. In: Alloway BJ (ed) Heavy metals in soils. Blackie and Son Ltd., Glasgow, pp 237–260
Oliver MA (1997) Soil and human health: a review. Eur J Soil Sci 48:573–592
Piñeros MA, Shaff JE, Kochian LV (1998) Development, characterization, and application of a cadmium-selective microelectrode for the measurement of cadmium fluxes in roots of Thlaspi species and wheat. Plant Physiol 116:1393–1401
Renkema H, Koopmans A, Kersbergen L, Kikkert J, Hale B, Berkelaar E (2012) The effect of transpiration on selenium uptake and mobility in durum wheat and spring canola. Plant Soil 354:239–250
Ríos JJ, Blasco B, Cervilla LM, Rubio-Wilhelmi MM, Ruiz JM, Romero L (2008) Regulation of sulphur assimilation in lettuce plants in the presence of selenium. Plant Growth Regul 56:43–51
Sandholm M, Oksanen HE, Pesonen L (1973) Uptake of selenium by aquatic organisms. Limnol Oceanogr 18:496–498
Sauheitl L, Glasera B, Weigelt A (2009) Uptake of intact amino acids by plants depends on soil amino acid concentrations. Environ Exp Bot 66:145–152
Sors TG, Ellis DR, Salt DE (2005) Selenium uptake, translocation, assimilation, and metabolic fate in plants. Photosynth Res 86:373–389
Terry N, Zayed AM, deSouza MP, Tarun AS (2000) Selenium in greater plants. Annu Rev Plant Physiol 51:401–432
Topper K, Kotuby-Amacher J (1990) Evaluation of a closed vessel acid digestion method for plant analyses using inductively coupled plasma spectrometry. Commun Soil Sci Plant Anal 21:1437–1455
van Dorst SH, Peterson PJ (1984) Selenium speciation in the soil solution and its relevance to plant uptake. J Sci Food Agric 35:601–605
Wallenda T, Read DJ (1999) Kinetics of amino acid uptake by ectomychorrhizal roots. Plant Cell Environ 22:179–187
Wang MC, Chen HM (2003) Forms and distribution of selenium at different depths and among particle size fractions of three Taiwan soils. Chemosphere 52:585–593
Williams MC, Mayland HF (1992) Selenium absorption by two-grooved milkvetch and western wheatgrass from selenomethionine, selenocystine, and selenite. J Range Manage 45:374–378
Wu L (2004) Review of 15 years of research on ecotoxicology and remediation of land contaminated by agricultural drainage sediment rich in selenium. Ecotoxicol Environ Saf 57:257–269
Ximénez-Embún P, Alonso I, Madrid-Albarran Y, Camara C (2004) Establishment of selenium uptake and species distribution in lupine, Indian mustard, and sunflower plants. J Agric Food Chem 52:832–838
Zayed A, Lytle CM, Terry N (1998) Accumulation and volatilization of different chemical species of selenium by plants. Planta 206:284–292
Zhang Y, Pan G, Chen J, Hu Q (2003) Uptake and transport of selenite and selenate by soybean seedlings of two genotypes. Plant Soil 253:437–443
Zhao C, Ren J, Xue C, Lin E (2005) Study on the relationship between soil selenium and plant selenium uptake. Plant Soil 277:197–206
Acknowledgments
The authors gratefully acknowledge the support of the Natural Sciences and Engineering Research Council of Canada Metals in the Human Environment Strategic Network and Redeemer University College (Internal Research Grant) for the funding of this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kikkert, J., Berkelaar, E. Plant Uptake and Translocation of Inorganic and Organic Forms of Selenium. Arch Environ Contam Toxicol 65, 458–465 (2013). https://doi.org/10.1007/s00244-013-9926-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-013-9926-0