Abstract
Aluminum (Al) toxicity is a major constraint for wheat production in acidic soils. An Al resistance gene on chromosome 4DL that traces to Brazilian wheat has been extensively studied, and can provide partial protection from Al damage. To identify potentially new sources of Al resistance, 590 wheat accessions, including elite wheat breeding lines from the United States and other American and European countries, landraces and commercial cultivars from East Asia, and synthetic wheat lines from CIMMYT, Mexico, were screened for Al resistance by measuring relative root elongation in culture with a nutrient solution containing Al, and by staining Al-stressed root tips with hematoxylin. Eighty-eight wheat accessions demonstrated at least moderate resistance to Al toxicity. Those selected lines were subjected to analysis of microsatellite markers linked to an Al resistance gene on 4DL and a gene marker for the Al-activated malate transporter (ALMT1) locus. Many of the selected Al-resistant accessions from East Asia did not have the Al-resistant marker alleles of ALMT1, although they showed Al resistance similar to the US Al-resistant cultivar, Atlas 66. Most of the cultivars derived from Jagger and Atlas 66 have the Al-resistant marker alleles of ALMT1. Cluster analysis separated the selected Al-resistant germplasm into two major clusters, labeled as Asian and American–European clusters. Potentially new germplasm of Al resistance different from those derived from Brazil were identified. Further investigation of Al resistance in those new germplasms may reveal alternative Al-resistance mechanisms in wheat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acidic soils occupy about 30% to 40% of world arable lands, and Al toxicity is a major constraint for crop production in these soils (von Uexküll and Mutert 1995). As soil pH falls below 5.0, Al3+ becomes the dominant form of Al in soils. Al3+ affects plant growth by inhibiting elongation and division of plant root tips, and subsequently reducing nutrient and water uptake. Besides the natural occurrence of soil acidity, the extensive use of ammonia fertilizers causes further soil acidification. Direct application of lime to acidic soils may increase soil pH to relieve Al toxicity in the plant, but transportation and material costs are often prohibitive. Fortunately, significant genetic variation in Al resistance has been found among wheat cultivars (Aniol and Gustafson 1984). The use of Al-resistant cultivars has been considered a cost-effective means to improve wheat production in acidic soils.
Genetic diversity is the foundation of genetic improvement in plants (Rejesus et al. 1996). Utilization of new variation for Al resistance traits could increase wheat yields in acidic soils without additional increases in inputs. The wheat gene pool consists of diverse biological species, including cultivated, wild, and weedy species. But, only a small proportion of the genetic variation of Al resistance has been studied and utilized in breeding programs. In wheat, well-known genetic sources of Al resistance originated from Brazil, where acid soils dominate in wheat production. A spring wheat cultivar BH 1146 (Ponta Grossa 1//Fronteira/Mentana) from Brazil and a winter wheat cultivar Atlas 66 (Frondoso//Redhart 3/Noll 28) from the United States have been extensively used as genetic materials to study the inheritance of Al resistance (Berzonsky 1992; Riede and Anderson 1996). These two cultivars share a common ancestor: Ponta Grossa 1. Ponta Grossa 1 was a selection from Polyssu, and Polyssu was a parent of Frondoso. Near-isogenic lines were developed from these sources and used for cloning the Al-activated malate transporter gene (Sasaki et al. 2004). For example, ET8 was derived from a cross between an Al-resistant cultivar, Carazinho (Colonista/Frontana), and an Al-sensitive cultivar, Egret (Fisher and Scott 1987). Frontana was derived from Fronteira (Polyssu/Alfredo Chaves 6-21). Many Al-resistant wheat lines currently used in genetic studies can be traced back to Brazilian ancestors, especially Polyssu (de Sousa 1998; Garvin and Carver 2003). New sources of Al-resistant germplasm would be desirable for the improvement of wheat Al resistance in modern breeding programs.
Landraces are old cultivars that were selected by farmers for their adaptation to local conditions. Outside their local area, they may not be suitable for commercial wheat production due to poor overall agronomic performance, but landraces are important sources of many stress-related traits for genetic improvement of modern cultivars. Aluminum resistance from Brazilian landraces has been used in wheat breeding programs, and common to that genetic base is the Al-activated malate release mechanism of Al resistance. Whether wheat accessions from other regions were derived from the same source and feature the same mechanism of resistance remains unknown. With the worldwide distribution of acid soils (von Uexküll and Mutert 1995; Borlaug and Dowswell 1997), natural selection for adaptation to low-pH soils might create diverse Al-resistant wheat germplasm in other regions. Screening a worldwide collection of wheat accessions may identify new sources of Al resistance in wheat (Stodart et al. 2006).
In addition to conventional wheat cultivars and landraces (Stodart et al. 2006), synthetic hexaploid wheat (SHW) (2n = 6× = 42, AABB–DD) can provide another source of Al resistance. Produced by artificially crossing durum wheat (Triticum turgidum, 2n = 4× = 28 AABB) with A. tauschii (2n = 2× = 14 DD) (Mujeeb-Kazi et al. 1996), SHW provides a convenient conduit for the introduction of desirable genes from A. tauschii to common wheat. Examples include genes for resistance to leaf rust resistance (Puccinia triticina Eriks.), Septoria blotch (Septoria tritici Roberge in Desmaz), Karnal bunt (Tilletia indica Mitra), and wheat curl mite (Eriophyes tulipae Keifer) (Cox 1998). But SHW as a potential new source for Al resistance has not been examined to date.
A major gene on chromosome 4DL has been identified in wheat cultivars BH1146, Atlas 66, and Chinese Spring (Riede and Anderson 1996; Luo and Dvorak 1996; Ma et al. 2005; Raman et al. 2005). Markers linked to this gene are available for screening wheat materials for this gene (Ma et al. 2005). Meanwhile, several markers for ALMT1 genes have been developed and mapped on the 4DL of Atlas 66 (Ma et al. 2005; Sasaki et al. 2004, 2006; Raman et al. 2006). They were proposed as diagnostic markers of Al resistance (Sasaki et al. 2004, 2006; Raman et al. 2006). The objectives in this study were to: (1) survey U.S. wheat breeding lines and cultivars for the presence of the 4DL Al resistance gene; (2) identify new sources of Al resistance from wheat germplasm that does not have a Brazilian origin; and (3) investigate the genetic diversity of selected Al-resistant germplasm.
Materials and methods
Plant materials
A total of 590 wheat accessions were evaluated for Al resistance in nutrient-solution culture, including 232 U.S. commercial wheat cultivars and elite breeding lines mainly from the Great Plains area. Other lines and sources were 31 cultivars from Mexico (CIMMYT); 13 cultivars from Italy; 1 cultivar each from Brazil (Frontana); Pakistan (Lu 26S) and Russia (Avrora); 20 cultivars/landraces from Japan; 40 landraces and 10 breeding lines from China; 2 cultivars from Korea; and 239 synthetic wheat lines from Mexico (CIMMYT).
Evaluation of Al resistance
Aluminum resistance was evaluated by measuring relative root elongation and by staining root tips with hematoxylin after 2 days of Al stress in nutrient-solution culture. Wheat seeds were placed on moist paper in a petri dish at 4°C for 24 h, and then were moved to room temperature (22–25°C) for an additional 24 h. Three germinated seeds with similar viability were transferred onto a nylon net at the bottom of a plastic cup that had an open bottom. Cups with germinated seeds supported by a plastic cup holder floated on deionized water at 22°C with 16 h of fluorescent light daily. Connected to an air pump were two bubble rods in the water, which provided aeration during nutrient-solution culture. After 48 h, the deionized water was replaced with a nutrient solution (pH 4.0) consisting of 4 mM CaCl2, 6.5 mM KNO3, 2.5 mM MgCl2·6H2O, 0.4 mM NH4NO3, 0.1 mM (NH4)2SO4, and 0.36 mM AlK(SO4)·2H2O. In the control treatment, deionized water was replaced with the same nutrient solution, without the addition of AlK(SO4)·2H2O.
Root growth in the Al treatment and the degree of hematoxylin staining on Al-treated root tips were measured for all accessions. The principal root of each seedling was measured twice, before and after seedlings were subjected to the 48-h Al or control treatments. The difference between the two measurements was calculated as net root growth (NRG) for Al-treated plants and control root growth (CRG) for control plants. Relative root growth (RRG) for each accession was calculated as 100 × NRG / CRG. After they were measured, the excess Al on roots was rinsed two to three times with deionized water for 1 h. Clean roots were then submerged in a hematoxylin solution consisting of 0.2% hematoxylin (w/v) and 0.02% (w/v) KIO3 for 15 min, followed by rinsing the roots three to four times with deionized water. Root tips of each stained seedling were visually scored according to three grades: no stain on root tips as grade 1, light stain as grade 2, and heavy stain as grade 3. The initial screen was accomplished with one replicate. The highly susceptible accessions were eliminated, and the remaining accessions were further evaluated by using a randomized complete-block design with three replicates.
Genetic diversity among Al-resistant germplasm
A total of 54 accessions were selected for genetic diversity analysis. This subset showed at least moderate Al resistance, based on performance in nutrient-solution culture. Most accessions were from the United States, except 13 from Japan and China. A total of 60 pairs of SSR primers, which included 6 CFD primers, 24 WMC primers, and 30 BARC primers, were selected to represent all chromosomes. Among the 60 SSRs, 20 markers were selected from genome A, 15 markers were from genome B, 18 markers were on genomes D, and the remaining 7 markers had no known chromosome location (Table 1).
PCR was performed in a DNA Engine Tetrad® Peltier Thermal Cycler (BioRad Lab, Hercules, CA). A 10-μl PCR mix contained 40 ng of template DNA, 0.2 mM of each dNTP, 1x PCR buffer, 2.5 mM MgCl2 and 0.6 U of Taq polymerase (Promega, Madison, WI). Forward primer was labeled with IRDye-700 or IRDye-800 (Li-Cor, Lincoln, NE). A touch-town program for PCR amplification started at 95°C for 5 min, followed by 5 cycles of 45 s of denaturing at 95°C, 5 min of annealing at 68°C, with a decrease of 2°C in each subsequent cycle, and 1 min of extension at 72°C; for another 5 cycles, the annealing temperature started at 58°C for 2 min, with a decrease of 2°C for subsequent cycles; then, PCR went through additional 25 cycles of 4 s at 94°C, 2 min at 50°C, and 1 min at 72°C, with a final extension at 72°C for 5 min. Amplified PCR fragments were separated in a Li-Cor 4300 DNA analyzer (Li-Cor, Lincoln, NE) with 1X TBE buffer (50 mM Tris, 50 mM boric acid, 1 mM EDTA). Before running gels, PCR products, mixed with 5 μl of formamide loading dye, were denatured at 95°C for 5 min and then cooled on ice immediately for loading. The gel was prepared with 6.5% Gel Matrix for 2 h before use (Li-Cor, Lincoln, NE). After 10 min pre-run, 0.8 μl of PCR-sample mix was loaded into the gel. The gel was run at 1,400 V and 40 W with consistent temperature of 45°C. Analysis of ALMT1 gene followed methods from Sasaki et al. (2004).
Marker data were scored as present (1) or absent (0) by using Saga™ Software (Li-Cor, Lincoln, NE). All data were visually inspected and ambiguous data were eliminated manually. Similarities for pairs of accessions were calculated using the SIMQUAL module of NTSYSpc software (Rohlf 1998). The unweighted pair-group method with arithmetic average (UPGMA) was used for cluster analysis, and the resulting cluster was presented as a dendrogram by using the same software. A principal coordinate analysis was performed with the DCENTER module of the NTSYSpc program. Polymorphic information content (PIC) was calculated according to Anderson et al. (1993), assuming that the wheat accessions are all homozygous.
Results
Al-resistant germplasm
The majority of the 590 accessions evaluated for root growth and hematoxylin stain were highly sensitive to Al toxicity in nutrient-solution culture, yet some accessions showed a highly resistant reaction. After 2 days of Al stress, NRG varied from 0 (‘SHW Septoria 6’) to 3.64 cm (‘Wesley’), with a mean of 0.93 cm. Hematoxylin staining scores ranged from 1 to 3 with a mean of 2.4. RRG ranged from 0 to 1.0, with a mean of 0.27.
A total of 88 accessions demonstrated a minimum of moderate Al resistance with HSS ≤2 and RRG >0.3 (Table 2). Mean RRG in these 88 selected cultivars was 0.56 and mean HSS was 1.4. Six were synthetics (mean RRG = 0.62, mean HSS = 1.2), although about one-half of the total accessions initially screened were synthetics. Eight accessions were from China, and most of them were moderately Al-resistant (mean RRG = 0.44, mean HSS = 1.7), except FSW, which had RRG = 1 and HSS = 1. Thirteen Japanese landraces showed high Al resistance (mean RRG = 0.59, mean HSS = 1.2). Avrora (RRG = 0.39, HSS = 1.3), from Russia, was moderately Al-resistant.
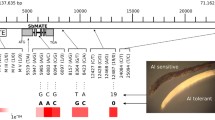
All selected Al-resistant accessions were analyzed with two ALMT1 gene markers (ALMT1-CAP and ALMT1-SSR3a) and two SSR flanking markers (Xwmc 331 and Xgdm125) for the 4DL Al resistance gene from Atlas 66. For the ALMT1-CAP marker, the restriction enzyme XmnI can only digest the PCR product of the ALMT1-2 allele (the allele in Al-sensitive NIL ES8, according to Sasaki et al. 2004) into two small fragments, but can not digest the ALMT1-1 allele (the allele in Al-resistant NIL ET8). When the PCR products from the 87 selected Al-resistant accessions (excluding Atlas 66) were digested with XmnI, only 41 accessions showed the same banding pattern as Atlas 66 (Table 2), whereas the PCR products from another 40 accessions were digested into two small fragments, as in ES8 (Fig. 1, Sasaki et al. 2004). The remaining six cultivars were heterogeneous and partly digested by XmnI. Among the 41 accessions, 17 showed the same ALMT1-SSR3a allele (232 bp) as that of Atlas 66 and Jagger (Table 3). The rest of 24 accessions carried different ALMT1-SSR3a alleles, ranging from 231 to 235. All other accessions that carry ALMT1-2 allele have smaller ALMT1-SSR3a allele sizes, ranging from 221 to 227 bp.
Cleavage-amplified polymorphism (CAP) marker patterns of ALMT1 (ALMT1-CAP) in selected wheat accessions. From left to right: Sobakomugi, Asozairaiii, Itoukomugi, Kikuchi, Shironankin, Sotome, Asotomea, Nobeokabouzu Komugi, Yangmai 5, Avrora, Abura, NyuBai, Tokai 66, Atlas 66, Chinese Spring, Karl 92, Scout 66. ALMT1-1 resistance allele is 107 bp, ALMT1-2 alleles are 57 and 50 bp
Variation in haplotypes of the two flanking SSR markers linked to the 4DL Al-resistance gene was also observed among the 87 Al-resistant accessions. Atlas 66 inherited its Al resistance from the same Brazilian source as ET8 (Sasaki et al. 2004). Twenty-one Al-resistant accessions had banding patterns completely different from that of Atlas 66 (Table 3). Only 4 accessions, Endurance, SD-789, TAM 111, and Fumai 3, showed the same banding pattern as Atlas 66 for SSR markers, Xwmc331 and Xgdm125, and for the ALMT1 gene marker. But 29 cultivars showed the same banding patterns as Jagger for the three markers, in which Xgdm125 amplified a fragment 2 bp longer (261 bp) than that (259 bp) of Atlas 66 (Table 2).
Genetic diversity of selected Al-resistant germplasm
To analyze genetic diversity of the selected germplasm, 54 accessions with at least moderate Al resistance were selected from the pool of 88 accessions described earlier to represent diverse sources of origin. These were analyzed with 60 SSR markers selected on the basis of their chromosome distribution (Table 1). A total of 471 SSR bands were scored, producing a mean of 7.9 bands per SSR primer pair, ranging from 2 to 17 scorable bands per primer pair. PIC of all markers ranged from 0.17 (Xwmc627) to 0.9 (Xbarc74), with an average PIC of 0.68, therefore the selected markers were highly polymorphic among the accessions analyzed. Cluster analysis revealed approximately two major clusters, classified as Asian (predominately Japanese and Chinese) and American–European (Fig. 2). The American–European cluster can be further divided into two subclusters according to the presence or absence of Jagger parentage. Atlas 66 and 2163 from the U.S.A. clustered independent of either major cluster, with about 80% similarity.
Discussion
To date, Al-resistant materials used in genetic research have been limited to cultivars with Brazilian ancestry, such as Atlas 66 or related cultivars (Tang et al. 2002; Samac and Tesfaye 2003; Vitorello et al. 2005; Kochian et al. 2005). Aluminum resistance in Brazilian landraces might be derived by natural selection because acidic soils are very common in wheat-growing areas in Brazil. Atlas 66 has many undesirable agronomic traits that prohibit its use as a modern commercial cultivar, although it has excellent Al resistance. Contemporary elite wheat cultivars such as Jagger and Endurance, which not only have a high level of Al resistance but also have other desirable agronomic traits, provide much better sources of Al resistance for developing new cultivars.
On the basis of their pedigrees, Jagger and Endurance seem to have no obvious lineage to Brazilian sources of Al resistance. Their haplotypes for molecular markers associated with the 4DL Al resistance gene suggested, however, that Endurance and Jagger may have the same Al resistance gene as that from Brazilian sources. Jagger had the same alleles at the ALMT1-CAP and Xwmc331 loci, and Endurance had the same alleles at all four marker loci linked to the 4DL Al resistance gene, as those in Atlas 66. In addition, Jagger is resistant to many diseases, including stripe rust (Puccinias striiformis f. sp. tritici), leaf rust (P. triticina), soil-borne mosaic, spindle streak mosaic, and tan spot (Pyrenophora tritici-repentis). It also has moderate resistance to glume blotch, bacterial streak, and wheat streak mosaic virus (Sears et al. 1997; Donmez et al. 2001). Endurance also has adult-plant resistance to leaf rust, broad adaptation, grazing tolerance, good yield potential, and acceptable quality traits. Therefore Endurance and Jagger, or their new derivatives, are preferred Al-resistant parents for improving wheat resistance to Al toxicity.
Other accessions showed Al resistance similar to that of Atlas 66, and some are commercial cultivars, or their derivatives with many desirable agronomic traits, and they have been used as parents in breeding programs. For example, Oasis, developed in Indiana, USA, was resistant to many biotypes of Hessian fly [Mayetiola destructor (Say)] in greenhouse and field tests at the time of its release, and has resistance to S. tritici as well (Patterson et al. 1975; Eyal and Talpaz 1990). It is a good source of Al resistance for soft winter wheat improvement. Wesley is a hard winter wheat cultivar, and has not only superior bread-making quality and high yield potential but also resistance to stem rust (Puccinia graminis), soil-borne mosaic virus, and wheat spindle streak virus (Peterson et al. 2001). It may also be a good source of Al resistance for wheat improvement.
The American/European cluster contained hard winter wheat cultivars from the southern Great Plains, which were closely related, such as OK04819, TX01A5936, Santa Fe, HV9W00-B140R, Jagalene, Overley, Cutter, Jagger, HV9W00-B243R, W98-159-7, and HV9W02-657R. Almost all of those contain Jagger in their pedigrees. Principal coordinate analysis classified Jagger and its derivatives into a different subcluster from most other American/European cultivars that have Brazilian sources in their pedigrees (data not shown), which again indicated that the Al resistance genes in Jagger may be derived from different sources outside of Brazil. One parent of Jagger, Stephens, has French and Japanese parentage, whereas the other parent is a sister line of the moderately susceptible cultivar, Karl. It is unknown if Jagger inherited its Al tolerance from Stephens; thus, the origin of Jagger’s Al resistance gene(s) needs further investigation.
Besides modern wheat cultivars, landraces from Europe and Asia also had Al resistance (Stodart et al. 2006). Acidic soils are predominant in both southern China and Japan and landraces from East Asia may have acquired Al resistance through natural selection in acidic soils. Aluminum resistance from Chinese and Japanese wheat has not been reported to date, so the genetic relationship between the Asian and American accessions is unknown. Cluster analyses based on molecular marker profiles may provide the best estimate of genetic relationships among cultivars, and can be used to reveal pedigree relatedness among plant accessions (Plaschke et al. 1995; Ahmad 2002). Results from cluster analysis in this study classified American/European and Asian accessions into two separate clusters, signifying distinct gene pools. The Asian cluster contained mainly spring wheat from China and Japan, with only one exception of TAM 111 from the United States; whereas the American/European cluster consisted of mainly winter wheat from the United States, plus one accession from Russia. The appearance of TAM 111 in the Asian cluster is not unexpected, given the occurrence of Japanese and Korean lines in its pedigree (Table 2). The parent, Sturdy, in TAM 111’s pedigree was derived from Sinvalocho/Wichita//Hope/Cheyenne/3/2* Wichita/4/Seu Seun 27, and the parent, Bluejay, was derived from Tezanos Pintos Precoz/Paloma//Siete Cerros 66. Seu Seun 27 in Sturdy’s pedigree is a Korean wheat cultivar, and Siete Cerros 66 has a Japanese cultivar, Norin 10, in its pedigree (Frontana/Kenya 58//Newthatch/3/Norin 10/Brevor/Gabo 55).
Analysis of the ALMT1 gene markers (Sasaki et al. 2004; Raman et al. 2006) showed that most U.S./European cultivars contain the same Al-resistant allele as those in Atlas 66, whereas most Asian accessions carry the Al-sensitive allele (Table 2 and Fig. 1). These results suggested that some Asian landraces might have different Al resistance QTL/genes than those in American accessions. Because most Asian accessions are not related to American accessions, the Al resistance in Asian accessions seems not to have originated from Brazilian sources. Further investigation of these Asian materials may facilitate identification of new sources of Al resistance for breeding application and discovery of alternative mechanisms of wheat resistance to Al toxicity.
In addition to common wheat, six synthetic wheat lines were also identified to have Al resistance. Among them, three have resistance to Fusarium head blight and one has resistance to Karnal bunt. Synthetic wheat seems to have no obvious lineage to those Al-resistant wheat accessions from Asia and Brazil.
The association between Al resistance in the cultivars and the presence of the ALMT1-1 allele, as described by Sasaki et al. (2004) and Raman et al. (2006), was low. More alleles were observed for ALMT1-SSR3a marker. However the result was the same as that for ALMT1-CAP when ALMT1-SSR3a marker with sizes over 230 bp were considered as resistant marker alleles. Less than one-half of Al-resistant or moderately resistant cultivars showed the Al-resistant marker allele at the ALMT1-CAP and ALMT1-SSR3a locus. This suggests that the sequence differences at the two ALMT1 marker loci reported by Sasaki et al. (2004) and Raman et al. (2006) may not be diagnostic for Al resistance in many cultivars, especially those from Asia. This discrepancy could be due to mutations that occurred in several sites within the ALMT1 gene (Raman et al. 2005) and different materials evaluated in those studies. The sequence differences that the gene markers captured seemed to be two of them. Sequence polymorphism in the promoter region was also reported to be important for this gene expression (Raman et al. 2005; Sasaki et al. 2006). It is also possible that those accessions without the ALMT1-CAP and ALMT1-SSR3a Al-resistant alleles contain an Al resistance gene for Al resistance different from the one on 4DL. But the ALMT1-CAP and ALMT1-SSR3a Al-resistant marker alleles appeared in most Jagger-derived cultivars, as well as in Atlas 66; hence, this marker is still a diagnostic marker of 4DL Al-resistance gene in many American hard winter wheat cultivars.
Because the ALMT1-CAP marker needs the additional step of restriction digestion, it is not a recommended marker for marker-assisted selection in breeding programs. More recently, Raman et al. (2005) identified SSR markers from intron 3 region of ALMT1 gene. This marker showed a high level of polymorphism among the accessions tested and was well correlated with ALMT1-CAP marker in this study. It can be used to replace ALMT1-CAP marker. Sasaki et al. (2006) reported that promoter sequence of ALMT1 determined the expression levels of ALMT1, therefore the capacity of Al-activated malate release. However, they also indicate that Japanese cultivars did not follow the specific pattern of promoter sequences as those of American cultivars. In another study, two SSR markers flanking the 4DL Al resistance gene have been identified (Ma et al. 2005). These markers can be used as high-throughput markers for marker-assisted breeding. Our results indicated that Jagger and Atlas 66 share the same allele at the Xwmc331 marker locus (151 bp), but a different allele at the Xgdm125 locus (159 bp for Atlas 66 and 161 bp for Jagger). If the haplotype of Atlas 66 is selected in this pool of genotypes, only seven cultivars would be considered to have the 4DL Al resistance gene, whereas if the Jagger haplotype is selected, 32 cultivars would be selected to have the 4DL Al resistance gene. Therefore, for marker-assisted selection of the 4DL Al resistance gene, both Jagger and Atlas 66 should be considered as the reference Al resistance genotypes. The haplotype of flanking markers for the 4DL Al resistance gene in Chinese/Japanese accessions is similar to Al-sensitive accessions from the U.S.A. such as Scout 66, suggesting that Al resistance in these wheat accessions might be controlled by a different allele of the 4DL Al-resistance gene, or possibly at a different locus. Further investigation of Al resistance in those cultivars may lead to discovery of new genes or mechanisms for Al resistance, and provide genetically diverse breeding parents for genetic improvement of wheat cultivars.
Abbreviations
- Al:
-
aluminum
- RRG:
-
relative root growth
- NRG:
-
net root growth
- HSS:
-
hematoxylin staining score
- ALMT :
-
Al-induced malate transporter
- SSR:
-
simple sequence repeat
References
Ahmad M (2002) Assessment of genomic diversity among wheat genotypes as determined by simple sequence repeats. Genome 45:646–651
Anderson JA, Churchill GA, Autrique JE, Sorrells ME, Tanksley SD (1993) Optimizing parental selection for genetic linkage maps. Genome 36:181–186
Aniol A, Gustafson JP (1984) Chromosome location of genes controlling aluminum tolerance in wheat, rye and triticale. Can J Genet Cytol 26:701–705
Berzonsky WA (1992) The genomic inheritance of aluminum tolerance in ‘Atlas 66’ wheat. Genome 35:689–693
Borlaug NE, Dowswell CR (1997) The acid lands: one of agriculture’s last frontiers. In: Moniz AC et al (eds) Plant–soil interactions at low pH. Brazilian Soil Science Society, Brazil, pp 5–15
Cox TS (1998) Deepening the wheat gene pool. J Crop Prod 1:1–25
de Sousa CNA (1998) Classification of Brazilian wheat cultivars for aluminium toxicity in acid soils. Plant Breed 117:217–221
Donmez E, Sears RG, Shroyer JP, Paulsen GM (2001) Genetic gain in yield attributes of winter wheat in the Great Plains. Crop Sci 41:1412–1419
Eyal Z, Talpaz H (1990) The combined effect of plant stature and maturity on the response of wheat and triticale accessions to Septoria tritici. Euphytica 46:133–141
Fisher JA, Scott BJ (1987) Response to selection for aluminium tolerance. In: Searle PGE, Davey BG (eds) Priorities in soil/plant relation. Research for plant production. The University of Sydney, Sydney, pp 135–137
Garvin DF, Carver BF (2003) Role of genotypes tolerant of acidity and aluminium toxicity. In: Rengel Z (ed) Handbook of soil acidity. Marcel Dekker, New York USA, pp 387–406
Kochian LV, Piñeros MA, Hoekenga AO (2005) The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 274:175–195
Luo MC, Dvorak J (1996) Molecular mapping of an aluminum tolerance locus on chromosome 4D of Chinese spring wheat. Euphytica 91:31–35
Ma H-X, Bai G-H, Carver BF, Zhou L-L (2005) Molecular mapping of a quantitative trait locus for aluminum tolerance in wheat cultivar Atlas 66. Theor Appl Genet 112:51–57
Mujeeb-Kazi A, Rosas AV, Roldan S (1996) Conservation of the genetic variation of Triticum tauschii (Coss) Schmalh (Aegilop squarrosa auct. non L.) in synthetic hexaploid wheats (T. turgidum L.s.lat. x T. tauschii; 2n = 6× = 42, AABB–DD) and its potential utilisation for wheat improvement. Genet Resour Crop Evol 43:129–134
Patterson FL, Roberts JJ, Finney RE, Shaner GE, Gallun RL, Ohm HW (1975) Registration of ‘Oasis’ wheat. Crop Sci 15:736–737
Peterson CJ, Shelton DR, Baenziger PS, Baltensperger DD, Graybosch RA, Worrall WD, Nelson LA, McVey DV, Watkins JE, Krall J (2001) Registration of ‘Wesley’ wheat. Crop Sci 41:260–261
Plaschke J, Ganal MW, Roder MS (1995) Detection of genetic diversity in closely related bread wheat cultivars using microsatellite markers. Theor Appl Genet 91:1001–1007
Raman H, Zhang K, Cakir M, Appels, RR, Garvin DF, Maron LG, Kochian LV, Moroni JS, Raman R, Imtiaz M, Drake-Brockman F, Waters I, Martin P, Sasaki T, Yamamoto Y, Matsumoto H, Hebb DM, Delhaize E, Ryan PR (2005) Molecular characterization and mapping of ALMT1, the aluminium-tolerance gene of bread wheat (Triticum aestivum L.). Genome 48:781–791
Raman H, Raman R, Wood R, Martin P (2006) Repetitive indel markers within the ALMT1 gene conditioning aluminium tolerance in wheat (Triticum aestivum L.). Mol Breed 18:171–183
Rejesus R, Smale M, Van Ginkel M (1996) Wheat breeders’ perspectives on genetic diversity and germplasm use: findings from an international survey. Plant Var Seeds 9:129–147
Riede CR, Anderson JA (1996) Linkage of RFLP markers to an aluminum tolerance gene in wheat. Crop Sci 36:905–909
Rohlf FJ (1998) Numerical taxonomy and multivariate analysis system, version 2.0. Exeter Software, Setauket, NY
Samac DA, Tesfaye M (2003) Plant improvement for tolerance to aluminum in acid soils – a review. Plant Cell Tissue Organ Cult 75:189–207
Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H (2004) A wheat gene encoding an aluminum-activated malate transporter. Plant J 37:645–653
Sasaki T, Ryan PR, Delhaize E, Hebb DM, Ogihara Y, Kawaura K, Noda K, Kojima T, Toyoda A, Matsumoto H, Yamamoto Y (2006) Sequence upstream of the wheat (Triticum aestivum L.) ALMT1 gene and its relationship to aluminum resistance. Plant Cell Physiol 47(10):1343–1354
Sears RG, Moffatt JM, Martin TJ, Cox TS, Bequette RK, Curran SP, Chung OK, Heer WF, Long JH, Witt MD (1997) Registration of ‘Jagger’ wheat. Crop Sci 37:1010
Stodart BJ, Raman H, Coombes N, Mackay M (2006) Evaluating landraces of bread wheat for tolerance to aluminium under low pH. Genet Resour Crop Evol DOI 10.1007/s10722-006-9150-0
Tang Y, Garvin DF, Kochian LV, Sorrells ME, Carver BF (2002) Physiological genetics of aluminum tolerance in the wheat cultivar Atlas 66. Crop Sci 42:1541–1546
Vitorello VA, Capaldi FR, Stefanuto VA (2005) Recent advances in aluminum toxicity and resistance in higher plants. Brazilian Journal of Plant Physiology 17:129–143
Von Uexküll HR, Mutert E (1995) Global extent, development and economic impact of acid soils. Plant Soil 171:1–15
Acknowledgements
We thank Dr. Gill in the Wheat Genetics Resources Center and Dr. Mujeeb-Kazi, formerly with CIMMYT, for providing synthetic hexaploid wheats. This paper reports the results of research only. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. This is contribution no. 07-95-J of the Kansas Agricultural Experiment Station, Manhattan, KS, USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Thomas B. Kinraide.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, LL., Bai, GH., Carver, B. et al. Identification of new sources of aluminum resistance in wheat. Plant Soil 297, 105–118 (2007). https://doi.org/10.1007/s11104-007-9324-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-007-9324-3