Abstract
Titanium (Ti) is a beneficial element that promotes growth and biomass production although the mechanism by which this improvement takes place is still unclear, as are other effects on plants, although it is believed that Ti can compensate for N deficiency. To prove this hypothesis, a hydroponic experiment was designed to investigate the effect of adding Ti to a nutrient solution on the nutrient uptake of tomato (Lycopersicon esculentum L.) by withholding N within the nutrient solution (NS) by 25 % (NS2) and by 50 % (NS1). Ti was added at 1 and 2 mg L−1. When Ti was added to nutrient solution, the elemental concentration in tomato changed significantly: K, Ca, Fe, and Zn decreased while Ti increased. As the concentration of N in nutrient solution decreased, the Ca and Ti concentration of tomato leaves decreased and the K, Mn, Fe, Cu, and Zn concentration increased. As the N concentration in nutrient solution increased, the Ca concentration decreased although the application of Ti compensated for Ca concentration in NS1. All the photosynthetic attributes and physiological characteristics, including flower induction, decreased when the N concentration of NS decreased by 50 %, although this decrease could be compensated by applying 1 mg L−1 Ti. This has valuable and practical applications and implications for tomato hydroponic culture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Titanium (Ti) forms 0.33 % of the earth’s crust and its level in soil ranges from 0.1 % to 0.7 %, although the average concentration in soil solution is low, around 30 g L−1 [1]. Despite the low availability of this element to plants, its beneficial effects on plants have already been proved. In oats (Avena sativa), Ti applied as a nutrient solution was more effective than spraying it on the leaves, benefiting various plant physiological parameters such as biomass yield, chlorophyll (chl) content, and growth [2]. Ti, when applied as a leaf spray, improved the growth of pepper [3].
Němec and Káš [4] conducted the first systematic research of the dose–response relationship between Ti and plant growth using mustard, pea, and alfalfa grown in soil, observing that at certain “optimal” Ti concentrations, the growth and development of plants were intensified as was the chl content (indicated by greener plants) while leaf area increased and ripening was accelerated. Kiss et al. [5] and Daood et al. [6] much later capitalized upon those findings being the first researchers to investigate the physiological effect of Ti on plant growth, indicating that 10−8 M Ti (applied as titanium chelate with ascorbic acid) increased the growth of Anacystis nidulans. These authors also showed that Ti could activate photosynthesis, probably by changing the redox state of specific regulatory proteins and eliciting an alteration in enzyme activity, the most important enzyme being fructose-1,6-bisphosphatase (F-1,6BP), which participates in the Calvin cycle, gluconeogenic, and oxidative pentose phosphate pathways of carbohydrate metabolism which are assumed to be associated with Ti.
Ti has also been shown to improve the quality and yield of several fruits and vegetables, possibly related to the effect of Ti on lipoxygenase activity during ripening. Evidence exists that when activated, Ti may induce changes in various metabolic pathways such as ethylene production and lipid oxidation, as well as chl and carotenoid biosynthesis and degradation. Daood et al. [6] reached the conclusion that Ti had an activating effect on tomato in vivo and in vitro through four key pieces of evidence: (a) decreased lipoxygenase (LOX) biosynthesis or the availability of some essential components such as Fe at the initial stages of ripening; (b) increased content of antioxidants of Ti-treated tomato fruits at the beginning of ripening which affected the utilization of molecular oxygen (O2) by LOX in the course of linoleic acid oxidation; (c) the high availability of several essential elements and cofactors such as Fe and Mn at the final stage of ripening which activated LOX when they were present at low concentrations, but the possibility that they would be more effective when at higher concentrations (0.1 M) such as in titanium ascorbate (TITAVIT)-treated fruit; and (d) a direct interaction between LOX and Ti, similar to that which occurs with Fe since Ti that was absorbed by the plant was found to be adsorbed onto the surface of some macromolecules such as proteins and since there was no difference in the Ti content of TITAVIT-treated and untreated fruit.
As for other elements such as iron (Zn), selenium (Se), and lead (Pb) [7], higher doses of Ti can negatively impact plant growth and physiology, the phenomenon of “hormesis” [8]. This sensitive balance between benefit and phytotoxicity might involve the replacement of essential elements (particularly Fe and magnesium (Mg)) from their binding sites since soluble Ti(IV) has a high affinity to ligands containing oxygen [2, 8]. If this was to happen, then Ti could cause an apparent deficiency in Fe resulting in significant changes in Fe uptake via roots [9]. This influence of Ti on Fe uptake and its effect on metabolism, including an increase in the content of photosynthetic pigments, are based on the premise that the low redox potential of the Ti(IV)/Ti(III) couple can influence the activity of metals in the chloroplast and in the cytoplasm [10, 11]. Since Fe is an essential component of photosystem II (PSII), any influence of Ti on Fe uptake and subsequent Fe deficiency would influence the biochemical and physiological balance of the photosynthetic apparatus in general, weakening it, for example, by lowering PSII efficiency [12, 13] and references therein). Cigler et al. [13] used chl fluorescence emission (Chl-F) as a noninvasive tool to determine the photosynthetic activity in Ti-treated spinach (Spinacia oleracea) plants in vivo. They found that when Fe was present in growth medium, it significantly decreased the Ti concentration in shoots; moreover, photosynthetic activity was lowered by Ti. Supposedly, Ti affects the components of the electron transport chain containing Fe in their structure (particularly PSI), decreasing thus PSII efficiency. These authors further claimed that if Ti and Fe were present at equal concentrations in the medium, the impact of Ti would be lowered, probably resulting from their competition for binding sites.
Although the beneficial effects of Ti were first noted 80 years ago, and then exploited by adding Ti to various complex micronutrient fertilizers [2], its effect on macronutrient and micronutrient uptake has not been sufficiently investigated. There is still, to date, no evidence to show that Ti has any significant effect on the K content in plant tissues. Kužel et al. [2] applied Ti with different levels of Mg in nutrient solution and found that Ti increased the Mg content of oat leaves. The Fe content of leaves fluctuated depending on the mode of application of Ti: Fe increased when Ti was applied in nutrient solution and decreased when it was sprayed on the leaves. The authors ascribed these trends to apparent Fe deficiency, which resulted in an increased uptake of Fe and other metals via roots when Ti was applied in nutrient solution. On the other hand, when Ti was applied to leaves, Fe transport within the plant was blocked, decreasing Fe uptake to leaves. The Mn content was strongly decreased by Ti in nutrient solution [2].
Nitrogen (N) is the most essential element for growth and development of tomato plants but tends to be provided to plants in excess [14], hence causing potential environmental pollution through fertilizer application. Estimates indicate that N wastage from soilless cultures amounts to 1 ton N ha−1 year−1 in the absence of drainage recycling [15]. The ecological price for N waste of tomato hydroponic culture is very high to pay since a large amount of fertilizers are leached from this agrosystem to ground water. Conversely, a reduction of N to 25–50 % of normal levels had no adverse effect on tomato fruit yield and quality [14, 15] since the storage capacity of the plant for N exceeds that required for growth. Consequently, Le Bot et al. [15] suggested terminating nutrient supply 2–3 weeks prior to final harvest or growing hydroponic tomato at reduced N concentrations to restrict the release of nitrogen waste. However, it is not easy to define the critical N concentration that allows maximum growth rate while avoiding deficiency symptoms [14]. Another possible strategy is to enhance N use and uptake efficiency by employing another nutrient.
Employing that rationale, Frutos et al. [3] investigated the uptake of N induced by Ti in N-stressed pepper seedlings in three stages. However, their research did not show evidence of nutrient absorption. Rather, they found that Ti could compensate N deficiency although it was most likely related to the level of N-stress and growth stage. Most experiments involving N restriction or withdrawal have been carried out on young vegetative plants with little biomass or few N reserves [15].
Considering this relatively large gap in our understanding of the mechanism and applicability of Ti to the growth of horticultural commodities, the primary objective of this research was to investigate the effect of withholding a portion of the N content of a nutrient solution and adding Ti to evaluate their possible beneficial effect of this element on nutrient (macro and micro) uptake. Furthermore, we demonstrate, for the first time, physiological changes of tomato plants in the early stages of growth.
Material and Methods
This experiment was conducted in an environmentally controlled greenhouse with an average day/night temperature of 2517 °C/17 °C at the Department of Horticulture, Isfahan University of Technology (18° 7′ 23″ N latitude; 51° 53′ 2″ E longitude), Iran. Tomato (Lycopersicon esculentum L. cv. “Halil”) seeds were seeded in and seedlings were grown in a mixture of vermiculite and perlite (2:1, v/v). When the plants had four to five leaves, they were transplanted into hydroponic nutrient solution (NS). The composition of NS (in mg L−1) was as follows: N = 116, P = 21, K = 82, Ca = 125, Mg = 21, S = 28, Fe = 6.8, Mn = 1.97, Zn = 0.25, B = 0.70, Cu = 0.07, and Mo = 0.05 provided by KH2PO4, NH4NO3, Fe EDTA, CuSO4, MnSO4, ZnSO4, Ca(NO3)2, Na2Mo4 and H3BO3. This NS served as the control, i.e., NSc.
A factorial experiment was arranged in a completely randomized design (CRD) with four replicates. For testing the impact of Ti on growth and N assimilation, 25 % (NS2; or a quarter dose) and 50 % (NS1; or a half dose) of total N were employed. In addition, Ti was applied at two levels, 1 (Ti1) and 2 mg L−1 (Ti2), 10 days after transplanting.
Leaf chl content (i.e., SPAD value) was measured from fully expanded leaves (similar leaves in all treatments) using a nondestructive dual-wavelength chl meter (SPAD-502, Minolta Corp., Ramsey, NJ, USA). Five measurements from the same leaves were taken per replicate. Photosynthetic rate (μmol m−2 s−1) and transpiration rate (mmol m−2 s−1) were determined using a portable area meter (LI-COR, Li-3000, USA). At the end of the experiment (i.e., 6 weeks after treatment application), plants were harvested and washed in tap water. The shoots were separated from the roots using a surgical blade, and the tissues were dried in an oven at 70 °C until constant weight. Fresh and dry weights (FW and DW) of shoots and roots were determined. Dried samples were used for nutrient analysis. The concentration of nutrients (K, Ca, Mn, Fe, Cu, Zn, and Ti) was measured with X-ray fluorescence (Unisantis XMF104, Germany). The time to first flower induction from the start of treatment application was measured. The number of flowers was counted at the end of experiment. The shoot diameter was measured with digital caliper (Model 16 ER 0-6).
The study was arranged in a factorial design based on CRD with four replications. All data were subjected to one-way ANOVA with Statistix 8 (Tallahassee, FL, USA) and the means were compared by LSD test with P < 0.05 indicating significantly different treatments.
Results
The Effect of Different Levels of N, Ti, and N–Ti on Physiological Characteristics of Tomato
As N concentration decreased, shoot DW and FW, root volume, flower number, and shoot diameter were significantly decreased, particularly at NS2 (Table 1). Ti and N–Ti did not change the shoot FW and DW, root volume, or shoot diameter significantly although Ti2 reduced flower number significantly (Table 2). Although a reduction in N level reduced chl content and transpiration significantly, this trend was only true for photosynthetic rate and intercellular CO2 when 25 % of total N was employed (i.e., NS2; Table 3). Chlorophyll content was unaffected by treatment with Ti or N–Ti, although Ti decreased photosynthetic rate and transpiration and increased intercellular CO2 (but only at 2 mg L−1 Ti) significantly while the response of these parameters was largely unaffected by N–Ti treatments (Table 4).
The Effect of Different Levels of N, Ti, and N–Ti on Nutrient Uptake of Tomato
As N concentration of the NS decreased, the Ca and Ti concentration of tomato leaves decreased significantly while the K, Mn, Fe, Cu, and Zn concentration increased significantly (Table 5). When Ti was added to NS, the concentration of elements in tomato changed significantly. The concentration of K, Ca, Fe, and Zn decreased significantly, that of Ti increased significantly while the concentration of Mn did not change (Table 6).
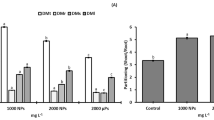
In a separate set of experiments, the interaction effects of N and Ti, applied to NS, were observed. K concentration increased significantly as N concentration decreased but when Ti was added to NS, the K concentration decreased more. These decreases were significantly higher in NS1 rather than in other treatments (Fig. 1a). A similar trend was observed for Mn (Fig. 1c) and Fe (Fig. 1d). Ca concentration decreased significantly as N concentration decreased but when Ti was added to NS, the Ca concentration increased significantly (Fig. 1b). Mn concentration in the leaves increased significantly as the N level of NS increased. Ti decreased the Mn concentration significantly in NS and did not affect Mn concentration at other levels of N in NS (Fig. 1c). The effect of withholding N and applying Ti on Fe concentration of leaves did not show any distinct change, except for Ti1 with a higher N level in NS, resulting in a significant increase in the Fe concentration. The lowest Fe concentration was observed at the highest concentration of Ti and without N in NS (Fig. 1d). There was no clear pattern for Zn, Cu, or Ti (Fig. 1e, f, g, respectively), although the following results could be highlighted: Zn concentration was significantly higher when N concentration remained high and even at low levels of Ti (Fig. 1e), Cu concentration was significantly stimulated by the addition of 1 mg/L Ti (Fig. 1f), and Ti concentration increased significantly as the concentration of N in NS decreased (Fig. 1g).
Nutrient concentration at different levels of N and Ti in tomato leaves. a–g The graphs show the K, Ca, Mn, Fe, Zn, Cu, and Ti concentrations, respectively. Different letters within a treatment are significantly different according to LSD test (P ≤ 0.05); n = 20. NSc (full nutrient solution), NS1 (50 % or half dose), and NS2 (25 % or a quarter dose) of total N were employed. Ti1 (1 mg L−1) and Ti2 (2 mg L−1) of Ti
The Effect of Different Levels of N with Ti on Physiological Characteristics of Tomato
Provided that N concentration was not lowered, the addition of Ti did not significantly impact shoot fresh weight (Fig. 2), dry weight (Fig. 3), or root fresh weight (Fig. 4). Ti, when added to NS, sped up the time to first flower induction, independent of the level of N (Fig. 5). Ti also increased the number of flowers, but only significantly so in the control (full N concentration in NS) and 50 % reduction in N level (Fig. 6). The addition of Ti, independent of the N level, neither negatively influenced nor promoted chl content (Fig. 7). However, the higher the level of Ti, the greater the photosynthetic capacity of plants, particularly as N level decreased (Fig. 8). The addition of Ti to NS had no influence on, or decreased transpiration, for all levels of N in NS (Fig. 9).
Nutrient concentration at different levels of N and Ti on fresh weight of tomato shoots; n = 20. Different letters within a treatment are significantly different according to LSD test (P ≤ 0.05). NSc (full nutrient solution), NS1 (50 % or a half dose), and NS2 (25 % or a quarter dose) of total N were employed. Ti1 (1 mg L−1) and Ti2 (2 mg L−1) of Ti. N–Ti1 (1 mg L−1) and N–Ti2 (2 mg L−1) of N–Ti
Nutrient concentration at different levels of N and Ti on dry weight of tomato shoots; n = 20. Different letters within a treatment are significantly different according to LSD test (P ≤ 0.05). NSc (full nutrient solution), NS1 (50 % or a half dose), and NS2 (25 % or a quarter dose) of total N were employed. Ti1 (1 mg L−1) and Ti2 (2 mg L−1) of Ti. N–Ti1 (1 mg L−1) and N–Ti2 (2 mg L−1) of N–Ti
Nutrient concentration at different levels of N and Ti on fresh weight of tomato roots; n = 20. Different letters within a treatment are significantly different according to LSD test (P ≤ 0.05). NSc (full nutrient solution), NS1 (50 % or a half dose), and NS2 (25 % or a quarter dose) of total N were employed. Ti1 (1 mg L−1) and Ti2 (2 mg L−1) of Ti. N–Ti1 (1 mg L−1) and N–Ti2 (2 mg L−1) of N–Ti
Nutrient concentration at different levels of N and Ti on time to first flowering of tomato; n = 20. Different letters within a treatment are significantly different according to LSD test (P ≤ 0.05). NSc (full nutrient solution), NS1 (50 % or a half dose), and NS2 (25 % or a quarter dose) of total N were employed. Ti1 (1 mg L−1) and Ti2 (2 mg L−1) of Ti. N–Ti1 (1 mg L−1) and N–Ti2 (2 mg L−1) of N–Ti
Nutrient concentration at different levels of N and Ti on the number of tomato flowers; n = 20. Different letters within a treatment are significantly different according to LSD test (P ≤ 0.05). NSc (full nutrient solution), NS1 (50 %;or a half dose), and NS2 (25 % or a quarter dose) of total N were employed. Ti1 (1 mg L−1) and Ti2 (2 mg L−1) of Ti. N–Ti1 (1 mg L−1) and N–Ti2 (2 mg L−1) of N–Ti
Nutrient concentration at different levels of N and Ti on tomato chlorophyll content; n = 20. Different letters within a treatment are significantly different according to LSD test (P ≤ 0.05). NSc (full nutrient solution), NS1 (50 % or a half dose), and NS2 (25 % or a quarter dose) of total N were employed. Ti1 (1 mg L−1) and Ti2 (2 mg L−1) of Ti. N–Ti1 (1 mg L−1) and N–Ti2 (2 mg L−1) of N–Ti
Nutrient concentration at different levels of N and Ti on photosynthesis of tomato; n = 20. Different letters within a treatment are significantly different according to LSD test (P ≤ 0.05). NSc (full nutrient solution), NS1 (50 % or a half dose), and NS2 (25 % or a quarter dose) of total N were employed. Ti1 (1 mg L−1) and Ti2 (2 mg L−1) of Ti. N–Ti1 (1 mg L−1) and N–Ti2 (2 mg L−1) of N–Ti
Nutrient concentration at different levels of N and Ti on transpiration of tomato; n = 20. Different letters within a treatment are significantly different according to LSD test (P ≤ 0.05). NSc (full nutrient solution), NS1 (50 % or a half dose), and NS2 (25 % or a quarter dose) of total N were employed. Ti1 (1 mg L−1) and Ti2 (2 mg L−1) of Ti. N–Ti1 (1 mg L−1) and N–Ti2 (2 mg L−1) of N–Ti
Discussion
The Effect of Withholding N on Physiological Characteristics and Photosynthetic Attributes of Tomato
In vegetable crops, excessive N generally leads to excessive vegetative growth rather than root or fruit development, reduced visual quality of fruits, and poor fruit set, indirectly delaying fruit development in tomato [16].
N fertilization in fruit production should be reduced to a minimum level without affecting yield and fruit quality of blackberry, peach, apple, apricot, wine grapes, and cherry [16] and their results showed no effect of N level on fruit production, rather only on vegetative growth similar to this study in which tomato vegetative growth was affected by excluding N from the hydroponic nutrient solution (an almost instinctively expected result).
With N deficiency, plants display a two-step response. In the first step, plants reduce leaf elongation rate without affecting photosynthesis and maintain or even stimulate root elongation because the carbohydrates produced are mainly allocated to roots. In the early period of N deficiency, plants are able to mobilize internal N reserves to maintain N metabolism and are able to adapt to temporary N shortage without seriously impacting growth. In the second step, there is irreversible leaf senescence and inhibition of plant growth [17]. In this study too, hydroponic solution without N resulted in growth inhibition.
Many studies have shown a strong positive correlation between N content and the photosynthetic capacities of leaves. N deficiency decreases the amount of thylakoids, carboxylation activity, photochemical efficiency, chl content, and stomatal conductance of leaves; these effects ultimately decrease biomass production [14]. In this study, this was partially true, but only when 25 % of total N was employed (i.e., NS2; Table 3).
Photosynthesis and internal CO2 decreased when N was applied at a quarter dose while transpiration decreased in both half and quarter dose of N treatments (Table 4). Nitrogen deficiency (i.e., 1 mM) decreased photosynthesis rate, transpiration, and stomatal conductance in the vegetative stage of another tomato cultivar (“Marmande”) which in turn resulted in a reduction of growth and dry matter [18]. These authors assumed that photosynthesis decreased due to accumulation of starch which decreased both the carbon demand from sinks and CO2 conductance in the mesophyll. They found that photosynthetic rate was reduced more than stomatal conductance as N deficiency increased since decreasing stomatal conductance also reduced photosynthesis.
Does Ti Affect Physiological Characteristics and Photosynthetic Attributes of Tomato?
Ti administered as TiO2 at 62 mL ha−1 increased seed production of cow pea (Vigna unguiculata) [19]. A similar increase in rice (Oryza sativa) seed production was due to the photocatalytic ability of the material which led to an increase in photosynthetic rate and reduction in the incidence of Curvularia leaf spot and bacterial leaf blight disease [20]. The application of 0.02–0.04 % Tytanit preparation, containing 46 % Ti, on yellow lupin (Lupinus sp) cv. “Idol” increased the number of pods and seeds as well as seed weight. Moreover, Ti stimulated pollination, fertilization, and fruit and seed set and increased yield, thus enhancing seed quality. The yield of apple tree (Malus domestica), corn (Zea mays), and sugar beet (Beta vulgaris subsp. vulgaris) increased by as much as 30 % and the content of chl by 15–65 % when Tytanit was used [21]. Yang et al. [22] observed that nano-TiO2 improved photosynthesis and growth of spinach (S. oleracea); these authors claimed that this improvement was related to the change of N metabolism observed by increasing nitrate reductase, glutamate dehydrogenase, glutamine synthase, and glutamic-pyruvic transaminase activities. Ti increased the absorption of nitrate, accelerated translated inorganic N into organic N, and resulted in an increase in protein and chl content and fresh and dry weights of leaves. Subsequently the same group of authors, and also using spinach as the target plant, suggested that nano-anatase TiO2 induced Rubisco through a Rubsico + Rubisco activase complex which promoted Rubsico carboxylation and increased the rate of photosynthetic carbon reaction [23]. Extending their research, Yang et al. [24] noted that nano-anatase TiO2, on exposure to sunlight, could chemisorb N2 directly or reduce N2 to NH3 in spinach leaves, transforming into organic nitrogen and improving spinach growth.
The Effect of Ti Application on Nutrient Uptake of Tomato
As Ti increased, Fe and Mn concentration decreased in Pieris japonica [25], and even though a similar decrease was not reported by Carvajal and Alcaraz [11] in Capsicum annuum, with Ti4+-ascorbate in this research on tomato, the same results were observed when Ti was used (Fig. 1c, d).
Ti, when added to medium, increased nutrient absorption when this was reported in beans considering that this increase in the macroelement and microelement uptake decreased along with these plants in optimal nutritional conditions. These results imply that Ti is more effective in suboptimal field conditions rather than in optimal conditions of hydroponic culture [26–28]. The absorption of Ca and Zn was improved in tomato when N was totally removed from NS after the application of Ti, although the absorption of other nutrients (K, Mn, Fe, and Cu) under optimal conditions (i.e., NSc) was higher than when N was reduced, even when Ti was added (Fig. 1). Frutos et al. [3] reported that as much as a 50 % reduction in N did not change the nutritional balance of apple trees when plants were treated with Ti one and two times with 0.042 mM Ti ascorbate except for Ca and Zn that did not change. Conclusively, Ti can improve K, Mn, Fe, and Cu absorption but inhibit Ca and Zn absorption when sufficient N is supplied to tomato in hydroponic conditions (Fig. 1).
Simon et al. [9] hypothesized that Ti promoted the better utilization of Fe due to an increase in intracellular Fe2+. Our results support this finding by adding that Ti can promote Fe utilization with 1 mg/L Ti when N concentration is optimized (Fig. 1d).
Kužel et al. [2] used the concept of hormesis to explain the mechanism of Ti’s beneficial effects: Ti causes apparent essential element deficiency in a plant that results in defense reactions (increased uptake of essential elements, etc.) that may increase the health status of the plant more than the toxic effects of Ti decrease in the plant’s health. An understanding of the increase in Zn, Mn, and Ca uptake can be explained by hormesis (Fig. 1b, c, e). The competitive antagonistic effect of Ti as a divalent ion with Cu, Zn and Fe can reduce the uptake of these nutrients. Ti induced Zn transport by the apparent deficiency of Zn in oats [2]. The hormesis argument used by Kužel et al. [2] in oats could be extended to tomato in this study in which Fe transport might be blocked within the plant thus decreasing Fe uptake to leaves. Another plausible reason for Fe deficiency is that Ti causes changes to roots that are typical of Fe deficiency [2].
Ti increased nutrient uptake and growth as a consequence of the corresponding increasing plant photosynthesis and increasing demand by the plant for nutrients [1].
On the other hand, there are reports that revealed that Ti can promote the growth and demand of nutrients under suboptimal nutrient conditions [26–28]. In tomato, in this study, when N was 50 % withdrawn, the absorption of Cu, Zn, Fe, Mn, and K increased compared to full nutrient solution, NSc (Fig. 1). Frutos et al. [3] also showed that when Ti was applied when N concentration was low, this had a compensatory effect thus stimulating growth, the higher N use efficiency being attributed to the promotive effect of Ti on photosynthesis. In tomato, photosynthetic rate increased as Ti concentration increased (data not shown) with a corresponding increase in element absorption, hence promoting growth.
When Ti is added to growth medium, the level of Ti in shoot increased in bean [29], maize [30], and tomato (this study). When Ti was added at 2 and 4 mg/L to NS, this did not affect the Ti concentration of leaves because of the low solubility of Ti at pH 5 in NS; Ti is most soluble at pH < 3, as observed in apple [1]. In conclusion, although N plays a vital role in plant growth and metabolism, in N deficiency, Ti can compensate their deleterious effect. Through hormesis, Ti can improve photosynthesis and compensate for N deficiency in media while improving efficiency in the use and absorption of nutrient such as Cu, Zn, and Mn. On the other hand, not in support of the hormesis hypothesis, Ti appears to be unable to weaken the antagonistic effect it has on Cu, Zn, and Fe (i.e., divalent ions). Decreasing N level by 50 % decreased the shoot fresh weight and root fresh weight and did not change root volume, flower number, and shoot diameter significantly. Thus, our results support previous studies indicating that lower concentration of nutrients in solution can be used to grow tomatoes [15, 31]. Moreover, and most likely, the most important take-away result of this study is the fact that 1 mg L−1 Ti can almost fully compensate the fresh and dry weight of shoots and roots, flowering time, number of flowers, chl content, and photosynthetic capacity when N is reduced by 50 %. This has very practical applications for tomato hydroponic culture by maximizing macronutrient and micronutrient absorption while lowering N concentration by half, thus making the system more cost-effective and ecologically friendly while maintaining the same productivity.
References
Wojcik P, Klamkowski K (2007) “Szampion” apple tree response to foliar titanium application. J Plant Nutr 27:203–204
Kužel S, Hruby M, Cígler P, Tlustoš P, Van PN (2003) Mechanism of physiological effects of titanium leaf sprays on plants grown on soil. Biol Trace Elem Res 91:179–189
Frutos MJ, Pastor JJ, Martínez-Sánchez F, Alcaraz CF (1996) Improvement of the nitrogen uptake induced by titanium leaf supply in nitrogen-stressed pepper seedlings. J Plant Nutr 19:771–783
Němec A, Káš V (1923) The physiological significance of titanium in the plant organism. Biochem 140:583–590
Kiss F, Gy D, Feher M, Balough A, Szabolcsi Pais L (1985) The effect of titanium and gallium on photosynthetic rate of algae. J Plant Nutr 8:825–831
Daood HG, Biacs P, Fehér M, Hajdu F, Pais I (1998) Effect of titanium on the activity of lipoxygenase. J Plant Nutr 11:505–516
Calabrese EJ, Baldwin IA (2003) Inorganics and hormesis. Crit Rev Toxicol 33:215–304
Hruby M, Cigler P, Kuzel S (2002) Titanium in plant nutrition: the contribution to understanding the mechanism of titanium action in plant. J Plant Nutr 25:577–598
Simon L, Hajdu F, Balogh A, Pais I (1988) Effect of titanium on growth and photosynthetic pigment composition of Chlorella pyrenoidosa (green alga). II. Effect of titanium ascorbate on pigment content and chlorophyll metabolism of chlorella. In: Pais I (ed) New results in the research of hardly known trace element and their role in the food chain. University of Horticultural and Food Science, Budapest, pp 87–101
Carvajal M, Alcaraz CF (1995) Effect of Ti(IV) on Fe activity in organs and organelles of Capsicum annuum L. J Plant Phytochem 35:977–980
Carvajal M, Alcaraz CF (1998) Why titanium is a beneficial element for plants. J Plant Nutr 21:655–664
Larbi A, Abadia A, Abadia J, Morales F (2006) Down co-regulation of light absorption, photochemistry, and carboxylation in Fe-deficient plants growing in different environments. Photosynth Res 89:113–126
Cigler P, Olejnickova J, Hrubyd M, Csefalvay L, Peterka J, Kužel S (2010) Interactions between iron and titanium metabolism in spinach: a chlorophyll fluorescence study in hydropony. J Plant Physiol 167:1592–1597
Kanai S, Adu-Gymf J, Lei K, Ito J, Ohkura K, Moghaieb REA, El-Shemy H, Mohapatra R, Mohapatra PK, Saneoka H, Fujita K (2008) N-deficiency damps out circadian rhythmic changes of stem diameter dynamics in tomato plant. Plant Sci 174:183–191
Le Bot J, Jeannequin B, Fabre R (2001) Growth and nitrogen status of soilless tomato plants following nitrate withdrawal from the nutrient solution. Ann Bot 88:361–370
Stefanelli D, Goodwin I, Jones R (2010) Minimal nitrogen and water use in horticulture: effects on quality and content of selected nutrients. Food Res Int 43:1833–1843
Wang X-L, Shan Y-H, Wang S-H, Du Y, Feng K (2011) Physiological responses of two wheat cultivars to nitrogen starvation. Agric Sci China 10:1577–1585
Guidi I, Lorefice G, Pardossi A, Malorgio F, Tognoni F, Soldatini GF (1998) Growth and photosynthesis of Lycopersicon esculentum (L.) plants as affected by nitrogen deficiency. Biol Plant 40:235–244
Owolade OF, Ogunleti DO, Adenekan MO (2008) Titanium dioxide affects diseases, development and yield of edible cowpea. J Agr Food Chem 7:2942–2947
Chao S-H, Choi H-S (2005) Method for providing enhanced photosynthesis. Korea Research Institute of Chemical Technology, Jeonju, South Korea. Bulletin 10 pp.
Prusiński J, Kaszkowiak E (2005) Effect of titanium on yellow lupin yielding (Lupinus luteus L.). EJPAU 8(2):36
Yang F, Hong F, You W, Liu C, Gao F, Wu C, Yang P (2006) Influences of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol Trace Elem Res 110:179–190
Gao F, Hong F, Liu C, Zheng L, Su M, Wu X, Yang F, Wu C, Yang P (2006) Mechanism of nano-anatase TiO2 on promoting photosynthetic carbon reaction of spinach: Inducing complex of Rubisco–Rubisco activase. Biol Trace Elem Res 111:239–253
Yang F, Liu C, Gao F, Su M, Wu X, Zheng L, Hong F, Yang P (2007) The improvement of spinach growth by nano-anatase TiO2 treatment is related to nitrogen photoreduction. Biol Trace Elem Res 119:77–88
Bedrosian AJ, Hanna WJ (1966) Trace element relationships in New Jersey soils. Soil Sci 101:50–56
Carvajal M, Martínez-Sánchez F, Alcaraz CF (1994) Effect of Ti (IV) on some physiological activity indicators of Capsicum annuum L. plants. J Hort Sci 69:427–432
Giménez JL, Martínez-Sánchez F, Moreno A, Fuentes JL, Alcaraz CF (1990) Titanium in plant nutrition III. Effect of Ti (IV) on yield of Capsicum annuum L. In: Barcello, PIJ (ed.), Proc. III. Symp. Nat. Nutr. Min., pp. 123–128.
Martínez-Sánchez F, Nunez M, Amoros A, Gimenez JL, Alcaraz CF (1993) Effect of titanium leaf spray treatments on ascorbic acid levels of Capsicum annuum L. fruits. J Plant Nutr 16:975–981
Ram N, Verloo M, Cottenie A (1983) Response of bean to foliar spray of titanium. Plant Soil 73:285–290
Pais I (1983) The biological importance of titanium. J Plant Nutr 6:3–131
Siddiqi MY, Kronzucker HJ, Britto DT, Glass ADM (1998) Growth of a tomato crop at reduced nutrient concentrations as a strategy to limit eutrophication. J Plant Nutr 21:1879–1895
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haghighi, M., Heidarian, S. & Teixeira da Silva, J.A. The Effect of Titanium Amendment in N-Withholding Nutrient Solution on Physiological and Photosynthesis Attributes and Micronutrient Uptake of Tomato. Biol Trace Elem Res 150, 381–390 (2012). https://doi.org/10.1007/s12011-012-9481-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9481-y