Abstract
In this study, we present the results of measurements and radiological impact of natural radioactivity in soil and groundwater of the Himalayan region in the Uttarakhand State of India. The concentrations of primordial radionuclides (226Ra, 232Th and 40K) in soil samples of the study area were determined using gamma-ray spectrometry by employing the NaI(Tl) detector. The concentrations of radon and uranium were also measured in potable groundwater samples using RAD7 and Inductively Coupled Plasma Mass Spectrometry (ICPMS) techniques, respectively. The average specific activities of 226Ra (116 Bq kg−1), 232Th (137 Bq kg−1) and 40K (735 Bq kg−1) in soil were found considerably higher than the corresponding global average values. The average concentrations of radon (35 Bq l−1) and uranium (1.3 µg l−1) in potable groundwater were found well within the safe limits recommended by the World Health Organization. The effects of natural radioactivity in soil and groundwater are discussed in terms of different risk assessment parameters and dose quantities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human population is continuously exposed to extra-terrestrial and terrestrial sources of radiation. Extra-terrestrial sources of radiation mainly include cosmic rays coming from earth’s outer atmosphere while the terrestrial radiation are gamma rays emitted from 40K and radionuclides of 226Ra and 232Th decay series present in soil, rocks and water. According to UNSCEAR (1982), cosmic and terrestrial radiations impart 40% and 50% of the total external radiation dose to humans, respectively [1]. The global mean value of radiation dose from the exposure to cosmic rays at sea level is 31 nGy h−1 [2, 3]. In India, the mean value of dose from the exposure to cosmic rays is 32 nGy h−1 [3, 4].The external exposure to gamma rays emitted from 226Ra, 232Th and 40K is an important contributor to natural background radiation dose. These radionuclides are distributed in varying concentrations in soil and rocks. Owing to their highly unstable nature, these radionuclides spontaneously decay into their daughters with the emission of alpha, beta and gamma radiations. Gamma rays can penetrate the body internally and externally as well. The harmfulness of external radiation exposure depends upon the abundance of naturally occurring gamma emitting radionuclides in soil and rocks which in turn is associated with the geology of a particular area [5,6,7]. The contributions of 40K, 238U, and 232Th to global mean value (54 nGy h−1) of absorbed gamma dose rate in outdoor environment is 35%, 25% and 40%, respectively [1]. Furthermore, the distribution of 226Ra and 232Th in a particular region is directly associated with the levels of 222Rn, 220Rn and their progeny which are major contributors to natural background radiation dose received by the human beings [8]. The variation of natural radionuclides is also useful in identifying geo-chemical processes. The natural radionuclides present in the soil also migrate and transfer to biological systems like plants, trees etc. and become a potential source of internal radiation via intake of vegetables, fruits etc. [9]. Thus, a systematic study of natural radionuclides plays a key role in radiation protection, geo scientific studies and in establishing guidelines for the mitigation of these radionuclides. It is, therefore, important to carry out systematic studies to determine the levels of 226Ra, 232Th and 40K and to estimate associated health risks for human beings.
In addition to soil radioactivity, radiation exposure via drinking water route is also an important parameter in radiation protection. In general, drinking water is mainly obtained from groundwater sources, which may be contaminated by several radioactive elements. The concentrations of these elements depend on a physical, chemical and geological feature of the aquifer [10, 11]. Radon and uranium in drinking water are key radioelements from the health risk point of view. The health risk associated with radon in drinking water depends on type of water sources such as groundwater, surface water, tap water etc. If the drinking water is made available from groundwater sources, the radiation risk becomes high due to usually found high radon concentration in groundwater. Further, the levels of radon dissolved in water depend upon rock formation such as alluvial rocks, hard rocks etc. Lower levels of dissolved radon are found in alluvial rock formation as compared to hard rock formation [12]. A positive relation of radon levels in groundwater with the soil depth below ground is reported for hard rock formation of Nalgonda, Andhra Pradesh, India [13]. Radon in drinking and household water is linked with ingestion and inhalation doses to the human population, respectively [14]. Radon ingested through drinking water route is recognized as one of the possible causes of stomach cancer [15, 16]. On the other hand, radon released from household water to indoor air becomes the main contributor to indoor radon and its short lived progeny which contributes more than 50% of the natural background radiation dose to human beings [8]. The radiation exposure to indoor 222Rn and its daughters on human health are indicated as the most important cause of lung cancer after smoking [17]. Furthermore, the groundwater plays a key role in the migration and redistribution of uranium in the earth’s crust. A decreasing trend of uranium concentration in groundwater with soil depth has been reported in a recent study carried out at Nalgonda area of Andhra Pradesh, India [13]. Uranium in drinking water is dangerous to the human population due to its radiological and chemically toxicity. The modes of intake of uranium by the human body are inhalation, ingestion and other (industrial and occupational) exposures. Among these routes of exposure, the ingestion mode is the most important one. Uranium ingested through drinking water route is linked with harmful radiological (carcinogenic) and chemical (non-carcinogenic) effects on human health. The radiological health risk is due to the ionizing radiation of uranium isotopes whereas the chemical risk is due to chemical toxicity of uranium as heavy metal. Some of the diseases associated with the exposure to uranium in drinking water are leukemia, stomach cancer, urinary track cancer, kidney toxicity etc. [18,19,20,21]. A reference level of 30 µg l−1 has been suggested by the World Health Organization (WHO) as a limit of maximum concentration for uranium in drinking water [22]. In India, the Atomic Energy Regulatory Board (AERB), Department of Atomic Energy, Govt. of India has suggested a value of 60 µg l−1 as a limit for uranium in drinking water on radiotoxicity [23]. It is therefore, very important to carry out systematic studies on radon and uranium in drinking water sources. In Garhwal Himalayan region, a number of surveys have been carried out in the past on the occurrence of radionuclides in soil [24,25,26,27]. However, Mandakini valley has not been covered in these surveys. The radionuclide surveys in this part of Garhwal Himalaya are important due to its different geological structure. The present study has been designed and performed to investigate the levels and radiological effects of natural radioactivity in soil and groundwater of the Himalayan region in the Uttarakhand state of India. For this purpose, activity levels of 226Ra, 232Th and 40K were determined in soil samples collected from Mandakini valley using gamma ray spectrometry employing NaI (Tl) detector. The concentrations of radon and uranium were also measured in potable groundwater samples using RAD7 and Inductively Coupled Plasma Mass Spectrometry (ICPMS) techniques, respectively. The radiological impact of natural radioactivity in soil and potable groundwater on human health is estimated in terms of various hazard assessment quantities. The present work will be helpful in providing baseline data of natural radioactivity levels in soil and groundwater for future studies as well as the radiation protection program of the country.
Geology of the study area
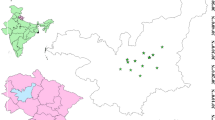
The geological map of the study area showing major formations and sampling locations is shown in Figs. 1 and 2. The investigated region comprises of Mandakini and Bhagirathi valleys of Garhwal Himalaya, India. A total of 38 soil samples were collected from Mandakini valley of Garhwal Himalaya for radionuclide analysis. The results and effects of radon and uranium measurements in water samples collected from Mandakini valley were reported in our recent studies [28, 29]. However, a total of 30 water samples collected from Bhagirathi valley were analyzed for radon and uranium measurements. The major geological formations in Garhwal Himalayan region along Mandakini valley are Berinag, Rautganga, Bhatwari and Munsiari formations. Origin of the Mandakini River is Chorabari glacier at an altitude of 3860 m above sea level. At Rudraprayag (895 m), the Mandakini River joins Alaknanda River. The investigated region is located in Vaikrita Group of Central Crystalline, which is made up of the Munsiari, Joshimath, Pandukeshwar and Pindari fromations [30]. The main rock composition in the Kedarnath region of Munsiari formation is mica shist, calc silicate lances, quartzite and phyllonites etc. [31]. The geological formation of Bhagirathi valley comprises of Lesser Himalayan Protozoic sequence and Higher Himalayan Crystalines (Bhatwari Group and Harshil Group). The Bhatwari group is composed of porphyroclastic granite gneiss, garnetiferous mica schist, amphibolite, mylonitized augen gneiss, mica schist, amphibolite, phyllonite and schist rocks [32].
Geological map [33] of Mandakini valley showing soil sampling locations
Geological map [32] of Bhagirathi valley showing water sampling locations (A1): porphyroclastic granite gneiss, garnetiferous mica schist, amphibolite, (A2): Mylonitized augen gneiss, mica schist, amphibolite, (A3): Phyllonite, schist
Materials and methods
Soil sample preparation
A total of 38 soil samples were collected from the top surface up to 15 cm below the ground. The collected samples were crushed to fine powder and dried in an oven at about 110 °C for about 24 h after removing organic material, stones, pebbles, roots and vegetation. The samples were then sieved through a sieve of 150 µm size to obtain the fine quality of samples so as to get large surface area. The samples processed in this way were sealed in Marinelli beakers and stored for a period of at least one month for establishment of secular equilibrium among 226Ra, 232Th and their decay products taking care to prevent 222Rn escaping from the beakers. The prepared samples were analyzed for radionuclides measurements using gamma ray spectrometry (Fig. 3).
Measurements of ambient dose equivalent rates
The ambient dose equivalent rates (µSv h−1) were measured at 1 m above the ground in all sampling locations using a GM tube based portable gamma survey meter (AT6130A, ATOMTEX, Australia). This instrument is capable of detecting gamma rays in the energy range of 20 keV to 3 MeV and can store 2000 measurement results in its non-volatile memory. The device is calibrated by the company and displays the results on its LCD screen. The geometric mean of gamma dose rate values over all sampling locations may be considered as representative value of gamma dose rate for the region.
Radionuclide analysis using gamma ray spectrometry
The levels of 226Ra, 232Th and 40K in the soil samples were determined by using a gamma-ray spectrometric system manufactured by ATOMTEX (Belarus) installed at Nuclear Research Laboratory, H.N.B. Garhwal University, Tehri Garhwal. A NaI(Tl) scintillation detector of size 63 mm × 63 mm is employed in the spectrometric system for the detection of natural radionuclides. The details of measurement procedure, calibration of detector etc. are given elsewhere [24,25,26,27, 34].
Measurements of uranium concentration in potable groundwater
The measurements of uranium in 30 potable groundwater samples were carried out using inductively coupled plasma mass spectrometry (ICPMS) technique (Perkin Elmer, model-ELAN DRC-e). ICPMS is a an analytical technique for accurate and reliable measurements of various elements. In this technique, the water samples to be analyzed are ionized with inductively coupled plasma source and then mass spectrometry is utilized for the detectection and quantification of various elements. The detailed description of sampling procedure and measurement technique is given elsewhere [28].
Measurements of radon concentrations in potable groundwater
Radon concentration in potable groundwater was measured using a semiconductor detecor based RAD7 monitor with RAD H2O accessory. The semiconductor detector used in this monitor is silicon detector which converts the alpha radiation into an electrical signal. The detailed methodology for the measurement of radon in water using RAD7 monitor is given Prasad et al. [29].
Risk assessment
The radiological effects of natural radionuclides present in soil are expressed in terms of following risk assessment quantitites.
Radium equivalent activity
The distribution of 226Ra, 232Th and 40K is not uniform in soil or rocks. The effects of these radionuclides on human body also vary from one radionuclide to other. The combined effect of these three radionuclides on human body can be expressed in terms of various hazard assessment quantities. Radium equivalent activity (Raeq) is one of these quantities which is expressed as follows [35]:
where ARa, ATh and AK are activities of 226Ra, 232Th and 40K in Bq kg−1, respectively.
External hazard index (Hex)
The external exposure to gamma radiations emitted from 226Ra series elements, 232Th series elements and 40K is expressed in terms of external hazard index (Hex). The external hazard index (Hex) is widely used in radiological protection and is defined as follows [35]:
where ARa, ATh and AK are the activities of 226Ra, 232Th and 40K in Bq kg−1, respectively. The external hazard index must be less than unity for the safety purpose [35]:
Internal hazard index (Hin)
226Ra, 232Th and 40K may be potential source of internal exposure to the households if the local soils and/or rocks are used as building material. The internal exposure is mainly due to inhalation of alpha radiation emmited from indoor 222Rn, 220Rn and their progeny. In radiological protection, the widely used unit of internal exposure is internal hazard index (Hin) which is defined as follows [35]:
Gamma index (Iϒ)
The gamma index (Iϒ) is the measure of external exposure gamma rays emitted from building materials. It takes into account the quantities of soil and/or rocks and the ways in which they are used in construction of a building. It is defined by European Commission as follows [35]:
The values of Iϒ ≥ 0.5 and Iϒ ≥ 0.5 correspond to a dose rate criteria of 0.3 mSv y−1 and 1 mSv y−1, respectively. The building materials with Iϒ ≥ 1 should be avoided for safety purpose.
Alpha index
The radiation exposure to inhaled 222Rn originated from 226Ra present in the building materials can be estimated in terms of alpha index (Iα), which is defined as follows [35]:
The recommended maximum value of 226Ra activity is 200 Bq kg−1, which gives Iα = 1. If activity concentration of 226Ra in building materials exceeds 200 Bq kg−1, the radon exhaled from building materials to indoor environment can be equal to higher than 200 Bq m−3 which may cause lung cancer in households. Therefore, 226Ra content in building materials should not exceed 200 Bq kg−1. In other words, Iα must lie within unity for safe use of soil or rocks as building materials.
Air absorbed dose rate
The air absorbed dose rate {D (nGy h−1)} from the exposure to 226Ra, 232Th and 40K at 1 m height from the earth’s surface can be calculated from activity concentrations of 226Ra, 232Th and 40K in soil as follows [35]:
It can be considered that 238U is in secular equilibrium with 226Ra in the given sample of soil. However, if any disequilibrium exists between 226Ra and 238U, it does not affect the dose assessment significantly if one calculates the dose from 226Ra instead of 238U. This is due to the fact that about 98% of the dose from 238U decay series is contributed by the elements of 226Ra sub-series [8].
Annual effective dose
The annual average effective dose rates can be calculated from air absorbed dose rate using following relation given by UNSCEAR [8].
where 0.7 Sv Gy−1 is the factor which is used to convert absorbed dose rate (nGy h−1) to equivalent effective dose rate (mSv y−1), 0.8 and 0.2 are the indoor and outdoor occupancy factors, respectively. The multiplication factor of 10−6 is used to convert the unit of effective dose from nSv to mSv.
Health risks associated with uranium in drinking water
Radiological risk assessment
The health risk associated with the ionizing radiation arising from radioactive isotopes of uranium in drinking water is known as radiological risk. It can be defined in terms of excess cancer (ECR) as follows [36]:
where AU represents activity concentration (Bq l−1) of uranium and R is the risk factor. Further, R is defined as follows:
where r is cancer risk constant (1.19 × 10−9) of uranium for mortality and I is per capita activity intake of uranium via drinking water. The value of I can be calculated by multiplying water consumption rate (4.05 l day−1) with average life (23250 days) of a person [37].
Chemical risk assessment
The health risk due to the chemical toxicity of uranium as a heavy metal is termed as chemical risk associated with uranium in drinking water. It can be defined in terms of lifetime average daily dose (LADD) as follows [38,39,40,41]:
where AU is uranium activity in µg l−1, IR is ingestion rate (4.05 l d−1), ED is exposure duration (63.7 years i.e. 23,250 days), EF is exposure frequency (365 d y−1), BW is average body weight (70 kg) and AT is the averaging time i.e. life expectancy (63.7 years i.e. 23,250 days).
Hazard quotient (HQ)
The extent of harm produced by the consumption of uranium via drinking water can be expressed in terms of hazard quotient (HQ) which is defined as a ratio of LADD to reference dose i.e.
where RfD is the reference dose [28, 41]. The reference doses (RfD) recommended by the World Health Organization (WHO), the Health Canada, the United States Environmental Protection Agency (USEPA) and the Atomic Energy Regulatory Board (AERB) are given in Table 4. In this study, the value of RfD (4.53 µg kg−1 d−1) recommended by AERB was used for HQ calculation.
Dose assessment from the exposure to uranium via drinking water
The ingestion doses associated with the consumption of uranium via drinking water can be calculated using following expression given by IAEA [28, 42, 43].
where AU, I and F is the uranium activity in water (Bq l−1), daily water intake (l d−1) and dose conversion factor (4.5 × 10−8 µSv y−1/Bq l−1 for 238U), respectively. The daily water intake (I) for the people of different age groups are given in Table 5.
Dose assessment from the exposure to 222Rn in water
Radon in water affects the human body in two ways. First, the radon ingested through drinking water route imparts radiation dose to stomach. Second, the radon escaped from household water to indoor air can be a potential contributor to inhalation dose to lungs. Thus, stomach and lungs both are affected by high levels of radon in water.
The annual ingestion dose (mSv y−1) due to radon in drinking water for the people of different age groups can be estimated as follows [29]:
where ARn is activity concentration of radon in water (Bq l−1), DWI is daily water intake (l d−1), DCF is dose conversion factor (10−8 Sv Bq−1) and t is time of exposure (365 days y−1) [29].
The inhalation dose associated with the exposure to 222Rn escaped from household water to indoor environment can be calculated as follows [29]:
where ARn is 222Rn concentration (Bq m−3) in water, Ra/Rw = 10−4, Ra is 222Rn concentration in air, Rw is 222Rn concentrations water, F is equilibrium factor (0.4) between 222Rn and progeny, TIndoor is indoor occupancy factor (0.8) and DCF is dose conversion factor (9 nSv Bq−1 h−1 m3).
Results and discussion
Radionuclides distribution in soil and its radiological implications
The measured values of 226Ra, 232Th and 40K and estimated hazard assessment quantities are given in Table 1.
The measured values of 226Ra, 232Th and 40K concentrations ranged from 34.2 ± 4.43 to 229 ± 23.4 Bq kg−1 with an average of 97 Bq kg−1, 28.7 ± 9.3 to 295 ± 29.5 Bq kg−1 with an average of 129 Bq kg−1 and 15.2 ± 3 to 1360 ± 194 Bq kg−1 with an average of 541 Bq kg−1, respectively. The average values of measured 226Ra, 232Th and 40K concentrations were found considerably greater than the corresponding values at global level given by UNSCEAR [8]. According to UNSCEAR, global average values of 226Ra, 232Th and 40K in soil are 35, 30 and 400 Bq kg−1, respectively [8]. A wide variation was observed in the distribution of natural radionuclides in the area. The radiological effects of natural radionuclides present in the soil of study area were determined in terms of radium equivalent activity (Raeq), external hazard index (Hex), internal hazard index (Hin), gamma index (ϒ) and alpha index (α). Raeq was found to vary from 146 to 599 Bq kg−1 with an average of 322 Bq kg−1. The mean value of estimated Raeq is well within the safe limit of 370 Bq kg−1 [4]. Frequency distributions for radium equivalent activity is shown in Fig. 4. It is worth noticing that 10 out of 38 analyzed samples have Raeq values higher that the safe limit of 370 Bq kg−1.
The calculated values of external hazard index (Hex), internal hazard index (Hin), gamma index (Iϒ) and alpha index (Iα) were found to range from 0.40 to 1.62 with an average of 0.87, 0.49 to 2.06 with an average of 1.13, 0.54 to 2.08 with an average of 1.15 and 0.17 to 1.15 with an average of 0.48, respectively. The variation of these hazard indices is shown in Fig. 5. It is clear from the figure that at significant number of locations the values of hazard indices are greater than unity which indicates the possibility of radiation hazard if the local soil and/or rocks is brought in use for construction of buildings. However, in majority of locations the values of hazard indices are well within unity (the safe limits of radionuclides exposure).
Radiation dose rates due to natural radionuclides in soil
The descriptive statistics for measured air absorbed gamma dose rates, estimated air absorbed gamma dose rates and annual effective dose rates (indoor and outdoor) due to natural radionuclides in soil are shown in Table 2. The estimated air absorbed gamma dose rate from the exposure to 226Ra, 232Th and 40K in soil was found to be in the range of 69 to 261 nGy h−1 with a mean value of 145 nGy h−1. The contributions of 226Ra, 232Th and 40K to average air absorbed gamma dose rate were found to be 45 nGy h−1, 78 nGy h−1 and 22 nGy h−1, respectively. The air absorbed gamma dose rate measured using a radiation survey meter directly at sampling locations was found to vary from 80 to 260 nGy h−1 with an average of 150 nGy h−1. The annual effective dose rates in the indoor and outdoor environments were found to vary from 0.3 to 1.3 mSv y−1 with an average of 0.7 mSv y−1 and 0.1 to 0.3 mSv y−1 with an average of 0.2 mSv y−1, respectively. The estimated annual effective dose rates at all locations are far less than the global average value of 2.4 mSv y−1 received from all natural background radiation sources [8]. According to European Commission (EC), building materials should be avoided if the excess gamma radiation originating from them exceeds the annual effective dose of an individual by 0.3 mSv y−1 [44].
The values of air absorbed gamma dose rate estimated using soil radioactivity was found in good agreement with the ambient dose equivalent rate values measured using portable survey meter. A correlation between the air absorbed gamma dose rates estimated from soil activity and ambient dose equivalent rates directly measured using pocket survey meter (GM detector) is shown in Fig. 6. A strong positive correlation (R = 0.99, slope = 0.97) between estimated and measured data shows a good agreement between two methods. This validates the accuracy and reliability of results.
Levels and effects of uranium in drinking water
The measurements of uranium concentration in drinking water samples collected from 30 groundwater sources (one sample from each source) located in Bhagirathi valley of Garhwal Himalaya, India were carried out using ICMPS. The descriptive statistics for uranium activity in drinking water samples of investigated region along with associated radiological (carcinogenic) and chemical (non-carcinogenic) health risks are shown in Table 3. The measured values of uranium concentration were found to vary from 0.02 ± 0 to 6.2 ± 0.15 µg l−1 with an average of 1.3 µg l−1. The concentration of uranium in all drinking water samples were found well within the safe limit of 30 µg l−1 recommended by World Health Organization (WHO) and United States Environmental Protection Agency (USEPA) [22, 45]. In India, the Atomic Energy Regulatory Board (AERB), Department of Atomic Energy, Govt. of India has prescribed a limit of 60 µg l−1 for uranium in drinking water on radiotoxicity [23]. Frequency distribution of uranium concentration in the analyzed drinking water samples is shown in Fig. 7. The concentration of uranium in 22 out of 30 analyzed samples was found in the range of 0.02 to 2 µg l−1. The calculated values of excess cancer risk (ECR) linked with the exposure to uranium in all drinking water samples were found well below the safe limits recommended by WHO, Health Canada, USEPA and AERB. The recommendations of these health agencies on uranium concentration and its radiological risk (ECR) in drinking water are given in Table 4. The hazard quotient (HQ) which is the measure of chemical toxicity of uranium in drinking water was found to range from 0.0003 to 0.08. No samples were found to have HQ ˃ 1, indicating that there is not risk due to chemical toxicity of due to ingestion of uranium drinking water.
The ranges and mean values of uranium concentrations in drinking water samples from different parts of India are shown in Table 5. In the Himalayan region of Himachal Pradesh (Kangra, Mandi, Kullu & Shimla areas), the uranium concentration is reported to be in the range of 0.56 to 10.11 µg l−1 with an average of 2.17 µg l−1 [47]. The uranium concentration in potable groundwater (ranging between 0.02 ± 0 to 63.7 ± 4.3 µg l−1 with a mean of 7 µg l−1) in Mandakini valley of Garhwal Himalaya is recently reported in our recent study [28]. It has been observed that the concentrations of uranium in potable groundwater of Mandakini valley are considerable higher than those observed in Bhagirathi valley. The higher values of uranium concentrations in Mandakini valley of Garhwal Himalaya are attributed to localised uranium mineralization in the form of granitic rocks in the upper part of Mandakini valley [28]. The estimated values of age dependent ingestion dose rates from the exposure to uranium through drinking water is presented in Table 6. The calculated dose rates were observed well within 100 μSv y−1, a reference value recommended by WHO [41].
Levels and effects of radon in drinking water
As stated earlier, the measurements of radon concentrations were carried out in potable groundwater samples collected from 30 locations in Bhagirathi valley of Garhwal Himalaya. The descriptive statistics for radon concentrations in potable groundwater samples and associated age dependent doses are shown in Table 7. Radon concentration in analyzed groundwater samples was found to vary from 22 to 58 Bq l−1 with an average of 35 Bq l−1. It has been observed that radon concentration in all the analyzed samples were found to exceed the safe limit of 11 Bq l−1 recommended by USEPA [46]. However, all water samples were found to have radon concentration well withing the maximum concetration limit of 100 Bq l−1 recommended by WHO for its safe use as drinking purpose [41]. The frequency distribution of radon concentration in water samples is shown in Fig. 8. The high levels of radon in analyzed water samples may due to reason that samples were directly taken from groundwater sources. The measurements were performed on the sampling spots immediately after taking samples from groundwater (springs and bore wells) sources so as to avoid the release of radon from water samples. The comparison of ranges and average values of radon concentrations from diffeent parts of India are shown in the Table 8. The average value of radon concentration (35 Bq l−1) in groundwater of Bhagirathi valley is lower than the recently reported [29] values of radon concentration in groundwater (45 Bq l−1) in Mandakini valley but higher than those in Karnprayag area (15 Bq l−1) and New Tehri area (31 Bq l−1) of Garhwal Himalaya. The higher values of radon concentration in Mandakini valley may be attributed to the fault fault-lineament (FL) type and fractured joint (FJ) type springs in this valley. These types of springs present an increased rock surface area to water volume ratio; thereby increasing the radon emission rate [29]. However, the springs in Karnparayag and New Tehri areas are colluvial (CL) and fluvial deposit (FD) type springs which possess high water carrying capability, high permeability and high porosity. The high porosity of these springs results in the migration of radon gas into atmosphere; thereby decreasing the radon concentration in water [29].
The measured values of radon cocentrations in potable groundwater samples of Bhagirathi valley were used to calculate annual effective dose due to ingestion for different age groups and inhalation of radon released from household water. The observed values of age dependent ingestion dose and inhalation dose are presented in Table 7.
Conclusions
The activity levels of natural radionuclides (226Ra, 232Th & 40K) in soil samples at majority of locations in Mandakini valley of Garhwal Himalaya are higher than the corresponding average values in the world. The high values of natural radionuclides in soil samples show the uranium and thorium mineralization in the region. The observed high values hazard assessment quantities calculated for the soil of the region indicate that use of local soil and/or rocks as building materials should be avoided in the investigated region in order to protect the dwellers from possibility of radiation hazards. The levels of uranium in all drinking water samples of Bhagirathi valley of Garhwal Himalaya are well within the safe limits recommended by WHO and USEPA. The activity levels of radon in potable groundwater at majority of locations are higher than the reference value of 11 Bq l−1 recommended by USEPA. However, radon levels in potable groundwater are well within the safe limit of 100 Bq l−1 recommended by WHO. The results of measurements of natural radioactivity levels in soil and groundwater will be useful in radiation protection, geo scientific research, exploration of radionuclide minerals and in establishing guidelines for radiation protection program of the country. In addition, the results of radioactivtiy in soil and groundwater will be helpful in understanding the geochemical processes in the Himalayan regions.
References
UNSCEAR (1982) United nations scientific committee on the effects of atomic radiation. Exposures resulting from nuclear explosions, New York
UNSCEAR (2008) United nations scientific committee on the effects of atomic radiation. report to the general assembly, vol. 1, Annex. B. United Nations Publication, United Nations, New York
Karunakara N, Yashodhara I, Kumara KS, Tripathi RM, Menon SN, Kadam S, Chougaonkar MP (2014) Assessment of ambient gamma dose rate around a prospective uranium mining area of South India–a comparative study of dose by direct methods and soil radioactivity measurements. Results Phys 4:20–27
Nambi KSV, Bapat VN, David M, Sundaram VK, Sunta CM, Soman SD (1986) Natural background radiation and population dose distribution in India. Heal. Phys. Div. Bhabha At. Res. Centre, Bombay
Papadopoulos A, Christofides G, Koroneos A, Poli G (2013) Concentration of 238U and 232Th among constituent minerals of two igneous plutonic rocks exhibiting elevated natural radioactivity levels. J Radioanal Nucl Chem 298:639–650
Quindos LS, Fernandez PL, Soto J, Rodenas C, Gomez J (1994) Natural radioactivity in Spanish soils. Health Phys 66:194–200
Radhakrishna AP, Somashekarappa HM, Narayana Y, Siddappa K (1993) A new natural background radiation area on the southwest coast of India. Health Phys 65:390–395
UNSCEAR (2000) United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and effects of ionizing radiation. Report to the General Assembly, Annexe B. United Nations Publication, United Nations, New York
Doyi IN, Essumang DK, Dampare SB, Duah D, Ahwireng AF (2017) Evaluation of radionuclides and decay simulation in a terrestrial environment for health risk assessment. Sci Rep 7:16537. https://doi.org/10.1038/s41598-017-16659-w
Przylibski TA, Gorecka J (2014) 222Rn activity concentration differences in groundwaters of three Variscan granitoid massifs in the Sudetes (NE Bohemian Massif, SW Poland). J Environ Radioact 134:43–53
Sharma DA, Rishi MS, Keesari T et al (2017) Distribution of uranium in groundwaters of Bathinda and Mansa districts of Punjab, India: inferences from an isotope hydrochemical study. J Radioanal Nucl Chem 313(3):625–633
Sharma DA, Keesari T, Rishi M et al (2019) Radiological and hydrological implications of dissolved radon in alluvial aquifers of western India. J Radioan Nucl Chem. https://doi.org/10.1007/s10967-019-06619-1
Keesari T, Mohokar HV, Sahoo BK et al (2014) Assessment of environmental radioactive elements in groundwater in parts of Nalgonda district, Andhra Pradesh, South India using scintillation detection methods. J Radioan Nucl Chem. 302:1391–1398
Aditya VS, Chidambaram S, Keesari T et al (2017) Occurrence of uranium in groundwater along the lithological contacts in central Tamilnadu. An Isotope Hydrogeochemical Perspective. Expo Health, India. https://doi.org/10.1007/s12403-017-0269-3
Todorovic N, Nikolov J, Forkapic S et al (2012) Public exposure to radon in drinking water in Serbia. Appl Radiat Isot 70:543–549
Ravikumar P, Somashekar RK (2014) Determination of the radiation dose due to radon ingestion and inhalation. Int J Environ Sci Technol 11:493–508
WHO (2009) WHO handbook on indoor radon: a public health perspective. World Health Organization, Geneva
Auvinen A, Salonen L, Pekkanen J, Pukkala E, Ilus T, Kurttio P (2005) Radon and other natural radionuclides in drinking water and risk of stomach cancer: a case-cohort study in Finland. Int J Cancer 114:109–113
Kurttio P, Salonen L, Ilus T, Pekkanen J, Pukkala E, Auvinen A (2006) Well water radioactivity and risk of cancers of the urinary organs. Environ Res 102:333–338
Prat O, Vercouter T, Ansoborlo E, Fichet P, Perret P, Kurttio P, Salonen L (2009) Uranium speciation in drinking water from drilled wells in southern Finland and its potential links to health effects. Environ Sci Technol 43:3941–3946
Zamora ML, Zielinski JM, Moodie GB, Falcomer RA, Hunt WC, Capello K (2009) Uranium in drinking water: renal effects of long-term ingestion by an aboriginal community. Arch Environ Occup Health 64:228–241
WHO (2011) Guidelines for drinking-water quality, 4th edn. World Health Organization, Geneva
AERB (2004) Drinking water specifications in India. Department of Atomic Energy, Govt. of India
Yadav M, Rawat M, Dangwal A, Prasad M, Gusain GS, Ramola RC (2014) Levels and effects of natural radionuclides in soil samples of Garhwal Himalaya. J Radioanal Nucl Chem 302:869–873
Ramola RC, Yadav M, Gusain GS (2014) Distribution of natural radionuclide along Main Central Thrust in Garhwal Himalaya. J Radiat Res Appl Sci 7:614–619
Yadav M, Rawat M, Dangwal A, Prasad M, Gusain GS, Ramola RC (2015) Analysis of natural radionuclides in soil samples of Purola area of Garhwal Himalaya, India. Rad Prot Dosim 167:215–218
Kandari T, Prasad M, Pant P, Semwal P, Bourai AA, Ramola RC (2018) Study of radon flux and natural radionuclides (226Ra, 232Th and 40K) in the Main Boundary Thrust region of Garhwal Himalaya. Acta Geophys 66:1243–1248
Prasad M, Kumar GA, Sahoo SK, Ramola RC (2018) Health risks associated with the exposure to uranium and heavy metals through potable groundwater in Uttarakhand state of India. J Radioan Nucl Chem. https://doi.org/10.1007/s10967-018-6281-70123456789
Prasad M, Kumar GA, Sahoo BK, Ramola RC (2018) A comprehensive study of radon levels and associated radiation doses in Himalayan groundwater. Acta Geophys 66:1223–1231
Heim A, Gansser A (1939) Central Himalaya: geological observations of the Swiss expedition 1936. Denkschriften der Schwieizerishen Naturforschenden Gasellschaft
Valdiya KS, Paul SK, Chandra T, Bhakuni SS, Upadhyay RC (1999) Tectonic and lithological characterization of Himadri (Great Himalaya) between Kali and Yamuna rivers, Central Himalaya. Him Geol 20:1–17
Manickavasagam RM, Jain AK, Singh S et al (1999) Metamorphic evolution of the northwest Himalaya, India: pressure temperature data, inverted metamorphism and exhumation in the Kashmir, Himachal and Garhwal Himalayas. Geol Soc Am Spec Pap 328:179–198
Choubey VM, Bartarya SK, Ramola RC (1999) Radon in Himalayan Springs: a geohydrological control. Environ Geol 39(6):523–530
Shanbhag AA, Sartandel SJ, Ramachandran TV, Puranik VD (2005) Natural radioactivity concentrations in Beach sands of Ratnagiri coast, Maharastra. J Assoc Environ Geochem 8:304–308
Ramola RC, Gusain GS, Badoni M, Prasad Y, Prasad G, Ramachandran TV (2008) 226Ra, 232Th and 40K contents in soil samples from Garhwal Himalaya, India and its radiological implications. J Radiol Prot 28:379–385
USEPA (2000) National primary drinking water regulations, radionuclides. United States Environmental Protection Agency, Washington, D.C.
HDR (2009) Human Development Report, National Resource Centre for urban poverty and All India institute of Local Self Government, Mumbai
Singh L, Kumar R, Kumar S, Bajwa BS, Singh S (2013) Health risk assessments due to uranium contamination of drinking water in Bathinda region, Punjab state, India. Radioprotection 48:191–202
Lee JS, Chon HT, Kim KW (2005) Human risk assessment of As, Cd, Cu and Zn in the abandoned metal mine site. Environ Geochem Health 27:185–191
Health Canada (1999) Uranium in drinking water. Document for public comment prepared by federal provincial subcommittee on drinking water
WHO (2004) Guidelines for drinking-water quality, vol 1, 3rd edn. World Health Organization, Geneva
US National Academy of Science, Institute of Medicine, Food and Nutrition Board (2004) Dietary reference intakes for water, potassium, sodium, chloride and sulphate. The National Academies Press, Washington
Bronzovic M, Marovic G (2005) Age-dependent dose assessment of 226Ra from bottled water intake. Health Phys 88:480–485
EC (1999) European Commission. Radiation protection 112, Radiological protection principles concerning the natural radioactivity of building material. Directorate–General Environment, Nuclear safety and civil protection
USEPA (2003) Current drinking water standards, Ground water and drinking water protection agency. United States Environmental Protection Agency, Washington, D.C.
Singh L, Kumar R, Kumar S et al (2013) Health risk assessments due to uranium contamination of drinking water in Bathinda region, Punjab state, India. Radioprotection 48(2):191–202
Rani A, Singh S, Duggal V et al (2013) Uranium estimation in drinking water samples from some areas of Punjab and Himachal Pradesh, India using ICP-MS. Radiat Prot Dosim 157(1):146–151
Patra AC, Mohapatra S, Sahoo SK et al (2013) Age- dependent dose and health risk due to intake of uranium in drinking water from Jaduguda, India. Radiat Prot Dosim 155(2):210–216
Rana BK, Dhumale MR, Lenka P et al (2015) A study of natural uranium content in groundwater around Tummalapalle uranium mining and processing facility, India. J Radioanal Nucl Chem 307(2):1499–1506
Yadav AK, Sahoo SK, Mahapatra S et al (2014) Concentrations of uranium in drinking water and cumulative, age-dependent radiation doses in four districts of Uttar Pradesh, India. Toxicol Environ. Chem 96(2):192–200
Jakhu R, Mehra R, Mittal HM (2016) Exposure assessment of natural uranium from drinking water. Environ Sci Process Impacts 18(12):1540–1549
Duggal V, Sharma S (2017) Chemotoxicity and radiotoxicity risk assessment from exposure to uranium in groundwater from Western Haryana, India. Int J Pure Appl Phys 13(1):107–112
Virk HS, Jakhu R, Bangotra P (2016) Natural uranium content in ground waters of Mohali and Fatehgarh Districts of North Punjab (India) for the assessment of excess cancer risk. Glob J Hum Soc Sci B 16:13–17
Kumar A, Kaur M, Sharma S et al (2016) A study of radon concentration in drinking water samples of Amritsar city of Punjab (India). Radiat Prot Environ 39:13–19
Mittal S, Rani A, Mehra R (2016) Radon levels in drinking water and soil samples of Jodhpur and Nagaur districts of Rajasthan, India. Appl Radiat Isot 113:53–59
Kumar M, Kaushal A, Sahoo SK et al (2017) Measurement of uranium and radon concentration in drinking water samples and assessment of ingestion dose to local population in Jalandhar district of Punjab, India. Indoor Built Environ 28(5):611–618
Duggal V, Sharma S, Mehra R (2017) Radon levels in drinking water of Fatehabad district of Haryana, India. Appl Radiat Isot 123:36–40
Prasad Y, Prasad G, Choubey VM et al (2009) Geohydrological control on radon availability in groundwater. Radiat Meas 44:122–126
Acknowledgements
Science and Engineering Research Board, Department of Science and Technology, Government of India is thankfully acknowledged for providing financial assistance in the form of National Post Doctoral Fellowship (File No: PDF/2016/000565).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Prasad, M., Ranga, V., Kumar, G.A. et al. Radiological impact assessment of soil and groundwater of Himalayan regions in Uttarakhand, India. J Radioanal Nucl Chem 323, 1269–1282 (2020). https://doi.org/10.1007/s10967-019-06827-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06827-9