Abstract
Repeated blocks in the TaALMT1 promoter as well as a transposon insertion in the TaMATE1B upstream region have been correlated with the level of gene expression, organic acid efflux, and ultimately aluminum (Al3+) resistance in wheat (Triticum aestivum L.). In this study, we investigated the allelic polymorphism related to the TaALMT1 and TaMATE1B promoter regions in 300 Brazilian wheat genotypes and the correlation of that variation with root growth on acid soil. In addition, SSR markers were used to determine the genetic variability of the genotypes. Seven TaALMT1 promoter alleles (Types I–VII) were detected based on size of PCR products. The most common alleles were Type V and Type VI (71.3 and 11.9 %, respectively), and these are generally associated with higher levels of TaALMT1 expression and Al3+ resistance. The promoter alleles Type I and Type II, which are usually associated with Al3+ sensitivity, were detected in 12.2 % of the genotypes. The insertion in the TaMATE1B promoter, associated with greater Al3+ resistance, was identified in 80 genotypes. Combination among the alleles allowed the separation in 12 haplotypes were 68 genotypes presented the TaALMT1 promoters Type V and Type VI along with the transposon insertion in the TaMATE1B promoter. However, the most represented haplotype was Type V without the insertion (176 genotypes). Short-term soil experiment, performed in 33 genotypes representing the 12 haplotypes, revealed that the higher relative root length was observed in some genotypes presenting TaALMT1 promoters Type V or Type VI and the transposon insertion in the TaMATE1B promoter. Moreover, when comparing genotypes inside the same haplotype, the transposon insertion was significantly advantageous for a few materials. However, the majority the genotypes presenting the insertion in the TaMATE1B promoter did not outperform the genotypes without the insertion but showing the same TaALMT1 promoter. Analysis using SSR markers, with an average PIC of 0.60, showed high genetic diversity among the genotypes belonging to different haplotypes. The alleles and the genetically diverse genotypes reported here should be considered for wheat-breeding programs aiming increments in wheat Al3+ resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many plant species cope with the aluminum (Al3+) present in acid soils (pH ≤ 5.5) using mechanisms classified as resistance (exclusion of Al3+ from root and shoot tissues) or tolerance (safe accommodation of Al3+ once it enters the cell). An Al3+ resistance mechanism present in many species is the efflux of organic acids (citrate, malate or oxalate) from root apices into the apoplasm (Liu et al. 2014). This mechanism relies on the formation of a complex between the organic acid(s) released by the root tips and the Al3+ present in the soil; these complexes are less damaging to the root tip, consequently reducing Al3+ toxicity to the plant (Kochian et al. 2015).
In wheat (Triticum aestivum L.), two organic anions (malate and citrate) have been reported to detoxify the Al3+ around the root tip (Delhaize et al. 1993; Ryan et al. 2009), and the membrane transporters encoded by the genes TaALMT1 (chromosome 4D) and TaMATE1B (chromosome 4B) are responsible for the efflux of these anions by the root apex (Sasaki et al. 2004; Raman et al. 2005; Tovkach et al. 2013). Although differences in the TaALMT1 and TaMATE1B structural region can be detected (Sasaki et al. 2004; Raman et al. 2005, 2008; Tovkach et al. 2013), it is the level of gene expression that shows higher correlation to the Al3+ resistance/sensitivity. For instance, the overexpression of those genes have been reported to transgenically enhance the amount of malate or citrate released by the root tip (Sasaki et al. 2004; Delhaize et al. 2004; Zhang et al. 2008; Pereira et al. 2010; Ryan et al. 2011; Tovkach et al. 2013). Ultimately, the increase in organic acid release enhances the Al3+ resistance. The link between higher TaALMT1 expression and higher Al3+ resistance was also observed in conventional wheat lines of non-Japanese origin where the number of repeats of four blocks (A, B, C and D) in the TaALMT1 promoter was correlated with the level of TaALMT1 expression, malate efflux, and Al3+ resistance (Sasaki et al. 2006). It is the presence of these tandem repeated elements in the TaALMT1 promoter region that contribute directly to the Al3+ resistance of hexaploid wheat (Ryan et al. 2010). Similarly, greater Al3+ resistance in wheat has also been correlated with citrate efflux from root tips and the expression of the TaMATE1B gene (Tovkach et al. 2013). Genotypes with a transposon-like insertion upstream of the TaMATE1B coding region have stronger expression at the root apex and greater citrate efflux (Tovkach et al. 2013; Garcia-Oliveira et al. 2014). The transposon-like insertion near TaMATE1B as well as the repeated elements in the TaALMT1 promoter can be discriminated by PCR (Sasaki et al. 2006; Garcia-Oliveira et al. 2014). Up to date, nine alleles for the TaALMT1 promoter have been described this way (Sasaki et al. 2006; Raman et al. 2008; Ryan et al. 2010; Delhaize et al. 2012b), while the transposon insertion in the TaMATE1B promoter can be distinguished by the presence or absence of a PCR product (Tovkach et al. 2013; Garcia-Oliveira et al. 2014). In general, genotypes with the Type V and Type VI alleles of TaALMT1 (greater number of repeats in the promoter) show intermediate to high levels of expression and are more resistant to Al3+ (Sasaki et al. 2006; Raman et al. 2008). The transposon-like insertion near TaMATE1B is associated with constitutive citrate efflux by the root tip and higher Al3+ resistance (Tovkach et al. 2013; Garcia-Oliveira et al. 2014).
Although a number of wheat genotypes have been analyzed for the TaALMT1 and TaMATE1B promoter polymorphism (Sasaki et al. 2006; Raman et al. 2008; Ryan et al. 2010; Garcia-Oliveira et al. 2014), only a few Brazilian wheat genotypes have been characterized, even though Brazilian wheat is an important source of Al3+ resistance. For instance, Brazilian genotypes BH 1146, Carazinho, Frontana, Fronteira, IAC 5 - Maringá, Toropi, Trintani, Trintecinco, and Veranópolis have been used in different studies investigating Al3+ resistance mechanisms, and all of them have been recognized as important Al3+-resistant genotypes (de Sousa 1998; Garvin and Carver 2003; Raman et al. 2008; Ryan et al. 2009; Tovkach et al. 2013). Because 60 % of the soil in tropical and subtropical regions is acidic (Von Uexküll and Mutert 1995), improved Al3+ resistance remains an important objective for wheat-breeding programs. In Brazil, 63 % of the territory is composed by acidic soil (FAO 2000), and Al3+ toxicity is an important constraint in south and central regions (Echart and Cavalli-Molina 2001; Yamada 2005) where practically all wheat is grown.
Different methodologies can be used to assess the Al3+ resistance/tolerance in wheat, and a high correlation has been reported between field trials and root length in hydroponics (Baier et al. 1995) and in pots in glasshouses (Tang et al. 2003). Although studies have demonstrated that Brazilian wheat show a diverse level of Al3+ resistance (Camargo and Oliveira 1981; Camargo et al. 1987, 2006; Bertan et al. 2006; Raman et al. 2008), no reports have evaluated the genetic variability among Al3+-resistant Brazilian wheat cultivars. Genetic diversity studies have been performed in wheat genotypes from Brazil using SSR markers (Huang et al. 2002; Warburton et al. 2006; Zhou et al. 2007; Hu et al. 2008; Schuster et al. 2009) as these markers have been reported to be highly suitable and very effective for the assessment of genetic diversity in wheat (Röder et al. 1998; Huang et al. 2002). Although most papers have used a small number of Brazilian genotypes, a highly variable wheat germplasm adapted for use in Brazil has been reported (Schuster et al. 2009). Because breeding programs depend on the genetic variability that can be exploited in their germplasm, it is important to access the genetic diversity among Brazilian genotypes with superior Al3+ resistance.
In this context, the objective of this work was to assess the allelic polymorphism in the TaALMT1 and TaMATE1B promoters in a collection of 300 Brazilian wheat genotypes and to link these polymorphisms with root growth on acidic soil and with genetic variability detected by SSR markers. Our goal also extended to the identification of TaALMT1 and TaMATE1B promoters that are associated with the highest levels of Al3+ resistance.
Materials and methods
Genotypes
Seeds of the 300 Brazilian wheat genotypes (Table 1), belonging to different wheat-breeding programs, were provided by the Embrapa Active Wheat Germplasm Bank.
DNA extraction
Seeds were pre-germinated in the dark at 23 °C for 48 h. Five to ten germinated seeds were transferred to pots containing a mix of soil, vermiculite, and sand (1:1:1). The pots were placed on a laboratory bench under ambient light and irrigated daily. After 7 days, leaves of the seedlings were collected, transferred to a 2-ml Eppendorf tube containing three stainless steel beads (2.3 mm diameter), and frozen in liquid nitrogen. Leaf tissue was triturated in a Mini-Beadbeater™ (Biospec Products) for 1 min. Total DNA was extracted using a CTAB-based protocol (Doyle and Doyle 1987). After quantification on an agarose gel, 100–150 ng of DNA was used for PCR.
Detection of the allelic variability in the TaALMT1 and TaMATE1B promoters
To amplify the different alleles of the TaALMT1 promoter, primers LPF-F (CCTGGTTTTCTTGATGGGGGCACA) and LPF-R (TGCCCACCATCTCGCCGTCGCTCTCTCT) were used according to Sasaki et al. (2006). If promoters Type I or II (1190 or 1221 bp) were identified, an additional amplification with primers SPF-F (GCTCCTACCACTATGGTTGCG) and SPF-R (CCAGGCCGACTTTGAGCGAG) was performed to allow better allele discrimination (612 or 643 bp). To detect the presence of the transposon insertion in the TaMATE1B promoter, primers TaMATE1-4B-SLT-F (ATCCATCCTCCTTCCCTCAC) and TaMATE1-4B-SLT-R (ATGAATGCTGTGTCCACCAA) were used according to Garcia-Oliveira et al. (2014). The reactions were carried out in a final volume of 20 µl containing 1× PCR buffer, 0.3 mM each dNTPs, 1× Q solution, 0.3 µM each primer, and one-unit Taq DNA Polymerase (Qiagen). Amplification programs were performed as described by Sasaki et al. (2006) and Garcia-Oliveira et al. (2014). PCR fragments were run on a 1 % (LPF primers), 1.25 % (SPF primers), or 1.5 % (TaMATE1B primers) agarose gel, stained with ethidium bromide, and detected as shown in Fig. 1. Since a negative amplification with the TaMATE1B primers is related to the absence of the insertion but could also be associated with poor DNA quality or mistakes in PCR preparation, we executed controls by adding a positive sample in each set of amplification, replicating the PCR (using the same DNA) and re-extracting and re-amplifying DNA from 121 samples.
TaALMT1 and TaMATE1B promoter alleles identified in this study. a Type I–VII as described in Sasaki et al. (2006) and Raman et al. (2008). ‘M’ indicates marker (shown at left in kb), ‘I’ indicates the Type I promoter (1190 bp/cultivar Caçador 2); ‘II’, Type II (1221 bp/cultivar IAS 15 - Campeiro); ‘III’, Type III (1993 bp/Ocepar 17); ‘IV’, Type IV (1470 bp/cultivar Ocepar 19); ‘V’, Type V (1750 bp/IAS 2); ‘VI’, Type VI (1600 bp/cultivar IAS 52); and ‘VII’, Type VII (1395 bp/cultivar Centelha). b Schematic representation of the TaALMT1 promoters alleles including the Type VII identified in cultivar Centelha (GenBank KF662919) and the Type I and V alleles identified in its parents (cultivars Centenário and Fronteira). Block A is represented in black (172 bp), block B in gray (108 bp), block C in white (97 bp) and block D in downward diagonal stripes (528 bp). A repeat (31 bp) within block B in the promoter Type II is represented by vertical stripes. The second repeat of block A in the promoter Type III is shorter (70 bp). Lines represent sequences not identified as repeats by Sasaki et al. (2006) (210 bp to the right and 75 bp to the left). Arrows indicate the coding region (not proportional). c Presence or absence of a transposon insertion in the TaMATE1B promoter as described in Garcia-Oliveira et al. (2014). ‘M’ indicates marker (shown at left in kb), ‘1’ indicates cultivar IAC 5 - Maringá; ‘2’, BRS 207; ‘3’, CNT 10; ‘4’, BRS 194; ‘5’, Trigo Chapéu; ‘6’, MGS 1 Aliança; ‘7’, Toropi; ‘8’, B 5; ‘9’, Cinquentenário; ‘10’, Fortaleza; ‘11’, MGS 3 Brilhante; and ‘12’, Horto
Sequencing and sequence analysis
To confirm the PCR fragment amplified for cultivar Centelha (Type VII), we sequenced the fragment. PCR products were precipitated, quantified on a 0.8 % agarose gel, and cloned into the pGEM-T easy vector (Promega). The plasmids were sequenced with primers LPF-F, LPF-R, T7 (TAATACGACTCACTATAGGG), SP6 (ATTTAGGTGACACTATAG), and VII-seq (CAGGTGCAAGGTTGTTAGAAGA) (3.2 µM), sequencing buffer and BigDye Terminator version 3.1. The sample was precipitated again, resuspended in Hi-Di formamide, denatured, and run on an ABI 3130 Sequence Analyzer. Sequencing Analysis Software version 5.1.1 and the Staden sequencing analysis package (Staden 1996) were used to analyze and align the sequences. The resulting sequence was deposited at GenBank with accession number KF662919.
Soil experiment
Short-term growth experiments were conducted to measure the root growth of 33 genotypes selected from each of the 12 haplotypes. Except for genotypes Ocepar 17, IAS 14 - Contestado, and Centelha, which are the unique representatives from their haplotypes, the selection inside each haplotype was random. The acidic soil (dystrophic red latosol of clayey texture) was collected at a depth of 0–20 cm from Embrapa Trigo (latitude-28,216979, longitude-52,408428). The soil pH in the water was 4.2, and the exchangeable aluminum (extracted by 1 mol L−1 KCl) was 58.5 mmolc dm−3 representing 78.9 % of the total cations exchange capacity (CEC). Half of the soil was limed, increasing the pH to 6.2 and reducing the soluble aluminum to 0 %. Five germinated seeds of each genotype containing roots of similar sizes were transferred to pots (25 cm high × 5 cm diameter) containing 450 g of limed and acidic soil and incubated in a glasshouse at 18 °C/24 °C (night–day) and natural light for 8 days. The pots were structured in randomized block designs, and the five plants constituting each repetition were watered to 90 % of field capacity every day. At the end of the experiment, the roots were carefully removed from the soil and washed. The length of the longest root on each plant was measured. The RRL was estimated as (root length in acidic soil/root length in limed soil) × 100. Errors associated with deriving the RRL were calculated by SERRL = RRL [(SE x /x)2 + (SE y /y)2]1/2 where x represents the mean root length in the acidic soil and y the mean root length in the limed soil. Statistical analyses were performed as detailed by Zhou et al. (2013).
Diversity analysis with SSR markers
Twelve SSR markers (Table 2) were used along with a M13 primer labeled with a fluorescent dye (FAM, NED, PET or VIC) as described in Schuelke (2000). For each SSR primer, part of the reaction mixture was identical (1× buffer, 0.2 μM reverse primer, 0.02 μM forward primer, and 0.2 μM labeled M13 primer), but other reagents were optimized for each primer and added as follows: mix A (1.5 mM MgCl2, 0.2 mM each dNTP, 0.5 U of Taq polymerase) for marker wms120; mix C (2.5 mM MgCl2, 0.2 mM each dNTP, 0.75 U of Taq polymerase) for barc71, barc130, wms539, wms3, and wms261; and mix D (2.5 mM MgCl2, 0.35 mM each dNTP, 0.75 U of Taq polymerase) for wmc215, barc96, wms18, psp3000, wmc331, and wms155. The amplification program was 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s, followed by four cycles at decreasing annealing temperatures of 1 °C per cycle and then 30 cycles at 94 °C for 30 s, 56 °C for 30 s, and 72 °C for 30 s. After amplification, the reactions were multiplexed (up to 4 primers combinations with each one containing different fluorescent dyes), diluted in water, mixed with Hi-Di formamide and GeneScan 500 LIZ size standard (Applied Biosystems), denatured (94 °C for 5 min), and run on an ABI 3130xl Genetic Analyzer containing a 36 cm capillary array with POP7 polymer. The program GeneMapper v3.5 was used to analyze the data. The SSR loci were used to build a similarity matrix using the simple matching coefficient and dendrogram construction was carried out using the hierarchical UPGMA method (Unweighted Pair Group Method with Arithmetic Mean) using the obtained matrix. The data were analyzed using the PowerMaker program (Liu and Muse 2005), and the polymorphism information content (PIC) value of each polymorphic marker was calculated according to Anderson et al. (1993).
Results
Analysis of TaALMT1 and TaMATE1B allelic variability
PCR primers specific for the TaALMT1 and TaMATE1B promoter regions were used to detected their allelic variability. The different types of TaALMT1 and TaMATE1B promoters detected in this study are listed in Table 1. Seven different TaALMT1 promoter alleles were detected based on amplicon size (Fig. 1) being promoters Type V (71.3 %) and Type VI (11.9 %) the most frequently detected. Those two types of promoter are usually associated with Al3+ resistance (Sasaki et al. 2006; Raman et al. 2008). On the other hand, promoters Type I and Type II, generally associated with Al3+ sensitivity, were identified in 21 (6.9 %) and 16 (5.3 %) genotypes, respectively. TaALMT1 promoters Type III and VII were detected in one genotype each (cultivars Ocepar 17 and Centelha, respectively), representing only 0.6 % of the detected alleles. The Type IV promoter was also uncommon (detected in 4.0 % of the analyzed genotypes).
The presence of the transposon insertion in the TaMATE1B promoter was observed in 80 genotypes where 68 of them showed TaALMT1 promoters Type V and VI. Considering the TaALMT1 and TaMATE1B allelic variation, the genotypes were separated in 12 haplotypes (Table 1). Only two haplotypes (TaALMT1 promoter Type III and presence of the insertion in the TaMATE1B promoter as well as TaALMT1 promoter Type VII and absence of the TaMATE1B promoter insertion) were not detected. Three haplotypes were represented by only one genotype, while the most frequent one was the TaALMT1 promoter Type V with the absence of insertion in the TaMATE1B promoter (176 genotypes). Interestingly, most of the genotypes presenting the TaALMT1 promoter Type VI also showed the insertion in the TaMATE1B promoter (28 in 36 genotypes), while in all others TaALMT1 promoter alleles the absence of the insertion was more frequent.
Some discrepancies related to the type of TaALMT1 promoter were found in the results published here compared to previous reports. The results of most genotypes were consistent although we detected the TaALMT1 promoter Type II in Trintani and Type VI in Cinquentenário and Trintecinco, while those same genotypes amplified alleles Type V, V and II, respectively, in Raman et al. (2008). For cultivars Trintani and Trintecinco, we further analyzed seeds multiplied in different years by the Embrapa Active Wheat Germplasm Bank and found the Type II promoter in both genotypes (data not shown). In this way, we detected two different TaALMT1 alleles (II and VI) in cultivar Trintecinco. We also identified two other genotypes where TaMATE1B alleles differed among the different entries. In that case, seeds from packets labeled as Carazinho and Londrina showed mixed alleles for the TaMATE1B promoter (Table 1). Different entries of the same genotype containing different TaALMT1 promoter alleles or Al3+ resistance phenotypes have been described previously (Sasaki et al. 2006; Raman et al. 2008), and this problem, regardless of the explanation, can lead to misinterpretation of the data.
Rare TaALMT1 promoter alleles
To understand the rare distribution of TaALMT1 promoters Type III and Type VII, we evaluated the pedigree (assessed on http://genbank.vurv.cz/wheat/pedigree/) of cultivars Ocepar 17 (Kalyansona/Bluebird//Alondra/3/B7408) and Centelha (Centenário/Fronteira). Two of the parents of Ocepar 17, Alondra and Kalyansona, have Type V and Type I promoters (Raman et al. 2008), and the Type III promoter should have been inherited from the other ancestors (Bluebird or B-7408). Unfortunately, we were not able to evaluate this because there were no seeds available in our Germplasm Bank. We assume that the ancestor containing the Type III promoter was not commonly used by the different wheat-breeding programs in Brazil. The parents of Centelha were also analyzed, and Centenário had a Type I promoter (data not shown), while Fronteira had a Type V allele (Table 1), meaning that the Type VII allele originated by merging the only B–C repeat from the Type I promoter with the only B–C repeat from the Type V promoter. This hypothesis should be viewed with caution as we were not able to evaluate the exact plants used in the particular cross that resulted in Centelha.
Relative root length (RRL) in short-term soil experiment
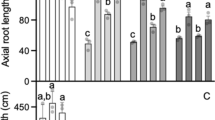
The length of the longest root of 33 genotypes, representatives of each haplotype, was measured after 8 days of growth in a short-term soil experiments (Fig. 2). Higher RRL was observed for some genotypes harboring the TaALMT1 Type V or VI and the insertion in the TaMATE1B promoter followed by genotypes carrying the TaALMT1 Type V without the insertion (Fig. 3). The impact of the transposon insertion in the TaMATE1B promoter could be evaluated by analyzing genotypes whose insertion was present or absent but showing the same TaALMT1 allele. That analysis could not be performed for the genotypes Ocepar 17 (TaALMT1 Type III with the TaMATE1B promoter insertion) and Centelha (TaALMT1 Type VII with the TaMATE1B promoter insertion) since they are the single representatives of their haplotypes. For the TaALMT1 promoter Type I and Type IV, the genotypes containing the insertion in the TaMATE1B promoter did not present greater RRL in comparison with the ones not presenting the insertion (Fig. 3). In fact, among the genotypes with the insertion in the TaMATE1B promoter, only five were statistically better than the ones without the insertion. It is important to note that the plants dealt with a high level of stress in the acidic soil used since it was chemically poor (low levels of phosphorus, potassium, and organic matter) and presented a high proportion of aluminum (78.9 % of the cations).
Wheat plants, representative of each haplotype, after 8 days of growth in the short-term soil experiment. The plants on the left were grown in limed soil, and the plants on the right were grown in acidic soil. ‘Alleles’ indicates the TaALMT1 (Types I–VI) and TaMATE1B (positive ‘+’ or negative ‘−’ for the transposon insertion) alleles detected
Relative growth of 33 cultivars, randomly selected from each of the 12 haplotypes, in the short-term soil experiment. The bars represent the corrected mean percentage ± SE. Numbers I to VII represent the different TaALMT1 promoter alleles. Gray bars represent genotypes lacking the transposon insertion in the TaMATE1B promoter while black bars represent genotypes showing the insertion. Asterisks indicate significant differences (P > 0.05) from genotypes with the same TaALMT1 allele but not presenting the TaMATE1B promoter insertion
Genetic diversity
The genetic diversity in the 300 genotypes was evaluated using 12 SSR markers spread across different chromosomes and genomes of the hexaploid wheat. The SSR markers were reasonably to highly informative (Table 2) showing PIC values from 0.46 (wms3 and barc130) to 0.79 (psp3000) with a mean of 0.60. A total of 113 SSR alleles generated an average of 9.4 alleles per primer. Primers specific for genome B (mean PIC of 0.66) were more informative than primers specific for genome D (mean PIC of 0.55). The dendrogram constructed based on the genotypic information of the markers showed a distribution of the internationally recognized Al3+ resistance sources (BH 1146, Carazinho, Frontana, Fronteira, IAC 5 - Maringá, Toropi, Trintani, Trintecinco, and Veranópolis) among different clusters (Fig. 4), although some of them exhibited a clear relationship. Cultivars Carazinho, Frontana, and Fronteira were clustered together, as were cultivars BH1146 and IAC 5 - Maringá. The genotypes Toropi, Trintani, and Trintecinco were also closely related. That indicates those cultivars probably present similar Al3+ tolerance/resistance mechanisms, and crossings among genetically diverse sources should be more useful to increase Al3+ resistance/tolerance. Besides that, genotypes belonging to the different haplotypes reported here are distributed across the dendrogram indicating a high genetic variability. One interesting distribution is related to the 28 genotypes showing TaALMT1 promoter Type VI and the insertion in the TaMATE1B promoter where 20 of them were clustered in the same group, even though they were developed by different breeding programs in Brazil.
Hierarchical analysis of the 300 Brazilian wheat genotypes with 12 SSR markers. The clustering dendrogram is based on a simple matching coefficient matrix. The open circles indicate genotypes with TaALMT1 promoter Type V and closed circles genotypes with TaALMT1 promoter Type VI. ‘+’ indicates the presence of the transposon insertion in the TaMATE1B promoter. Arrows indicate nine genotypes internationally recognized as Al3+ resistant (BH 1146, Carazinho, Frontana, Fronteira, IAC 5 - Maringá, Toropi, Trintani, Trintecinco, and Veranópolis)
Discussion
Soil acidity was one of the first challenges for Brazilian wheat breeders trying to introduce that crop in the South region of Brazil, and this is likely to be why Al3+ resistance is relatively common in Brazilian wheat genotypes (de Sousa 1998; Garvin and Carver 2003). Indeed, most Brazilian wheat varieties analyzed by others were considered resistant (de Sousa 1998; Raman et al. 2008). In this paper, we detected a high proportion (83.2 %) of TaALMT1 promoters Type V and VI among Brazilian wheat genotypes. As reported by Sasaki et al. (2006), wheat genotypes containing those alleles generally show intermediate to high levels of TaALMT1 expression and Al3+ resistance. Additionally, our study revealed that 27.0 % of the genotypes presenting TaALMT1 promoters Type V and VI also showed the transposon insertion in the TaMATE1B promoter. That insertion is associated with constitutive citrate efflux by the root tip and higher Al3+ resistance (Tovkach et al. 2013; Garcia-Oliveira et al. 2014). Aluminum resistance is not usually found in genotypes from other countries; at one time, Al3+ sensitivity was dominant in 250 genotypes from 21 countries from Africa, Europe, and Asia analyzed by Stodart et al. (2007).
Two genotypes analyzed here that presented TaALMT1 promoter Type V and the insertion in the TaMATE1B promoter (MGS 1 Aliança and MGS 3 Brilhante) are important varieties for rainfed cropping at the Brazilian Cerrado (Scheeren et al. 2008; Coelho et al. 2010), showing that Al3+ resistance should have a higher impact on wheat productivity for that condition. Heat and terminal drought are also found during the wheat-growing season in the Cerrado region (Scheeren et al. 2008), and genotypes presenting higher Al3+ resistance and, consequently, better root growth in acidic subsurface soil will most likely cope better when these stresses are combined. In fact, the most productive wheat genotypes in acid soil and rainfed conditions at the São Paulo state in Brazil were also Al resistant (Camargo et al. 2003). Besides that, the TaALMT1 promoter Type V was also shown to be advantageous in lower-rainfall regions of southern Australia, and it is likely to be most beneficial under high temperature when soil moisture is expected to be limiting (Eagles et al. 2014). In this context, wheat cultivars recommended for rainfed conditions at the Brazilian Cerrado will benefit from the joint action of superior TaALMT1 and TaMATE1B alleles.
Of the two Al-resistance mechanisms reported in wheat, the release of malate from root tips (encoded by TaALMT1) has a greater impact than the release of citrate (encoded by TaMATE1B); even though citrate is approximately eightfold more effective than malate in chelating Al3+ (Zheng et al. 1998). The reason for this is that malate release from root tips is ~tenfold higher than citrate release (Ryan et al. 2009; Tovkach et al. 2013). Indeed, even transgenic wheat and barley lines overexpressing citrate transporters (Magalhães et al. 2007; Zhou et al. 2013, 2014) do not appear to be as resistant as lines overexpressing TaALMT1 (Delhaize et al. 2004; Pereira et al. 2010; Zhou et al. 2014). Currently, the TaALMT1 gene confers the most effective level of Al3+ resistance in transgenic barley and wheat, and the effectiveness of organic acids in chelating Al3+ appears to be less determinant than the amount of organic acid released from root apices (Zhou et al. 2013, 2014). Here, we were not able to detect a clear correlation between a higher relative root length (RRL) under short-term soil experiments and the insertion in the TaMATE1B promoter. Although, when comparing genotypes with the same TaALMT1 alleles, the presence of the insertion in the TaMATE1B promoter was advantageous for a few genotypes, that advantage was not significant for most of the genotypes containing the insertion (Fig. 3). Important to note that the presence of the insertion in the TaALMT1 promoter had no penalty for the plants since the genotypes performed at least similar to the ones not showing the insertion.
When considering the best combinations for root growth on acid soil, the genotypes containing the TaALMT1 promoters Types V and VI and the transposon insertion in the TaMATE1B promoter presented the highest RRL since those genotypes are theorized to combine a higher malate and citrate efflux by the root tip (Fig. 3). That additive effect of TaALMT1 promoter Types V or VI and the insertion in the TaMATE1B promoter could be used to increase the accuracy of Al3+ resistance prediction when analyzed together with phenotyping methods. Besides that, organic acids released by the roots have been reported to have a role in phosphorus mobilization from the soil (Kochian et al. 2004). For instance, transgenic barley expressing TaALMT1 was more efficient at taking up phosphorus on acid soil (Delhaize et al. 2009), and theoretical modeling indicated a beneficial correlation, under certain conditions, between citrate efflux in wheat and phosphorus mobilization (Ryan et al. 2014). In this context, the incorporation of those alleles should be focused by the wheat-breeding programs targeting improvements in acid soil resistance. Additionally, breeders should also consider genes contributing to the formation of rhizosheaths (Delhaize et al. 2012a) and chromosomal regions not related to TaALMT1 and TaMATE1B that have been linked to the Al3+ resistance/tolerance in wheat (Ma et al. 2006; Cai et al. 2008; Raman et al. 2010), even though the mechanisms underlying these regions have not yet been fully described.
It is important to point out that the primers used here to distinguish between the presence and absence of the transposon-like insertion upstream of the TaMATE1B coding region could not result in effective amplification if the annealing sites are mutated. In that case, other TaMATE1B alleles could exist. In fact, these primers do not discriminate a single nucleotide polymorphism (SNP) located ~2 kb upstream the TaMATE1B coding region that was observed by Tovkach et al. (2013). That SNP was responsible for up to threefold difference in the TaMATE1B expression between cultivars presenting the transposon-like insertion (Tovkach et al. 2013) and could be one explanation why wheat genotypes presenting the same TaALMT1 allele, and the insertion in the TaMATE1B promoter showed different root growth in the acidic soil (Fig. 3). Besides that, other genes could also explain that difference. A number of metabolic pathways are activated in wheat under Al3+ stress including the ones related to root elongation, disease resistance response, fatty acid degradation, and response to oxidative stress (Guo et al. 2007; Houde and Diallo 2008). Plant genotypes differing for those traits, but with the same TaALMT1 and TaMATE1B alleles, could show different resistance to Al3+. Further studies should address these differences.
Genetic analysis of wheat genotypes collected from various regions indicates that Al3+ resistance and the TaALMT1 promoters could have multiple origins (Stodart et al. 2007; Zhou et al. 2007; Hu et al. 2008; Raman et al. 2008; Ryan et al. 2010), and the alleles described here for the TaALMT1 promoter are the same as those described for wheat genotypes from different countries (Sasaki et al. 2006; Raman et al. 2008). The Type III (cultivar Ocepar 17) and Type VII (cultivar Centelha) alleles were the rarest TaALMT1 promoters detected in Brazilian wheat genotypes and also the rarest ones detected by Raman et al. (2008). The origin of the tandem repeats in the TaALMT1 promoter is unclear, but they most likely appeared in T. aestivum within the last 10,000 years, and the alleles with different repeat patterns most likely originated independently (Ryan et al. 2010). However, some of the TaALMT1 alleles (presenting the same pattern of repeats but in a different number) are likely to have been derived from one another by unequal crossovers during recombination (Delhaize et al. 2007; Raman et al. 2008; Ryan et al. 2010). The Type VII allele (two repeats of blocks B-C) can be seen as a variant of Type VI allele that lost one B–C repeat (Raman et al. 2008); however, in this study, it appears to be a variant of Type I that acquired a new repeat because we found that the parents of Centelha, cultivars Centenário and Fronteira, had Type I and Type V alleles, respectively. Although one way to explain the origin of the Type VII promoter in Centelha is that it arose from a Type I allele, other explanations, such as heterozygosis and a mix-up of seeds, which have been detected in this study and by Raman et al. (2008), should also be considered.
A reduced amount of information is available for TaMATE1B alleles in comparison with the TaALMT1 alleles. The transposon insertion in the TaMATE1B promoter region appears to extend the TaMATE1B expression to the root apex, where it confers citrate efflux and increases aluminum resistance (Tovkach et al. 2013). Constitutive citrate efflux from roots of the Brazilian wheat cultivars Carazinho, Maringa, Toropi, and Trintecinco has been reported, while Portuguese wheat cultivars (Barbela 7/72/92 and Viloso Mole) showed different levels of that efflux (Ryan et al. 2009; Garcia-Oliveira et al. 2014). The transposon insertion in the TaMATE1B promoter was described for Carazinho (Tovkach et al. 2013) but not for another Brazilian cultivar, BH 1146 (Garcia-Oliveira et al. 2014). Here we confirmed that BH 1146 lack the transposon-like insertion in the TaMATE1B promoter. We also reported the presence of the insertion for the genotypes Carazinho, IAC 5 - Maringá, Toropi, and Trintecinco, the same ones that Ryan et al. (2009) detected constitutive citrate efflux. The insertion was not detected in Veranópolis and Fronteira, which did not present citrate efflux when analyzed by Ryan et al. (2009). In this way, the presence or absence of the TaMATE1B promoter insertion detailed here matched the citrate efflux results obtained by Ryan et al. (2009).
Nevertheless, discrepancies for the alleles of the other organic acid transporter gene, TaALMT1, were found for cultivar Trintani when compared to a previously published study (Raman et al. 2008) and for cultivar Trintecinco when comparing different seeds multiplied at the Embrapa Active Wheat Germplasm Bank. Besides that, discrepancies were also found for the genotypes Carazinho and Londrina that showed different TaMATE1B promoter alleles when seeds from different packets were analyzed. This is possibly due to the mix-up of seeds and misidentification and/or release of the cultivars in generations earlier than F6 fixing different alleles in loci (especially for older cultivars—the years of release for Trintecinco and Trintani are 1936 and 1949, respectively), where some alleles could still be segregating. Regardless of the explanation, these discrepancies can lead to misinterpretations, and breeding programs should take that into consideration.
Although the Al3+ resistance/tolerance of Brazilian wheat has been traced to a small number of landraces introduced in South Brazil in the beginning of the twentieth century (de Sousa 1998) and the promoter alleles associated with Al3+ resistance in Brazilian cultivars were detected in European landraces possibly introduced in Brazil by Europeans immigrants (Raman et al. 2008), we were able to detect genetic diversity among internationally recognized Al3+-resistant Brazilian wheat (Fig. 4). Some of these cultivars are more similar (Carazinho, Frontana, and Fronteira, as well as BH1146 and IAC 5 - Maringá or Toropi, Trintani, and Trintecinco), and those genotypes are most likely to share similar Al3+-resistance/tolerance mechanisms. That relationship reflects the cultivar pedigree since Fronteira is one of the genitors of Frontana (Fronteira/Mentana) while Frontana was used in the cross that generated Carazinho (Colonista/Frontana). Moreover, Trintecinco is one of the genitors of Trintani (Trintecinco/Guarany) while BH 1146 (PG 1//Fronteira/Mentana) and IAC 5 - Maringá (Frontana/Kenya 58//PG 1) share common ancestors. In fact, BH 1146, Frontana, IAC 5 - Maringá, and Trintecinco are among the most commonly used genotypes to generate Brazilian cultivars (de Sousa and Caierão 2014). Besides that diversity among recognized Al3+-resistant cultivars, our analysis revealed that other genotypes (presenting TaALMT1 promoters Type V or VI and the insertion in the TaMATE1B promoter) were quite different (Fig. 4), and these genetically diverse Al3+-resistant cultivars are important for the improvement of Al3+ resistance or as a source for studying the Al3+ resistance mechanisms. The SSR markers used here are located in different chromosomes and genomes of the hexaploid wheat (Table 2), and one of them, wmc331, is located in the chromosome 4DL, and it was reported to be closely linked to the TaALMT1 gene (Raman et al. 2005). In our analysis with these SSR markers, in which the highest number of Brazilian wheat genotypes has been genotypically analyzed, we were able to detect an average PIC of 0.60 (Table 2), indicating high genetic diversity among the analyzed genotypes. Although we used a small number of SSR markers, the polymorphism detected here is consistent with the results reported by Schuster et al. (2009), where a mean PIC of 0.49 was found.
In conclusion, for the first time a large number of Brazilian wheat genotypes was genotypically analyzed with markers linked to the TaALMT1 and TaMATE1B promoter. A high proportion of TaALMT1 promoter Types V and VI was found although the majority of the genotypes did not present the transposon insertion in the TaMATE1B promoter. Besides that, genotypes showing specific combinations of TaALMT1 and TaMATE1B alleles presented higher root growth on acid soil. In this context, to increase the frequency of the insertion in the TaMATE1B promoter is a target to be pursuit by different wheat-breeding programs in Brazil. Analysis using SSR markers indicated an important genetic variability among different Al3+-resistant sources and among genotypes harboring superior TaALMT1 and TaMATE1B alleles. This information is useful for studies regarding Al3+ resistance mechanisms and for wheat breeders targeting an improvement in root growth under acidic soil.
References
Anderson JA, Churchill GA, Autrique JE, Tanksley SD, Sorrells ME (1993) Optimizing parental selection for genetic linkage maps. Genome 36:181–186
Baier AC, Somers DJ, Gusiafson JP (1995) Aluminium tolerance in wheat: correlating hydroponic evaluations with field soil performances. Plant Breeding 114:291–296
Bertan I, Carvalho FIF, Oliveira AC, Silva JAG, Benin G, Vieira EA, Silva GO, Hartwig I, Valério IP, Finatto T (2006) Dissimilaridade genética entre genótipos de trigo avaliados em cultivo hidropônico sob estresse por alumínio. Bragantia 65:55–63
Cai S, Bai GH, Zhang D (2008) Quantitative trait loci for aluminum resistance in Chinese wheat landrace FSW. Theor Appl Genet 117:49–56
Camargo CEO, Oliveira OF (1981) Tolerância de cultivares de trigo a diferentes níveis de alumínio em solução nutritiva e no solo. Bragantia 40:21–31
Camargo CEO, Felicio JC, Rocha Junior LS (1987) Trigo: tolerância ao alumínio em solução nutritiva. Bragantia 46:183–190
Camargo CEO, Ferreira-Filho AWP, Ramos LCS, Pettinelli-Junior A, Castro JL, Felicio JC, Salomon MV, Mistro JC (2003) Comportamento de linhagens diaplóides de trigo em dois locais do Estado de São Paulo. Bragantia 62:217–226
Camargo CEO, Felicio JC, Ferreira Filho AWP, Lobato MTV (2006) Tolerância de genótipos de trigo comum, trigo duro e triticale à toxicidade de alumínio em soluções nutritivas. Bragantia 65:43–53
Coelho MAO, Condé ABT, Yamanaka CH, Corte HR (2010) Avaliação da produtividade de trigo (Triticum aestivum L.) de sequeiro em Minas Gerais. Biosci J 26:717–723
de Sousa CNA (1998) Classification of Brazilian wheat cultivars for aluminium toxicity in acid soils. Plant Breed 117:217–221
de Sousa CAN, Caierão E (2014) Cultivares de trigo indicadas para cultivo no Brasil e instituições criadoras—1922 a 2014, 2nd edn. Embrapa, Brasília
Delhaize E, Ryan PR, Randall PJ (1993) Aluminum tolerance in wheat (Triticum aestivum L.). II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol 103:695–702
Delhaize E, Ryan PR, Hebb DM, Yamamoto Y, Sasaki T, Matsumoto H (2004) Engineering high-level aluminum tolerance in barley with the ALMT1 gene. Proc Acad Sci USA 101:15249–15254
Delhaize E, Gruber BD, Ryan PR (2007) The roles of organic anion permeases in aluminium resistance and mineral nutrition. FEBS Lett 581:2255–2262
Delhaize E, Taylor P, Hocking PJ, Simpson RJ, Ryan PR, Richardson AE (2009) Transgenic barley (Hordeum vulgare L.) expressing the wheat aluminium resistance gene (TaALMT1) shows enhanced phosphorus nutrition and grain production when grown on an acid soil. Plant Biotech J 7:391–400
Delhaize E, James RA, Ryan PR (2012a) Aluminium tolerance of root hairs underlies genotypic differences in rhizosheath size of wheat (Triticum aestivum) grown on acid soil. New Phytol 195:609–619
Delhaize E, Ma JF, Ryan PR (2012b) Transcriptional regulation of aluminium tolerance genes. Trends Plant Sci 17:341–348
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf material. Phytochem Bull 19:11–15
Eagles HA, Cane K, Trevaskis B, Vallance N, Eastwood RF, Gororo NN, Kuchel H, Martin PJ (2014) Ppd1, Vrn1, ALMT1 and Rht genes and their effects on grain yield in lower rainfall environments in southern Australia. Crop Pasture Sci 65:159–170
Echart CL, Cavalli-Molina S (2001) Fitotoxicidade do alumínio: efeitos, mecanismo de tolerância e seu controle genético. Ciência Rural 31:531–541
FAO (2000) Land resource potential and constraints at regional and country levels. Land and Water Development Division, Rome
Garcia-Oliveira AL, Martins-Lopes P, Tolrá R, Poschenrieder C, Tarquis M, Guedes-Pinto H, Benito C (2014) Molecular characterization of the citrate transporter gene TaMATE1 and expression analysis of upstream genes involved in organic acid transport under Al stress in bread wheat (Triticum aestivum). Physiol Plant 152:441–452
Garvin DF, Carver BF (2003) Role of the genotype in tolerance to acidity and aluminum toxicity. In: Rengel Z (ed) Handbook of soil acidity. Marcel Dekker Inc., New York, pp 387–406
Guo P, Bai G, Carver B, Li R, Bernardo A, Baum M (2007) Transcriptional analysis between two wheat near-isogenic lines contrasting in aluminum tolerance under aluminum stress. Mol Genet Genomics 277:1–12
Houde M, Diallo AO (2008) Identification of genes and pathways associated with aluminum stress and tolerance using transcriptome profiling of wheat near-isogenic lines. BMC Genom 9:400
Hu SW, Bai GH, Carver BF, Zhang DD (2008) Diverse origins of aluminum-resistance sources in wheat. Theor Appl Genet 118:29–41
Huang XQ, Börner AB, Röder MS, Ganal MW (2002) Assessing genetic diversity of wheat (Triticum aestivum L.) germplasm using microsatellite markers. Theor Appl Genet 105:699–707
Kochian LV, Hoekenga OA, Piñeros MA (2004) How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol 55:459–493
Kochian LV, Piñeros MA, Liu J, Magalhaes JV (2015) Plant adaptation to acid soils: the molecular basis for crop aluminum resistance. Annu Rev Plant Biol 66:23.1–23.28
Liu K, Muse SV (2005) PowerMarker: integrated analysis environment for genetic marker data. Bioinformatics 21:2128–2129
Liu J, Piñeros MA, Kochian LV (2014) The role of aluminum sensing and signaling in plant aluminum resistance. J Integr Plant Biol 56:221–230
Ma HX, Bai GH, Lu WZ (2006) Quantitative trait loci for aluminum resistance in wheat cultivar Chinese spring. Plant Soil 283:239–249
Magalhães JV, Liu J, Guimarães CT, Lana UGP, Alves VMC, Wang YH, Schaffert RE, Hoekenga OA, Piñeros MA, Shaff JE, Klein PE, Carneiro NP, Coelho CM, Trick HN, Kochian LV (2007) A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat Genet 39:1156–1161
Pereira JF, Zhou G, Delhaize E, Richardson T, Zhou M, Ryan PR (2010) Engineering greater aluminium resistance in wheat by over-expressing TaALMT1. Ann Bot 106:205–214
Raman H, Zhang K, Cakir M, Appels R, Garvin DF et al (2005) Molecular characterization and mapping of ALMT1, the aluminium-tolerance gene of bread wheat (Triticum aestivum L.). Genome 48:781–791
Raman H, Ryan PR, Raman R, Stodart BJ, Zhang K, Martin P, Wood R, Sasaki T, Yamamoto Y, Mackay M, Hebb DM, Delhaize E (2008) Analysis of TaALMT1 traces the transmission of aluminum resistance in cultivated common wheat (Triticum aestivum L.). Theor Appl Genet 116:343–354
Raman H, Stodart B, Ryan PR, Delhaize E, Emebiri L, Raman R, Coombes N, Milgate A (2010) Genome-wide association analyses of common wheat (Triticum aestivum L.) germplasm identifies multiple loci for aluminium resistance. Genome 53:957–966
Röder MS, Korzum V, Wendehake K, Plashke J, Tixier MH, Leroy P, Ganal MW (1998) A microsatellite map of wheat. Genetics 149:2007–2023
Ryan PR, Raman H, Gupta S, Horst WJ, Delhaize E (2009) A second mechanism for aluminum resistance in wheat relies on the constitutive efflux of citrate from roots. Plant Physiol 149:340–351
Ryan PR, Raman H, Gupta S, Sasaki T, Yamamoto Y, Delhaize E (2010) The multiple origins of aluminium resistance in hexaploid wheat include Aegilops tauschii and more recent cis mutations to TaALMT1. Plant J 64:446–455
Ryan PR, Tyerman SD, Sasaki T, Furuichi T, Yamamoto Y, Zhang WH, Delhaize E (2011) The identification of aluminium-resistance genes provides opportunities for enhancing crop production on acid soils. J Exp Bot 62:9–20
Ryan PR, James RA, Weligama C, Delhaize E, Rattey A, Lewis DC, Bovill WD, McDonald G, Rathjen TM, Wang E, Fettell NA, Richardson AE (2014) Can citrate efflux from roots improve phosphorus uptake by plants? Testing the hypothesis with near-isogenic lines of wheat. Physiol Plant 151:230–242
Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H (2004) A wheat gene encoding an aluminum-activated malate transporter. Plant J 37:645–653
Sasaki T, Ryan PR, Delhaize E, Hebb DM, Ogihara Y, Kawaura K, Noda K, Kojima T, Toyoda A, Matsumoto H, Yamamoto Y (2006) Sequence upstream of the wheat (Triticum aestivum L.) ALMT1 gene and its relationship to aluminum resistance. Plant Cell Physiol 47:1343–1354
Scheeren PL, Caierão E, Silva MS, Nascimento AJ, Caetano VR, Bassoi MC, Brunetta D, Albrecht JC, Quadros WJ, Sousa PG, Trindade MG, Sobrinho JS, Wiethölter S, Cunha GR (2008) Challenges to wheat production in Brazil. In: Reynolds MP, Pietragalla J, Braun HJ (eds) International symposium on wheat yield potential. CIMMYT, pp 167–170
Schuelke M (2000) An economic method for the fluorescent labeling of PCR fragments. Nat Biotechnol 18:233–234
Schuster I, Vieira ESN, Silva GJ, Franco FA, Marchioro VS (2009) Genetic variability in Brazilian wheat cultivars assessed by microsatellite markers. Genet Mol Biol 32:557–563
Staden R (1996) The Staden sequence analysis package. Mol Biotechnol 5:233–241
Stodart BJ, Raman H, Coombes N, Mackay M (2007) Evaluating landraces of bread wheat Triticum aestivum L. for tolerance to aluminium under low pH conditions. Genet Resour Crop Evol 54:759–766
Tang C, Nuruzzaman M, Rengel Z (2003) Screening wheat genotypes for tolerance of soil acidity. Aust J Agr Res 54:445–452
Tovkach A, Ryan PR, Richardson AE, Lewis DC, Rathjen TM, Ramesh S, Tyerman SD, Delhaize E (2013) Transposon-mediated alteration of TaMATE1B expression in wheat confers constitutive citrate efflux from root apices. Plant Physiol 161:880–892
Von Uexküll HR, Mutert E (1995) Global extent, development and economic impact of acid soils. Plant Soil 171:1–15
Warburton ML, Crossa J, Franco J, Kazi M, Trethowan R, Rajaram S, Pfeiffer W, Zhang P, Dreisigacker S, van Ginkel M (2006) Bringing wild relatives back into the family: recovering genetic diversity in CIMMYT improved wheat germplasm. Euphytica 149:289–301
Yamada T (2005) The Cerrado of Brazil: a success story of production on acid soils. Soil Sci Plant Nutr 51:317–620
Zhang WH, Ryan PR, Sasaki T, Yamamoto Y, Sullivan W, Tyerman SD (2008) Characterization of the TaALMT1 protein as an Al3+-activated anion channel in transformed tobacco (Nicotiana Tabacum L.) cells. Plant Cell Physiol 49:1316–1330
Zheng SJ, Ma JF, Matsumoto H (1998) Continuous secretion of organic acids is related to aluminum resistance during relatively long-term exposure to aluminium stress. Physiol Plant 103:209–214
Zhou LL, Bai GH, Carver B, Zhang DD (2007) Identification of new sources of aluminum resistance in wheat. Plant Soil 297:105–118
Zhou G, Delhaize E, Zhou M, Ryan PR (2013) The barley MATE gene, HvAACT1, increases citrate efflux and Al3+ tolerance when expressed in wheat and barley. Ann Bot 112:603–612
Zhou G, Pereira JF, Delhaize E, Zhou M, Magalhaes JV, Ryan PR (2014) Enhancing the aluminium tolerance of barley by expressing the citrate transporter genes SbMATE and FRD3. J Exp Bot 65:2381–2390
Acknowledgments
We are thankful to Embrapa (Empresa Brasileira de Pesquisa Agropecuária) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for financial support (project Embrapa 02.11.08.001.00.00 and PNPD 560516/2010-0 fellowship to Jorge González Aguilera). We thank Dr Sirio Wietholter and his team for the soil analysis, and Dr. Douglas Lau for supplying reagents. We are grateful to Dr. Emmanuel Delhaize and Dr. Peter R. Ryan (CSIRO Agriculture) and to Dr. Caroline Turchetto for valuable discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pereira, J.F., Barichello, D., Ferreira, J.R. et al. TaALMT1 and TaMATE1B allelic variability in a collection of Brazilian wheat and its association with root growth on acidic soil. Mol Breeding 35, 169 (2015). https://doi.org/10.1007/s11032-015-0363-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-015-0363-9