Abstract
We investigated the functional properties of two 1Sl-encoded high molecular weight glutenin subunits (HMW-GS), 1Slx2.3* and 1Sly16*, from Aegilops longissima L., and developed single-nucleotide polymorphism (SNP)-based molecular markers. Two HMW-GS near-isogenic lines (NILs), HMW-NIL1 (1Ax1, 1Slx2.3* + 1Sly16*, and 1Dx2 + 1Dy12) and HMW-NIL2 (1Ax1, 1Bx17 + 1By18, and 1Dx2 + 1Dy12), were developed by crossing the ‘Chinese Spring’ (CS)-Ae. longissima 1Sl/1B substitution line (CS-1Sl/1B) with the spring wheat cultivar CB037A, and consecutive selfing combined with selection and identification. Quality analysis of the two NILs showed that the 1Slx2.3* + 1Sly16* subunits conferred better dough rheological properties and breadmaking quality than the 1Bx17 + 1By18 subunits; in particular, they significantly improved dough strength, loaf volume and texture, and final bread score. Allele-specific polymerase chain reaction markers for both genes were developed based on SNP variations and successfully amplified 290-bp and 283-bp specific fragments from the 1Slx2.3* and 1Sly16* genes, respectively. Both SNP-based molecular markers were further validated using 38 wheat varieties, two recombination inbred lines, and four F2 generations and showed high specificity and accuracy. Thus, these markers can be used for molecular marker-assisted selection in the early generations of wheat quality improvement programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wheat (Triticum aestivum L.), one of the three main cereal crops cultivated worldwide, represents a major source of energy and nutrients for humans. To support a growing global population and economy, it is vitally important to increase wheat production and enhance grain quality (Tilman et al. 2002; Pfeifer et al. 2014). The wheat endosperm contains gluten proteins, which account for 80–85% of total flour protein and are the major seed storage proteins, found as monomeric gliadins and polymeric glutenins.
Glutenins are formed from high and low molecular weight glutenin subunits (HMW-GS and LMW-GS, respectively) and are vital in dough viscoelasticity (Payne 1987; Payne et al. 1988; Weegels et al. 1996). Although HMW-GS only account for about 5–10% of total flour protein, they are particularly important in conferring dough strength and breadmaking quality (Payne et al. 1988; Ma et al. 2005). HMW-GS are encoded by Glu-A1, Glu-B1, and Glu-D1 loci and each locus consists of two tightly linked genes, named x- and y-type genes according to their size and the sequence of the conserved N-terminal domain (Payne and Lawrence 1983; Halford et al. 1987). Differences among HMW-GS are mainly the result of repeat motif variations in the central repeat domain (Gianibelli et al. 2001).
Considerable work has confirmed the extensive allelic variations present at Glu-1 loci in wheat and related species, which are closely related to dough properties and breadmaking quality (Rasheed et al. 2014). Early work identified and classified 20 alleles at Glu-A1, Glu-B1, and Glu-D1 in around 300 bread wheat cultivars (Payne and Lawrence 1983). Among them, 1Dx5 + 1Dy10 (Glu-D1d), 1Bx7 + 1By8 (Glu-B1b), and 1Bx17 + 1By18 (Glu-B1i) have higher quality scores, whereas 1Dx2 + 1Dy12 (Glu-D1a), 1Bx20 (Glu-B1e), and 1Bx7 + 1By9 (Glu-B1c) are related to poor quality performance (Shewry et al. 1992; Weegels et al. 1996; Gianibelli et al. 2001; Jiang et al. 2018; Guo et al. 2019). However, studies have shown that the allelic variations at Glu-1 loci in the A, B, and D genomes of bread wheat are limited. Thus, discovery of new HMW-GS variations in species related to wheat is important for wheat quality improvement programs.
Among 20 Triticeae groups, Aegilops is the closest relative to Triticum. The genus Aegilops includes 22 species with S, M, C, U, N, D, and T genomes. Among them, Aegilops longissima L. (SlSl, 2n = 2x = 14) is a diploid Aegilops species that has potential genetic resources for wheat genetic improvement, such as resistance genes against powdery mildew (Ceoloni et al. 1992; Cenci et al. 1999; Alberto et al. 2003), eyespot (Sheng et al. 2012; Sheng and Murray 2013), and drought stress (Zhou et al. 2016). In addition, hybridization of Ae. longissima and common wheat yielded hybrid offspring with higher trace element contents (e.g., iron, zinc, copper, manganese, calcium, magnesium, and potassium) (Neelam et al. 2013).
Furthermore, extensive allelic variation in storage protein composition has been found in Aegilops species, including Ae. tauschii (Xie et al. 2001; Yan et al. 2003a; Jiang et al. 2008; Zhang et al. 2008a), Ae. longissima (Wang et al. 2013; Zhu et al. 2015; Hu et al. 2019), Ae. speltoides (Wang et al. 2018), Ae. umbellulata (Wang et al. 2017), Ae. caudata (Wang et al. 2016), Ae. kotschyi, and Ae. juvenalis (Li et al. 2008). In particular, 1Sl-encoded HMW-GS in the 1Sl/1B substitution line had positive effects on breadmaking quality (Wang et al. 2013). Through 1Sl/1A substitution, the grain hardness was reduced, and the gluten strength, formation time, stability time, dough bread volume, and bread score were improved (Garg et al. 2014). These results indicate that Ae. longissima contains potential genetic resources for wheat quality improvement.
In this work, we used two Glu-1 near-isogenic lines (NILs), HMW-NIL1 and HMW-NIL2, to investigate the functional properties of two 1Sl-encoded HMW-GS, 1Slx2.3* and 1Sly16*, and developed single-nucleotide polymorphism (SNP)-based molecular markers. Our results demonstrate that the 1Slx2.3* and 1Sly16* subunits from Ae. longissima confer superior breadmaking quality through improved mixing properties, bread texture, and loaf score and have potential value for wheat quality improvement. The developed SNP-based molecular markers for the 1Slx2.3* and 1Sly16* subunit genes show high specificity and reliability, making them useful for molecular marker-assisted selection in the early generations of wheat quality improvement programs.
Materials and methods
Plant materials and field trial
The experimental materials used in this study included a pair of Glu-B1 NILs (HMW-NIL1 and HMW-NIL2), 38 bread wheat cultivars, two recombination inbred lines (NILs) derived from the crossing between CB037C and the ‘Chinese Spring’ (CS) substitution line (CS-1Sl/1B), and four F2 generation populations from CS-1Sl/1B × ‘Ningchun 4’, CS-1Sl/1B × ‘Wanmai 33’, CS-1Sl/1B × ‘Yumai 34’, and CS-1Sl/1B × ‘Zhongmai 8601’ (Table 1). The NILs HMW-NIL1 (1Ax1, 1Slx2.3* + 1Sly16*, and 1Dx2 + 1Dy12) and HMW-NIL2 (1Ax1, 1Bx17 + 1By18, and 1Dx2 + 1Dy12) were developed by our group through crossing CS-1Sl/1B and the spring wheat cultivar CB037A and consecutive selfing for seven times combined with selection and identification. CS-1Sl/1B with a HMW-GS composition of 1Ax1, 1Slx2.3* + 1Sly16*, and 1Dx2 + 1Dy12 was developed by the Institute for Plant Breeding, Technical University Munich, Germany, in which the chromosome 1Sl from Ae. longissima was substituted by 1B from CS (Wang et al. 2013). CB037A is a wheat-Haynaldia villosa 6VS/6AL translocation line developed at the Capital Normal University and Institute of Crop Science, Chinese Academy of Agricultural Science, China, which carries the Pm21 gene with a HMW-GS composition of 1Ax1, 1Bx17 + 1By18, and 1Dx2 + 1Dy12 and has high resistance to powdery mildew and superior gluten quality (Wu et al. 2017).
Both HMW-NIL1 and HMW-NIL2 were planted in the experimental fields of the China Agricultural University during the 2017–2018 wheat growing season. Field experiments were performed in a randomized block design with three biological replicates (each plot: 20 m2). The crops were cultivated and managed following the same procedures as local field cultivation practices.
Agronomic trait assessment
The mature plants of HMW-NIL1 and HMW-NIL2 from the three biological replicates were harvested, and their main agronomic traits were measured, including plant height, ear length, sturdy spikelet number per spike, spikelet number per spike, grain number per spike, and 1000-grain weight.
HMW-GS extraction and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
We used an improved procedure for seed HMW-GS extraction based on the method of Mackie et al. (1996). The extracted glutenin proteins (0.02 mL of the supernatant) were subjected to SDS-PAGE, as described previously Yan et al. (2003a).
Quality testing
The following grain quality parameters were tested in three biological replicates: ash, protein, and gluten contents; grain hardness; water absorption; Rapid Visco Analyzer (RVA) parameters; Farinograph parameters; C-cell parameters related to bread inner structure; loaf volume; and appearance score. Student’s t test was used for data evaluation. The quality parameters were tested according to Sun et al. (2010) with minor modifications. Grain hardness was measured using the Perten Single Kernel Characterization System 4100 (Perten Instruments, Springfield, IL, USA) according to American Association of Cereal Chemists (AACC) method 55-31. Flour moisture and ash content (% dry basis) were determined according to AACC (2000) methods 44-15A and 08-02, respectively. The flour protein content was determined using a near-infrared analyzer (Instalab 610 NIR Analyzer; DICKEY-john Co. Ltd., Auburn, IL, USA) according to AACC method 39-10A. The RVA profile was obtained using AACC (2000) method 76-21 using a RVA (Series 3; Newport Scientific, Jessup, MD, USA). AACC (2000) methods 54-21 and 54-10 were followed to determine the Farinograph parameters (Farinograph-E; Brabender GmbH & Co. KG, Duisburg, Germany) and tensile strength (Brabender GmbH & Co. KG). Bread baking and evaluation were performed based on AACC method 10-10B according to Jongh (1953). Bread crumb image analysis was performed using C-cell image analysis software and equipment (Calibre Control International Ltd., Warrington, UK) according to Zhen et al. (2016).
Development and validation of SNP-based molecular markers
BioEdit 7.0 was used to compare the sequences of HMW-GS genes cloned from Ae. longissima with previously cloned HMW-GS genes from common wheat varieties. Based on SNP variations, specific primers were designed to develop SNP-based molecular markers. The materials used for marker development and verification included 40 bread wheat varieties and related species with different HMW-GS compositions, four F2 generations, and two RILs (Table 1 ). In total, 50–80 grains from each variety or RIL and 250–300 grains from each F2 generation were detected.
Genomic DNA was extracted using the Phanta Super-Fidelity DNA Polymerase system (Vazyme Biotech, Nanjing, China). The PCR protocol was as follows: initial denaturation at 95 °C for 3 min, 30 cycles at 94 °C for 15 s, 61 °C for 15 s, and 72 °C for 10 s, and a final extension at 72 °C for 5 min. PCR products were analyzed by 1% agarose electrophoresis in Tris-acetic acid-EDTA buffer.
The amplified fragments were purified from the gel using a Gel Extraction Kit (Omega Bio-Tek, Norcross, GA, USA). Purified products were ligated to the CE Vector (Vazyme) and transformed into trans 2-blue receptor cells (TransGen Biotech, Beijing, China). DNA sequencing from five positive clones for each PCR fragment was carried out by TaKaRa Biotechnology (Dalian, China).
Results
Development and characterization of HMW-NIL1 and HMW-NIL2
Two HMW-GS NILs, HMW-NIL1 and HMW-NIL2, were developed via hybridization of the CS-1Sl/1B substitution line with common wheat CB037A and consecutive selection. Phenotype and main agronomic trait analysis showed clear differences between HMW-NIL1 and HMW-NIL2. The phenotypes of plants and ears (with awns) of HMW-NIL1 were similar to those of CB037A, whereas the phenotypes of HMW-NIL2 (without awns) were more similar to those of CS-1Sl/1B (Fig. 1a, b). Compared with HMW-NIL2, spike length, grain number per spike, and 1000-grain weight were highly significantly reduced in HMW-NIL1. Plant height, sturdy spikelet number per spike, and spikelet number per spike showed no significant differences between the NILs (Table 2).
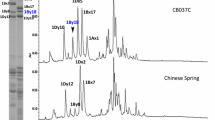
SDS-PAGE analysis of the HMW-GS showed that both HMW-NIL1 and HMW-NIL2 had the same Glu-A1a allele-encoded 1Ax1 subunit and Glu-D1a allele-encoded 1Dx2 + 1Dy12 subunits, but the Glu-B1 locus had different HMW-GS compositions. HMW-NIL1 contained a pair of 1Sl-encoding HMW-GS subunits, 1Slx2.3* + 1Sly16*, whereas HMW-NIL2 had the Glu-B1i-encoded 1Bx17 + 1By18 subunit (Fig. 1c). These results indicate that HMW-NIL1 and HMW-NIL2 are a pair of typical Glu-B1 NILs.
Functional properties of 1Sl-encoded 1Slx2.3* and 1Sly16* subunits
A comprehensive quality analysis of HMW-NIL1 and HMW-NIL2 was performed to assess the functional properties of the 1Sl-encoded 1Slx2.3* and 1Sly16* subunits, including basic quality, RVA, Farinograph, C-cell, and loaf parameters. The basic quality parameters included protein, moisture, and ash contents, gluten over sievies, total gluten, gluten index, and hardness. The results showed that HMW-NIL1 had significantly higher hardness (+ 5%) and highly significantly higher gluten sieving and gluten index (each + 18%) compared to HMW-NIL2. The NILs showed no significant differences in protein, moisture, ash, and total gluten contents (Table 3).
The RVA parameters included peak viscosity, final viscosity, and setback, which are closely associated with starch pasting and dispersion during heating/stirring and cooling/stirring (Qiao et al. 2008). The results revealed significantly lower peak viscosity (− 22%) and final viscosity (− 22%) and significantly increased setback (+ 6%) in HMW-NIL1 compared to HMW-NIL2, indicating inferior starch pasting properties of HMW-NIL1. Farinograph parameters, including water absorption, development time, stability time, and softening degree, reflect the flour mixing properties and dough strength (Liu et al. 2004). Compared to HMW-NIL2, HMW-NIL1 had significantly higher development and stability times, which are positively related to dough strength, but a significantly lower softening degree (− 60%), which is negatively correlated with dough strength. Water absorption did not differ between the NILs (Table 3).
C-cell image and parameter analyses were performed to evaluate the inner structural features of bread slices from HMW-NIL1 and HMW-NIL2, including slice brightness, slice area, wrapper length, attenuation ratio, cell contrast, volume of course cells, average cell elongation, cell diameter, number of cells, and cell density (Table 4). HMW-NIL1 had a significantly higher cell diameter (+ 12%) and significantly lower cell number (− 9%) and density (− 17%). Although the differences in the other parameters between HMW-NIL1and HMW-NIL2 were not significant, several important indicators related to loaf volume (e.g., slice area, wrapper length, and attenuation ratio) were non-significantly increased in HMW-NIL2 (Fig. 2a, Table 4). Loaf parameters, determined according to the evaluation standard of wheat flour bread baking quality in China (GB/T16411-2008), showed that the loaf volume of HMW-NIL1 (963 mL3) was significantly higher than that of HMW-NIL2 (918 mL3) (+ 12%). Ultimately, the loaf inner structure scores and total loaf scores of HMW-NIL1 were 34 (+ 6%) and 96 (+ 5%), respectively, both of which significantly exceeded those of HMW-NIL2 (Fig. 2b, Table 4). These results demonstrate that the 1Slx2.3* + 1Sly16* subunits result in better quality performance than the 1Bx17 + 1By18 subunits, particularly in terms of dough strength, cell size, loaf volume, texture, and score.
Development and validation of SNP-based molecular markers for 1S l x2.3* and 1S l y16* genes
To develop SNP-based molecular markers for the 1Slx2.3* and 1Sly16* genes, BioEdit 7.0 was used to compare different HMW-GS gene sequences to identify specific SNP variations. Then, specific primers were designed and used to amplify the targeted genes. Sequence alignment was performed for the 1Slx2.3* gene and 14 other x-type HMW-GS genes deposited in GenBank (Supplementary Fig. S1): X61009 (1Ax1), M22208 (1Ax2*), X13927 (1Bx7), DQ119142 (1Bx7OE), EF540764 (1Bx13), KF733216 (1Bx14), AB263219 (1Bx17), AJ437000 (1Bx20), AY553933 (1Bx23), X03346 (1Dx2), AY517724 (1Dx2.1), AY159367 (1Dx2.2), AJ893508 (1Dx2.2*), and JX12928 (1Dx5). Two specific SNP sites (A-G and A-G) in 1Slx2.3* were identified, which were located at the signal peptide region (11th base) and the N-terminal non-repeat region (285th base), respectively. A pair of specific primers (1Slx2.3*F: 5′-GGTTAGTCCTCTTTGTGGCGA-3′; 1Slx2.3*R: 5′-CCAAAATATACTTTGTTG GAGTTGT-3′) for amplifying 1Slx2.3* (fragment from bases 11–309) was designed based on the SNP variations. In addition, sequence alignment was performed for the 1Sly16* gene and eight other y-type HMW-GS genes: FJ404595 (1Ay), AY245797 (1By8), X61026 (1By9), KF733215 (1By15), EF540765 (1By16), KF430649 (1By18), X12929 (1Dy10), and BK006459 (1Dy12). Similarly, two specific SNP sites (C-T and T-C) present in 1Sly16* were identified, which were located at the signal peptide region (38th base) and the N-terminal non-repeat region (281th base), respectively (Supplementary Fig. S2). Based on the SNP variations, a pair of specific primers (1Sly16*F: 5′-GTCCTCTTTGCGACAGTAGTAAC-3′; 1Sly16*R: 5′-AGGATCCGC CCTTGGGCA-3′) for amplifying 1Sly16* (fragment from bases 16–298) was designed.
The designed primers were used to amplify the specific fragments of 1Slx2.3* and 1Sly16*, and the SNP-based molecular markers were developed in combination with SDS-PAGE, allele-specific polymerase chain reaction (AS-PCR) amplification, and sequencing. First, the HMW-GS compositions of 38 wheat varieties and two RILs were identified by SDS-PAGE (Fig. 3a), and then, AS-PCR was used to amplify the 1Slx2.3* and 1Sly16* genes (Fig. 3b, c). The results showed that a 290-bp specific fragment was amplified using the 1Slx2.3*F/1Slx2.3*R primer pair from the varieties containing the 1Slx2.3* subunit, whereas no amplified products were obtained in wheat varieties lacking the 1Slx2.3* subunit. Similarly, a 283-bp specific fragment was amplified by the 1Sly16*F/1Sly16*R primer pair from varieties containing the 1Sly16* subunit, but no amplified fragments were obtained from varieties lacking the 1Sly16* subunit. These results were consistent with those from the SDS-PAGE identification. Furthermore, both amplified fragments were collected and sequenced, and the results were consistent with their amplified regions.
Development of SNP-based molecular markers for the 1Slx2.3* and 1Sly16* genes. a Identification of HMW-GSs from different wheat cultivars and RILs by SDS-PAGE. Lanes 1–40 correspond to material numbers 1–40 in Table 1. b AS-PCR amplification of the 1Slx2.3* gene from wheat material numbers 1–40. The 290-bp specific amplified fragment is indicated. c AS-PCR amplification of the 1Sly16* gene from wheat material numbers 1–40. The 283-bp specific amplified fragment is indicated. M: DNA size markers at 10 kb, 8 kb, 6 kb, 5 kb, 4 kb, 3 kb, 2 kb, 1.5 kb, 1 kb, 750 bp, 500 bp, 250 bp, and 100 bp
To further validate the developed molecular markers, two pairs of specific primers were used to amplify the target genes in four F2 generations derived from CS-1Sl/1B × ‘Ningchun 4’, CS-1Sl/1B × ‘Wanmai 33’, CS-1Sl/1B × ‘Yumai 34’, and CS-1Sl/1B × ‘Zhongmai 8601’ crosses. SDS-PAGE was also used to verify the AS-PCR results (Figs. 4 and 5). The results showed that the same 290-bp and 283-bp specific fragments were amplified by the 1Slx2.3*F/1Slx2.3*R and 1Sly16*F/1Sly16*R primer pairs from the four F2 generations containing the 1Slx2.3* and 1Sly16* subunits, respectively. Similarly, no amplified fragments were obtained from the four F2 generations lacking the respective subunits. These results were consistent with the SDS-PAGE identification. Overall, these findings indicate that the developed SNP-based molecular markers have high specificity and accuracy and can effectively identify the 1Slx2.3* and 1Sly16* genes.
Validation of SNP-based molecular marker for 1Slx2.3* and 1Sly16* genes in two F2 generations of CS-1Sl/1B × Ningchun 4 and CS-1Sl/1B × Wanmai 33. a Identification of HMW-GS from the F2 generations (lanes 1–24) of CS-1Sl/1B × Ningchun 4 by SDS-PAGE. b AS-PCR amplification of 1Slx2.3* gene from the F2 generations of CS-1Sl/1B × Ningchun 4 (lane No. 1-24 are same as a). c AS-PCR amplification of 1Sly16* gene from F2 generations of CS-1Sl/1B × Ningchun 4 (Lane No. 1-24 are same as a and b). M: DNA markers with 10 kb, 8 kb, 5 kb, 2 kb, 1.6 kb, 1 kb, 700 bp, 500 bp, 400 bp, 300 bp, 200 bp, and 100 bp. d Identification of HMW-GS from the F2 generations (lanes 1–24) of CS-1Sl/1B × Wanmai 33 by SDS-PAGE. e AS-PCR amplification of 1Slx2.3* gene from the F2 generations of CS-1Sl/1B × Wanmai 33 (lane Nos. 1–24 are same as d). f AS-PCR amplification of 1Sly16* gene from the F2 generations of CS-1Sl/1B × Wanmai 33 (lane Nos. 1–24 are same as d and e). M: DNA markers with 10 kb, 8 kb, 6 kb, 5 kb, 4 kb, 3 kb, 2 kb, 1.5 kb, 1 kb, 750 bp, 500 bp, 250 bp, and 100 bp
Validation of the SNP-based molecular markers for 1Slx2.3* and 1Sly16* genes in two F2 generations of CS-1Sl/1B × Yumai 34 and CS-1Sl/1B × Zhongmai 8601. a Identification of HMW-GS from the F2 generations (lanes 1–24) of CS-1Sl/1B × Yumai 34 by SDS-PAGE. b AS-PCR amplification of 1Slx2.3* gene from the F2 generations of CS-1Sl/1B × Yumai 34 (lane Nos. 1–24 are same as a). c AS-PCR amplification of 1Sly16* gene from F2 generations of CS-1Sl/1B × Yumai 34 (lane Nos. 1–24 are same as a and b). M: DNA markers with 10 kb, 8 kb, 5 kb, 2 kb, 1.6 kb, 1 kb, 700 bp, 500 bp, 400 bp, 300 bp, 200 bp, and 100 bp. d Identification of HMW-GS from the F2 generations (lanes 1–24) of CS-1Sl/1B × Zhongmai 8601 by SDS-PAGE. e AS-PCR amplification of 1Slx2.3* gene from the F2 generations of CS-1Sl/1B × Zhongmai 8601 (lane Nos. 1–24 are same as d). f AS-PCR amplification of 1Sly16* gene from the F2 generations of CS-1Sl/1B × Zhongmai 8601 (lane Nos. 1–24 are same as d and e). M: DNA markers with 10 kb, 8 kb, 6 kb, 5 kb, 4 kb, 3 kb, 2 kb, 1.5 kb, 1 kb, 750 bp, 500 bp, 250 bp, and 100 bp
Discussion
Function of the 1Slx2.3* and 1Sly16* subunits and their potential value in wheat quality improvement
HMW-GS have been shown to be more important than gliadins and LMW-GS in determining dough rheological properties (Ma et al. 2005). Breadmaking quality largely depends on the number and composition of HMW-GS (Burnouf and Bouriquet 1980; Altpeter et al. 1996; Yan et al. 2004). Furthermore, the yield factors such as spike grain number and grain weight have no significant effects on processing quality (Zhao et al. 1994; Wang et al. 1995). Our results from a pair of Glu-B1 NILs indicated that the 1Sl-encoded HMW-GS 1Slx2.3* and 1Sly16* from Ae. longissima conferred superior dough rheological and bread qualities, including significantly higher grain hardness, gluten index, development and stability times, loaf cell size, volume, inner structure, and score compared to the 1Bx17 + 1By18 subunits (Fig. 2, Tables 3 and 4). Thus, although two NILs have significant differences in main yield traits (Table 2), the allelic variations at Glu-B1 locus could be responsible for their quality differences.
Grain hardness is closely related to the end-use quality of wheat flour. According to endosperm texture, common wheat is usually classified as soft wheat, hard wheat, or mixed wheat. Soft wheat generally has a lower damaged starch content and smaller flour granules, whereas hard wheat has a higher damaged starch content and larger flour granules due to the smaller intercellular space of the starch granules. Therefore, flour made from hard wheat is generally more suitable for making bread (Ma et al. 2010). The gluten-sieving parameter is determined via centrifugation of a gluten solution (total gluten), which forces gluten through a sieve plate, where a lower value of sieved gluten indicates higher gluten strength. The related gluten index is the ratio of gluten on the sieve to the total gluten content, and a higher gluten index represents stronger gluten (Wang 2013). Development time and stability time are two important parameters for estimating dough strength (Wang et al. 1997; Kari et al. 2003). Development time refers to the mixing time from the start of adding water to the maximum consistency of the dough. A higher gluten content in dough results in greater elasticity, increased mechanical energy consumption, and a prolonged time for final dough development (Wang et al. 1997). Dough stability time refers to the time from the initial time until reaching 500 BU, which reflects dough resistance. Longer stability times represent better dough toughness and greater gluten strength (Wang et al. 1997). The significant promotion of grain hardness, gluten index, and dough rheological properties by the 1Sl-encoded 1Slx2.3* and 1Sly16* subunits led to significant improvements in bread texture, loaf volume, and score (Table 4).
The allelic variations at the Glu-1 loci are closely related to flour quality, and individual subunits and subunit combinations have different contributions to quality formation. Previous studies found that the 1Dx5 + 1Dy10 subunit combination encoded by Glu-D1d was associated with the highest quality scores, whereas the 1Bx17 + 1By18 subunit combination encoded by Glu-B1i ranked second (Shewry et al. 1992). A recent report from our group based on analysis of a set of Glu-1 NILs confirmed that the 1Bx17 + 1By18 subunit combination is second only to the Dx5 + Dy10 subunit combination (Jiang et al. 2018). In the present study, the 1Sl-encoded 1Slx2.3* + 1Sly16* subunits endowed better dough rheological properties and breadmaking quality than the 1Bx17 + 1By18 subunits (Table 4), indicating that 1Slx2.3* + 1Sly16* may represent a new superior set of subunits for the improvement of breadmaking quality.
Molecular characterization revealed that both 1Slx2.3* and 1S1y16* subunits have relatively long repeat domains. In particular, 1Slx2.3* has an additional 102-amino acid residue insertion in the central repeat domain, and 1S1y16*, as one of the largest y-type HMW subunits characterized to date, contains 749 amino acid residues (Wang et al. 2013). The long repeat domain is thought to have a positive effect on wheat flour quality (Masci et al. 1998, 2000; Belton 1999), which can form more β-turn structures, imparting protein molecular elasticity (Tatham et al. 1985; Gianibelli et al. 2001). Insertions in the central repeat domain can directly affect functional properties (Hassani et al. 2005). Thus, the additional 102-amino acid residue insertion in the central repeat domain of the 1Slx2.3* subunit may have a positive effect on the viscoelastic properties of dough. The y-type HMW-GSs are also considered to be main components of the technical characteristics of flour (D’Ovidio and Anderson 1994). These structural features may reflect the important roles of 1Slx2.3* and 1S1y16* in determining gluten quality for improving wheat breadmaking quality.
SNP-based molecular markers for 1S l x2.3* and 1S l y16* genes and wheat quality improvement applications
Various methods have been developed and used to separate and identify HMW-GS, including SDS-PAGE, reversed-phase high-performance liquid chromatography, high-performance capillary electrophoresis, reversed-phase ultra-performance liquid chromatography, and mass spectrometry (Yan et al. 2003a, b; Zhang et al. 2008b; Gao et al. 2010; Yan et al. 2014). However, these methods for identifying individual protein subunits are based on protein physicochemical properties, such as isoelectric point and molecular mass, and present various disadvantages. For example, the widely used SDS-PAGE cannot accurately distinguish subunits with similar molecular weights and electrophoretic mobilities because the electrophoretic mobility of the radical is not always consistent with its molecular weight. It is also time-consuming, requires the use of toxic reagents, and is destructive. Meanwhile, liquid chromatography, capillary electrophoresis, and mass spectrometry require expensive analytical instruments, restricting their use.
SNPs are considered third-generation molecular markers and are a powerful tool in marker-assisted breeding (Rafalski 2002). SNP-based AS-PCR markers appear to be capable of effectively identifying HMW-GS genes. In general, PCR-based molecular markers are fast, cost-efficient, and subject to few restrictions, allowing for high-throughput selection during marker-assisted selection for wheat quality improvement. Various molecular markers for HMW-GS genes have been developed (Ahmad 2000; Liang et al. 2015; Shiri et al. 2015). These markers can effectively screen and identify the target HMW-GS genes from different varieties and isolated populations and have potential value for marker-assisted selection in wheat quality improvement programs.
In this study, we developed and validated two specific SNP-based markers for the 1Slx2.3* and 1Sly16* genes using different wheat cultivars, RILs, and F2 generations (Figs. 3, 4, and 5). Both SNP-based markers showed high specificity and accuracy and can be used for marker-assisted selection of the superior 1Slx2.3* and 1Sly16* genes in wheat quality improvement programs. Such molecular markers are expected to accelerate breeding processes and promote gluten quality improvement.
Conclusion
Analysis of two NILs, HMW-NIL1 (1Ax1, 1Slx2.3* + 1Sly16*, and 1Dx2 + 1Dy12) and HMW-NIL2 (1Ax1, 1Bx17 + 1By18, and 1Dx2 + 1Dy12), showed that the 1Sl-encoded 1Slx2.3* + 1Sly16* subunits confer better dough rheological properties and breadmaking quality than 1Bx17 + 1By18 subunits via improvements in dough strength, cell size, loaf volume, texture, and score. Subsequently, two specific SNP-based molecular markers for 1Slx2.3* (290 bp) and 1Sly16* (283 bp) were developed and validated. These markers can be used for molecular marker-assisted selection in early generations for wheat quality improvement to improve breeding efficiency.
Abbreviations
- AS-PCR:
-
allelic-specific polymerase chain reaction
- HMW-GS:
-
High molecular weight glutenin subunits
- HPCE:
-
high-performance capillary electrophoresis
- LMW-GS:
-
Low molecular weight glutenin subunits
- MAS:
-
Marker-assisted selection
- MS:
-
mass spectrometry
- NILs:
-
near-isogenic lines
- RILs:
-
recombination inbred lines
- RP-HPLC:
-
reversed-phase high-performance liquid chromatography
- RP-UPLC:
-
reversed-phase ultra-performance liquid chromatography
- RVA:
-
Rapid Visco Analyzer
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SNPs:
-
Single-nucleotide polymorphisms
References
Ahmad M (2000) Molecular marker-assisted selection of HMW glutenin alleles related to wheat bread quality by PCR-generated DNA markers. Theor Appl Genet 101:892–896
Alberto C, Renato D, Antonio TO, Carla C, Marina P, Enrico P (2003) Genetic analysis of the Aegilops longissima, 3S chromosome carrying the Pm13, resistance gene. Euphytica 130:177–183
Altpeter F, Vasil V, Srivastava V, Vasil IK (1996) Integration and expression of the high molecular weight glutenin subunit 1Ax1 gene into wheat. Nat Biotechnol 14:1155–1199
Belton PS (1999) On the elasticity of wheat gluten. J Cereal Sci 29:103–107
Burnouf T, Bouriquet R (1980) Glutenin subunits of genetically related European hexaploid wheat cultivars: their relationships with breadmaking quality. Theor Appl Genet 58:107–111
Cenci A, D’Ovidio R, Tanzarella OA, Ceoloni C, Porceddu E (1999) Identification of molecular markers linked to Pm13, an Aegilops longissima gene conferring resistance to powdery mildew in wheat. Theor Appl Genet 98:448–454
Ceoloni C, Signore GD, Ercoli L, Donini P (1992) Locating the alien chromatin segment in common wheat Aegilops longissima mildew resistant transfers. Hereditas 116:239–245
D’Ovidio R, Anderson O (1994) PCR analysis to distinguish between alleles of a member of a multigene family correlated with wheat bread making quality. Theor Appl Genet 88:759–763
Gao LY, Ma WJ, Chen J, Wang K, Li J, Wang SL, Bekes F, Appels R, Yan YM (2010) Characterization and comparative analysis of wheat high molecular weight glutenin subunits by SDS-PAGE, RP-HPLC, HPCE, and MALDITOF-MS. J Agric Food Chem 58:2777–2786
Garg M, Kumar R, Singh RP, Tsujimoto H (2014) Development of an Aegilops longissima, substitution line with improved bread-making quality. J Cereal Sci 60:389–396
Gianibelli MC, Larroque OR, Macritchie F, Wrigley CW (2001) Biochemical, genetic, and molecular characterization of wheat glutenin and its component subunits. Cereal Chemistry 78:635–646
Guo HY, Wu JS, Lu YX, Yan YM (2019) High molecular weight glutenin 1Bx17 and 1By18 subunits encoded by Glu-B1i enhance wheat dough rheological properties and breadmaking quality. Journal of Food Quality ID1958747
Halford NG, Forde J, Anderson OD, Greene FC, Shewry PR (1987) The nucleotide and deduced amino acid sequences of an HMW glutenin subunit gene from chromosome 1b of bread wheat (Triticum aestivum L.) and comparison with those of genes from chromosomes 1A and 1D. Theor Appl Genet 75:117–126
Hassani ME, Gianibelli MC, Shariflou MR, Sharp PJ (2005) Molecular structure of a novel y-type HMW glutenin subunit gene present in Triticum tauschii. Euphytica 141:191–198
Hu JX, Jian W, Deng X, Yan YM (2019) Cloning and characterization of special HMW glutenin subunit genes from Aegilops longissima L. and their potential for wheat quality improvement. 3 Biotech 9:267
Jiang CX, Pei YH, Zhang YZ, Li XH, Yao DN, Yan YM, Ma WJ, Hsam SLK, Zeller FJ (2008) Molecular cloning and characterization of four novel LMW glutenin subunit genes from Aegilops longissima, Triticum dicoccoides and T zhukovskyi. Hereditas 145:92–98
Jiang PH, Xue JS, Duan LN, Gu YS, Mu JY, Han SC, Chen L, Li YX, Ma WJ, Yan YM, Li XH (2018) Effects of high-molecular-weight glutenin subunit combination in common wheat on the quality of crumb structure. J Sci Food Agric 99:1501–1508
Jongh G (1953) The baking value of wheat varieties grown in the Netherlands. Euphytica 2:6–14
Kari MT, Ellen MM, Pernille B (2003) Comparison of small and large deformation rheologic properties of wheat dough and gluten. Cereal Chemistry 80:587–595
Li XH, Ma WJ, Gao LY, Zhang YZ, Wang AL, Ji KM, Wang K, Appels R, Yan YM (2008) A novel chimeric low-molecular-weight glutenin subunit gene from the wild relatives of wheat Aegilops kotschyi and Ae. juvenalis: evolution at the Glu-3 loci. Genetics 180:93–101
Liang XN, Zhen SM, Han CX, Wang C, Li XH, Ma WJ, Yan YM (2015) Molecular characterization and marker development for hexaploid wheat-specific HMW glutenin subunit 1By18 gene. Mol Breed 35:221
Liu YL, Tian JC, Han XM, Deng ZY (2004) Comparison of different dough rheological measurement and path coefficient analysis of bread quality. Agicultural Sciences in China 3:468–474
Ma W, Appels R, Bekes F, Larroque O, Morell MK, Gale KR (2005) Genetic characterisation of dough rheological properties in a wheat doubled haploid population: additive genetic effects and epistatic interactions. Theor Appl Genet 111:410–422
Ma DY, Zhang Y, Xia XC, Morris MCF, He ZH (2010) Wheat flour, pan bread, and steamed bread qualities of common wheat near-isogenic lines differing in puroindoline b alleles: wheat flour, pan bread, and steamed bread qualities of common wheat near-isogenic lines differing in puroindoline b alleles. Acta Agron Sin 36:261–266
Mackie AM, Sharp PJ, Lagudah ES (1996) The nucleotide and derived amino acid sequence of a HMW glutenin gene from Triticum tauschii and comparison with those from the D genome of bread wheat. J Cereal Sci 24:73–78
Masci S, D’Ovidio R, Lafiandra D, Kasarda DD (1998) Characterization of a low-molecular-weight glutenin subunit gene from bread wheat and the corresponding protein that represents a major subunit of the glutenin polymer. Plant Physiol 118:1147–1158
Masci S, D’Ovidio R, Lafiandra D, Kasarda DD (2000) A 1B coded low-molecular-weight glutenin subunit associated with quality in durum wheats show strong similarity to subunits present in some bread wheat cultivars. Theor Appl Genet 100:396–400
Neelam K, Rawat N, Tiwari VK, Ghandhi N, Arun PC, Kumar S, Tripathi SK, Randhawa GS, Prasad R, Dhaliwal HS (2013) Development and molecular characterization of wheat-Aegilops longissima derivatives with high grain micronutrients. Aust J Crop Sci 7:508–514
Payne PI (1987) Genetics of wheat storage proteins and the effect of allelic variation on bread-making quality. Annu Rev Plant Physiol 38:141–153
Payne PI, Lawrence GJ (1983) Catalogue of alleles for the complex gene loci, Glu-A1, Glu-B1 and Glu-D1 which code for high molecular weight subunits of glutenin in hexaploid wheat. Cereal Res Commun 11:29–35
Payne PI, Holt LM, Krattiger AF, Carrillo JM (1988) Relationships between seed quality characteristics and HMW glutenin subunit composition determined using wheats grown in Spain. J Cereal Sci 7:229–235
Pfeifer M, Kugler KG, Sandve SR, Zhan BJ, Rudi H, Hvidsten TR, Mayer KFX, Olsen O (2014) Genome interplay in the grain transcriptome of hexaploid bread wheat. Science 345:1250091
Qiao YQ, Ma CX, Huang ZL, Si HQ, Cai H, Xia YX (2008) Stability analysis on rva and relationship with major quality traits in common wheat. Acta Laser Biology Sinica 17:587–592
Rafalski A (2002) Applications of single nucleotide polymorphisms in crop genetics. Curr Opin Plant Biol 5:94–100
Rasheed A, Xia XC, Yan YM, Appels R, Mahmood T, He ZH (2014) Wheat seed storage proteins: advances in molecular genetics, diversity and breeding applications. J Cereal Sci 60:11–24
Sheng HY, Murray TD (2013) Identifying new sources of resistance to eyespot of wheat in Aegilops longissima. Plant Disease 97:346–353
Sheng HY, See DR, Murray TD (2012) Mapping QTL for resistance to eyespot of wheat in Aegilops longissima. Theor Appl Genet 125:355–366
Shewry PR, Halford NG, Tatham AS (1992) High molecular weight subunits of wheat glutenin. J Cereal Sci 15:105–120
Shiri Y, Solouki M, Forootan M (2015) Analysis of allelic variation in hmw-glu-1 gene blocks in iranian wheat cultivars using alp molecular marker. Cercetari Agronomice in Moldova 48:51–60
Sun H, Yan SP, Jiang WL, Li GT, MacRitchie F (2010) Contribution of lipid to physicochemical properties and Mantou-making quality of wheat flour. Food Chem 121:332–337
Tatham AS, Miflin BJ, Shewry PR (1985) The beta-turn conformation in wheat gluten proteins: relationship to gluten elasticity. Cereal Chem 62:405–412
Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S (2002) Agricultural sustainability and intensive production practices. Nature 418:671–677
Wang MF (2013) The relationship between protein quality and starch pasting parameters and bread baking quality in common wheat. Journal of Nuclear Agricultural Sciences 27:792–799
Wang R, Li SB, Zhao CP, Wang GR, Pene RJ, Jorge ZH (1995) The relation between more spikelet and hige molecular weight subunit compositions in hexaploid wheat. Acta Botanica Boerall-Occidentalla Sinica 15:265–269
Wang GR, Zhou GY, Wang R (1997) The correlation between baking quality and dough development time and stability time. Journal of the Chinese Cereal and Oil Association 12:1–6
Wang SL, Yu ZT, Cao M, Shen XX, Li N, Li XH, Ma WJ, Weißgerber H, Zeller F, Hsam S, Yan YM (2013) Molecular mechanisms of HMW glutenin subunits from 1Sl genome of Aegilops longissima positively affecting wheat breadmaking quality. PLoS One 8:e58947
Wang C, Shen XX, Wang K, Liu YL, Zhou JW, Hu YH, Zeller FJ, Hsam SLK, Yan YM (2016) Molecular characterization and functional properties of two novel x-type HMW-GS from wheat line CNU608 derived from Chinese spring × Ae. caudata cross. J Cereal Sci 68:16–24
Wang J, Wang C, Zhen SM, Li XH, Yan YM (2017) Low molecular weight glutenin subunits from the 1U genome of Aegilops umbellulata confer superior dough rheological properties and improve breadmaking quality of bread wheat. J Sci Food Agric 98:2156–2167
Wang W, Wang K, Chen X, Prodanovic S, Li XH, Ye XG, Yan YM (2018) Molecular characterization and phylogenetic analysis of one Omega-gliadin gene from Aegilops speltoides L. Genetika 50:503–517
Weegels PL, Pijpekamp AMVD, Graveland A, Hamer RJ, Schofield JD (1996) Depolymerisation and re-polymerisation of wheat glutenin during dough processing and relationships between glutenin macropolymer content and quality parameters. J Cereal Sci 23:103–111
Wu JS, Lu XB, Yu ZT, Han CX, Li XH, Prodanovic S, Yan YM (2017) Effects of Glu-1 and Glu-3 allelic variations on wheat glutenin macropolymer (GMP) content as revealed by size-exclusion high performance liquid chromatography (SE-HPLC). Genetika 49:677–691
Xie RL, Wan YF, Zhang WY, Wang DW (2001) HMW glutenin subunits in multiploid Aegilops species: composition analysis and molecular cloning of coding sequences. Chinese Science Bulletin 4:309–313
Yan YM, Hsam SLK, Yu JZ, Jiang Y, Zeller FJ (2003a) Allelic variation of the HMW glutenin subunits in Aegilops tauschii accessions detected by sodium dodecyl sulphate (SDS-PAGE), acid polyacrylamide gel (A-PAGE) and capillary electrophoresis. Euphytica 130:377–385
Yan YM, Yu JZ, Jiang Y, Hu YK, Cai MH, Hsam SLK, Zeller FJ (2003b) Capillary electrophoresis separation of high molecular weight glutenin subunits in bread wheat (Triticum aestivum L.) and related species with phosphate-based buffers. Electrophoresis 24:1429–1436
Yan Y, Zheng J, Xiao Y, Yu J, Hu Y, Cai M, Li Y, Hsam SLK, Zeller FJ (2004) Identification and molecular characterization of a novel y-type Glu-D t 1 glutenin gene of Aegilops tauschii. Theor Appl Genet 108:1349–1358
Yan X, Liu W, Yu ZT, Han CX, Zeller FJ, Hsam SLK, Yan YM (2014) Rapid separation and identification of wheat HMW glutenin subunits by UPLC and comparative analysis with HPLC. Aust J Crop Sci 8:140–147
Zhang YZ, Li X, Yan YM, Xiong X, An XL, Zhang Q, Pei YH, Gao LY, Hsam SLK, Zeller FJ (2008a) Molecular characterization of two novel x-type HMW glutenin genes from Aegilops tauschii and implications for the evolution of Glu-D1-1 alleles. Genetics 178:23–33
Zhang Q, Dong YM, An XL, Wang AL, Zhang YZ, Li XH, Gao LY, Xia XC, He ZH, Yan YM (2008b) Characterization of HMW glutenin subunits in common wheat and related species by matrix-assisted laser desorption/ionization time of-flight mass spectrometry (MALDI-TOF-MS). J Cereal Sci 47:252–261
Zhao H, Lu SY, Li ZZ (1994) Studies on inheritance and variation of hmw glutenin subunits and their correlation with quality and other agronomic characters in wheat. Acta Agronomica Sinica 20:67–75
Zhen SM, Zhou JX, Deng X, Zhu GR, Cao H, Wang ZM, Yan YM (2016) Metabolite profiling of the response to high-nitrogen fertilizer during grain development of bread wheat (Triticum aestivum L.). J Cereal Sci 69:85–94
Zhou JX, Ma CY, Zhen SM, Cao M, Zeller FJ, Hsam SLK, Yan YM (2016) Identification of drought stress related proteins from 1S1(1B) chromosome substitution line of wheat variety Chinese Spring. Bot Stud 57:2–10
Zhu GR, Wang SL, Zhen SM, Shen XX, Prodanovic S, Yan YM (2015) Molecular characterization and phylogenetic analysis of unusual x-type HMW glutenin subunits from 1Sl genome of Aegilops longissima. Genetika 47:185–203
Acknowledgments
The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/9VVQDl.
Funding
This research was financially supported by grants from National Key R. & D. Program of China (2016YFD0100502) and the National Natural Science Foundation of China (31771773).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cui, D., Wang, J., Li, M. et al. Functional assessment and SNP-based molecular marker development of two 1Sl-encoded HMW glutenin subunits in Aegilops longissima L.. Mol Breeding 39, 120 (2019). https://doi.org/10.1007/s11032-019-1025-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-019-1025-0