Abstract
The paper reports the effects of selenium (Se) supply on growth and some physiological traits of wheat (Triticum aestivum L. cv Shijiazhuang NO. 8) seedlings exposed to drought stress. The growth and physiological responses of seedlings were different depending on the Se concentration. The higher (3.0 mg Se kg−1) and lower amount used (0.5 mg Se kg−1) did not significantly affect on biomass accumulation. Treatments with 1.0 and 2.0 mg Se kg−1 promoted biomass accumulation of wheat seedlings. Treatments at 1.0, 2.0, and 3.0 mg Se kg−1 significantly increased root activity, proline content, peroxidase (POD), and catalase (CAT) activities, carotenoids (Car) content, chlorophyll content, and reduced malondialdehyde (MDA) content of wheat seedlings. Lower Se treatment did not significantly effect on chlorophyll content and MDA content, although it also increased some antioxidant index (proline and Car content, POD and CAT activities) in wheat seedlings. These results suggest that optimal Se supply is favorable for growth of wheat seedlings during drought condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water stress is considered to be one of the most important agricultural problems. Plants are often exposed to periods of soil and atmospheric water deficit during their life cycle [1]. Researches have shown that drought has very many adverse impacts on plant growth parameters [2–4]. Together, these combine to reduce plant fitness and function.
Searching for suitable ameliorants or stress alleviant is one of the tasks of plant biologists. Recent researches have identified several beneficial effects of selenium (Se) in plants, although Se is not considered to be required by higher plants [5, 6]. Positive effects of Se on plants mainly exhibited: promoting plant growth [5], alleviating UV-induced oxidative damage [5, 7, 8], improving the recovery of chlorophyll from light stress [9], increasing antioxidative capacity of senescing plants, and regulating the water status of plants exposed to drought [10, 11]. However, to our knowledge, there has been limited effort to understand the role of Se in plant under drought condition.
Wheat is one of the most important crops in world. At present, the growth of wheat has been seriously influenced by drought in many regions. The paper studies the short-term effects of Se supply on growth and physiological traits of wheat seedlings grown under drought stress. This will be helpful for development of improved plant tolerance toward stressful environmental factors. We hypothesize that Se supply will ease the adverse effects of water stress on wheat seedlings.
Materials and Methods
Plant Material and Experimental Design
The research was conducted with outdoor pot in Hebei University, Baoding, China. The selected seeds of wheat (Triticum aestivum L. cv Shijiazhuang NO. 8) from Baoding agricultural market were disinfected by immersion in a 2.5% solution of sodium hypochlorite for 5 min and washed five times with distilled [12]. Seeds were sowed in plastic pots (25 × 20 cm), 30 seeds per pot. The substrate used for growing the seedlings was sieved topsoil from farmland. Sodium selenite (Na2SeO3) was added at concentrations from 0.5 mg Se kg−1 to 3 mg Se kg−1 before sowing. Fifteen seedlings of similar size were saved in each pot after germination, and water stress (30% of maximum field capacity) was begun. Each treatment had five replicates.
All of the following measurements were carried out using samples collected around 20 days after seedling selection.
Measurements
Samples from five replicates (pots) were harvested. Shoots and roots were separated, and roots were rinsed free of soil. The fresh weight was measured immediately after harvest. Values were calculated to gram per pot.
Leaf tissue was ground in 80% acetone for chlorophyll and carotenoids determination. Total chlorophyll (Chl (a + b)), chlorophyll a (Chl a), chlorophyll b (Chl b), and total carotenoids (Car) contents were determined according to Lichtenthaler [13].
Root activity was measured by the improvement of triphenyl tetrazolium chloride (TTC) method [14]. Root tips were dipped in the mixed solution, 5 ml of 0.4% TTC and 5 ml of 0.1 mol L−1 sodium phosphate buffer (pH = 7.0), and incubated at 37°C for 60 min. Then, 2 ml of 1 mol L−1 H2SO4 was added to the mixture to stop the reaction, and root tips were taken out. Root tips were dipped in 20 ml of methanol and incubated at 37°C until they bleached. The supernatant was recorded at 485 nm.
The degree of lipid peroxidation in leaf tissue was assessed by malondialdehyde content (MDA). MDA content was determined by the thiobarbituric acid (TBA) reaction. Leaves (0.5 g) were homogenized with 5 ml of 20% (w/v) trichloroacetic acid (TCA). The homogenate was centrifuged at 3,500×g for 20 min. To 2 ml of the aliquot of the supernatant, 2 ml of 20% TCA containing 0.5% (w/v) TBA and 100 μl 4% (w/v) butylated hydroxytoluene in ethanol was added. The mixture was heated at 95°C for 30 min and then quickly cooled on ice. The contents were centrifuged at 10,000×g for 15 min, and the absorbance was measured at 532 nm. The value for nonspecific absorption at 600 nm was subtracted. The concentration of MDA was calculated using an extinction coefficient of 155 mM−1 cm−1. Results were expressed as μmol g−1 fresh weight (FW).
The free proline content was determined according to the method described by Bates et al. [15]. 0.5 g leaves were homogenized using a pestle and mortar with 5 ml of sulfosalicylic acid (3% w/v). After centrifugation (5 min at 20,000×g), 0.5 ml of the supernatant was incubated at 100°C for 60 min with 0.5 ml of glacial acetic acid and 0.5 ml of ninhydrin reagent. After cooling, 1 ml of toluene was added to the mixture, and the absorbance of the chromophore containing toluene was recorded at 520 nm.
Catalase activity (CAT, EC 1.11.1.6) was determined in the homogenates by measuring the decrease in absorption at 240 nm in a reaction medium containing 50 mM potassium phosphate buffer (pH 7.2), 10 mM H2O2 and 50 μl enzyme extract. The activity was calculated using the extinction coefficient (40 mM−1 cm−1) for H2O2.
Peroxidase activity (POD, EC 1.11.1.7) was based on the determination of guaiacol oxidation (extinction coefficient 26.6 mM−1 cm−1) at 470 nm by H2O2. The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.0), 20.1 mM guaiacol, 12.3 mM H2O2, and enzyme extract in a 3-ml volume.
All data were subjected to an analysis of variance using the Software Statistical Package for the Social Science (SPSS) version 11.0. Individual treatment means were compared with Duncan’s test to determine whether they were significantly different at the 0.05 probability level.
Results
Effects of Se Supply on Growth Parameters
When subjected to drought stress, the growth of seedlings was different depending on the Se concentration (Table 1). The lower amount used (0.5 mg Se kg−1) caused a slight increase in shoot weight, root weight, and total biomass compared with the control but was not statistically significant. The treatments with 1.0 and 2.0 mg Se kg−1 resulted in a significant increase in growth parameters. The higher Se treatment (3.0 mg Se kg−1) only increased root weight. Shoot/root ratio of seedlings was not affected by Se treatments.
Effects of Se Supply on Photosynthetic Pigments Content
As shown in Table 2, treatments at 1.0, 2.0, and 3.0 mg Se kg−1 induced an evident increase in Chl a, Chl b, total Chl (a + b), and Car content of seedlings compared with the control. Lower Se treatment only increased Car content.
Effects of Se Supply on Root Activity
Compared with the control, Se treatments significantly increased root activity of seedlings, and the increased amount depended on the Se concentration (Fig. 1). There was no significant difference in root activity between 2.0 and 3.0 mg Se kg−1 treatments.
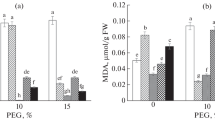
Effects of Se Supply on MDA and Free Proline
Significant increase in free proline content was observed by different Se treatments over the control (Fig. 2). Treatments with 1.0, 2.0, and 3.0 mg Se kg−1 also significantly reduced MDA content (Fig. 2).
Effects of Se Supply on Enzyme Activity
The effects of different Se treatments on CAT and POD activities in seedlings exposed to drought stress were shown in Fig. 3. The activities exhibited similar changes in response to Se supply. Se treatments all significantly increased CAT and POD activities compared with the control.
Discussions
Drought is an important abiotic factor that could influence the growth and physiological traits of plants [16, 17]. In present study, some reports showed that Se could increase the tolerance of plants to stressful environment, such as UV-B stress, salt stress, and light stress [8, 9, 18]. Our results suggested that the effects of Se on growth of wheat seedlings subjected to drought stress depended on the Se concentration (Table 1). Treatments with 1.0 and 2.0 mg Se kg−1 promoted the biomass accumulation of seedlings, while higher Se treatment (3.0 mg kg−1) and lower Se treatment (0.5 mg kg−1) did not significantly affect on biomass accumulation.
Root is not only a vital organ of plants for absorbing salt and water but also is very important for assimilation, synthesis, and translation of many materials. So, root activity directly affected growth and development of plants [14]. In our study, Se supply significantly increased root activity of seedlings (Fig. 1), which may be beneficial for the growth of roots (Table 1).
The production of reactive oxygen species (ROS) is the important cause of damage to plants when exposed to environmental stress, which resulted in the growth inhibition, the breakdown of lipid membrane, and reduction in photosynthetic parameters [4, 18, 19]. In our study, Se treatments (except for 0.5 mg Se kg−1) significantly reduced MDA content and increased total Chl (a + b) content in seedlings under water deficit. The reduction in MDA content indicated the decline of lipid peroxidation. The result was in agreement with other studies [10]. An increase in chlorophyll content was observed in here, which may be the result of the increase in Car content, since Car protects chlorophyll from photooxidative destruction [20]. The increase in chlorophyll content is beneficial for growth of seedlings.
When subjected to environmental stress, plants also form some protective mechanisms by increasing antioxidant enzymes’ activities and antioxidants content. Proline accumulation is a very common response in plants exposed to drought stress [21, 22]. It could function as a hydroxyl radical scavenger to prevent membrane damage and protein denaturation [23]. In our study, Se supply significantly increased free proline content and POD and CAT activities (Figs. 2 and 3), which may provide an ecological adaptation for seedlings under stress conditions.
References
M.M. Chaves, J.S. Pereira, J. Maroco, M.L. Rodrigues, C.P.P. Ricardo, M.L. Osório, I. Carvalho, T. Faria and C. Pinheiro, How plants cope with water stress in the field? Photosynthesis and growth. Ann Bot, 89, 907–916 2002.
P.D. Jamieson, R.J. Martin and G.S. Francis, Drought influences on grain yield of barley, wheat, and maize. New Zeal J Crop Hort, 23: 55–56 1995.
X. Tian and Y. Lei, Nitric oxide treatment alleviates drought stress in wheat seedlings. Biol. plantarum, 50, 775–778 2006.
H. Xu, D.K. Biswas, W.D. Li, S.B. Chen, L. Zhang, G.M. Jiang and Y.G. Li, Photosynthesis and yield responses of ozone-polluted winter wheat to drought. Photosynthetica, 45, 582–588 2007.
H. Hartikainen and I. Xue, The promotive effect of selenium on plant growth as triggered by ultraviolet radiation. J. Environ. Qual., 28, 1372–1375 1999.
N. Terry, A.M. Zayed, M.P. de Souza and A.S. Tarun, Selenium in higher plants, Annu. Rev. Plant Physiol. Plant Mol. Biol., 51, 401–423 2000.
H. Hartikainen, T. Xue and V. Piironen, Selenium as an antioxidant and pro-oxidant in ryegrass. Plant Soil, 225, 193–200 2000.
E. Valkama, M. Kivimäenpää, H. Hartikainen and A. Wulff, The combined effects of enhanced UV-B radiation and selenium on growth, chlorophyll fluorescence and ultrastructure in strawberry (Fragaria × ananassa) and barley (Hordeum vulgare) treated in the field. Agr. Forest. Meteorol., 120, 267–278 2003.
M. Seppänen, M. Turakainen and H. Hartikainen, Selenium effects on oxidative stress in potato. Plant Sci., 165, 311–319 2003.
M. Djanaguiraman, D. Durga, A.K. Shanker, A. Sheeba and U. Bangarusamy, Selenium—An antioxidative protectant in soybean during senescence. Plant and Soil, 272, 77–86 2005.
V.V. Kuznetsov, V.P. Kholodova, V.V. Kuznetsov and B.A. Yagodin, Selenium regulates the water status of plants exposed to drought. Dokl. biol. Nauk, 390: 266–268 2003.
I.A. Bakke, A.L. de Oliveira Freire, O.A. Bakke, A.P. de Andrade, R. de Lucena Alcântara Bruno, Water and sodium chloride effects on Minosa Tenuiflora (Willd.) poiret seed germination. Caatinga (Mossoró, Brasil), 19: 261–267 2006.
H.K. Lichtenthaler, Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148: 350–382 1987.
B.Z. Bai, J.Z. Jin, S. Bai and L.P. Huang, Improvement of TTC method determining root activity in corn. Maize Science. 2, 44–47 1994 (in Chinese with english abstract)
L.S. Bates, R.P. Waldren and I.D. Teare, Rapid determination of free proline for water stress studies. Plant Soil. 39, 205–208 1973.
N. Tadina, M. Germ, I. Kreft, B. Breznik and A. Gaberščik, Effects of water deficit and selenium on common buckwheat (Fagopyrum esculentum Moench.) plants. Photosynthetica, 45: 472–476 2007.
J. Ren, W.R. Dai, Z.Y. Xuan, Y.A. Yao, K. Helena and C.Y. Li, The effect of drought and enhanced UV-B radiation on the growth and physiological traits of two contrasting poplar species. Forest Ecol. Manage., 239, 112–119 2007.
L.G. Kong, M. Wang and D.L. Bi, Selenium modulates the activities of antioxidant enzymes, osmotic homeostasis and promotes the growth of sorrel seedlings under salt stress. Plant growth regul, 45, 155–163 2005.
X.Q. Yao, Q. Liu, Changes in photosynthesis and antioxidant defenses of Picea asperata seedlings to enhanced ultraviolet-B and to nitrogen supply. Physiol. Plant., 129, 364–374 2007.
A. Singh, Growth, physiological, and biochemical responses of three tropical legumes to enhanced UV-B radiation. Can. J. Bot., 74, 135–139 1996.
P.H. Yancey, M.E. Clark, S.C. Hand, R.D. Bowlus, G.N. Somero, Living with water stress: evolution of osmolyte systems. Science 217, 1214–1222 1982.
V. Alexieva, I. Sergiev, S. Mapellis and E. Karanov, The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 24, 1337–1344 2001.
F. Ain-Lhout, M. Zunzunegui, M.C. Diaz Barradas, R. Tirado, A. Clavijo and F. Carcia Novo, Comparison of proline accumulation in two Mediterranean shrubs subjected to natural and experimental water deficit. Plant Soil. 230, 175–183 2001.
Acknowledgments
This study was supported by Dr. Fund, Hebei University, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yao, X., Chu, J. & Wang, G. Effects of Selenium on Wheat Seedlings Under Drought Stress. Biol Trace Elem Res 130, 283–290 (2009). https://doi.org/10.1007/s12011-009-8328-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-009-8328-7