Abstract
Uranium (U) concentration and other associated risks have been studied in ground water around a newly established U mining and processing facility at Tummalapalle, India. The U concentration in groundwater samples was found to vary between 0.38 and 79.70 μg l−1. Data analysis revealed that more than 85 % of the samples had U concentration lower than the USEPA and current WHO recommended guideline value of 30 µg l−1. The annual effective dose, the lifetime excess cancer risk and chemical toxicity risk from drinking of this water have been found to be less and were within the prescribed limit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Different human activities such as combustion of fossil fuels, exploration and processing of phosphate rocks, use of phosphate fertilizer in agriculture, smelting of metals, extraction of thorium, mining and processing of uranium (U) ore etc. can release natural U to the surrounding environment and may contaminate nearby underground aquatic system. In particular to a U mining and processing industry, groundwater contamination due to U could be a major concern and therefore ground water needs to be monitored for U content around the site prior to the establishment and operation of the industry. As we know intake of water is an essential part of the human diet and therefore, it can contribute to chronic natural radioactivity exposure due to presence of various dissolved radionuclides in it. Being a primordial radionuclide, U is omnipresent and poses some radiation risk to human life according to ICRP recommendation [1]. U readily dissolves in oxygen-rich water which accounts for its presence in surface water, groundwater and sea water. In an oxidising environment where ground water contain appreciable amount of dissolved oxygen, oxidises U present in the rocks to more mobile +6 state (UO 2+)2 and the leached U gets into the solution and transported through the groundwater. The health effects of U can be divided into carcinogenic and non-carcinogenic effects [2] and these classifications are based on the radiological risk by radiation of U isotopes and the chemical risk as a heavy metal. For ingested U, the main target organ of toxicity is the kidney [3, 4]. An exposure of about 0.1 mg kg−1 of body weight of soluble natural U results in transient chemical damage to the kidneys [5]. The United Nations Scientific Committee on the Effects of Atomic Radiation [6] has estimated that exposure to natural sources contributes more than 98 % of radiation dose to the human population (excluding medical exposure). Worldwide average annual exposure to natural radiation sources has been estimated to be in the range of 1–10 mSv, with 2.4 mSv being the present estimate of the mean value [6]. However, there is a large local variation in this exposure depending on a number of factors, such as height of the study area above sea level, the amount and type of radionuclides present in the soil and rocks, the amount taken into the body through inhalation of air and ingestion of food and drinking water. U concentration in groundwater mainly depends on lithology, geomorphology and other geological conditions of the area [7–9]. Contamination of groundwater due to U around several uranium mines has been studied worldwide [10–12]. In India U mining has been started in mid-1960’s in the East Singhbhum district of Jharkhand and recently a new U mine and processing plant has been started at Tummalapalle in the district of Kadapa of Andhra Pradesh. In India several studies have also been conducted to estimate natural U concentrations in groundwater around different proposed and operational U deposits and also in general areas where a wide range of U concentrations have been reported. [13–18]. The major objectives of the present study are to estimate U concentration in groundwater in the surrounding environment of the U mining and processing facility and associated health risk, if any, to local population through ingestion of water. Furthermore the generated data could be served as a baseline data for the study region and can be helpful in the future to assess the impact on adjoining groundwater if any because of U mining and milling activities after a longer period of time.
Study area
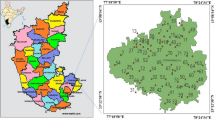
Groundwater sampling locations are shown in Fig. 1. Tummalapalle uranium deposit is located in the Vemula Mandal of Kadapa district in the state of Andhra Pradesh, which is about at a distance of 70 km from district headquarters. The nearest town is Pulivendula, which is about 15 km from the site (by road) towards the North West. The nearest railway station is Muddanuru on the south central railway, Hyderabad–Chennai BG line which is about 50 km towards the north east. The average ore grade of the deposit is about 0.040 % as U3O8 and 82 % of the rock is composed of carbonate and therefore, the favoured leaching of the ore has been planned to be carried out by a high temperature pressurised alkali leaching process. The study area is in the tropical region where the climate is characterised by very hot summers, mild winters and monsoon rains. In summer (March to June) the monthly temperatures range from maximum 46 °C during day time to a minimum of 15 °C in night and the winter is from November to February and the temperature varies from 40.6 °C in day time to 10.6 °C in night. The average rain fall recorded by India meteorological department (IMD) of Kadapa is 783.3 mm and 80 % of the rain fall took place during mid-June to mid-November. Further in the study region the average rain is still relatively less as compared to the Kadapa.
Geology of the study area
The area lies in the south western part of the crescent shaped to late Proterozoic Kadapa basin. The basin constitutes a metamorphosed to slightly metamorphosed thick (1500 m) arenaceous and argillaceous sedimentary sequence overlying the profound eparchean unconformity. Highly metamorphosed and deformed late archean to early proterozoic granite gneisses and dharwarian schists lie under this thick sedimentary pile. The sediments are mostly undisturbed on the SW margin of the basin while these are thrust over by Dharwarian schist and gneisses on the eastern margin. Tummalapalle U deposit is located in the middle of the south-western margin of Kadapa basin. Vempalle carbonate rock formation is the host rock of U mineralisation, forms the upper part of papaghni group of the rocks and is underlain by gulcheru quartzite.
Materials and methods
Sample collection and analysis
For this study, 106 groundwater (borehole and hand pump) samples were collected from 58 sampling locations within a 15 km radius around the Tummalapalle U mining and processing facility during the year 2013–2014 for a brief study of U content in groundwater. From each locations water sample were collected before and after the monsoon. 500 ml dry and pre cleaned polyethylene bottle was used for collection of water samples. After collection of sample it was filtered through 0.45 µm whatman filter paper and pH was measured. U content in groundwater sample was measured by LED based UV Fluorimeter (Model No. UA2, M/s. Quantalase Enterprises Pvt. Ltd., Indore, India) in which a pulsed LED UV light was used to excite uranyl species at 405 nm. A standard stock solution of 973 mg l−1 U (Sigma Aldrich make) was diluted to working concentrations for regular calibration and checking the performance of the instrument. Water sample analysis was carried out by standard addition method to avoid matrix effect and any other interference by different ions. 5 % sodium pyrophosphate solution was used as a fluorescence enhancing reagent that forms uranyl phosphate complexes which is more stable in the solution [19]. The pH of the reagent was maintained at 7.0 by ortho-phosphoric acid. 0.1 M HCl/0.1 M NaOH was used to adjust the pH of water samples prior to analysis of U in water sample. 5 ml water sample and 0.5 ml of 5 % sodium pyrophosphate were taken in cleaned cuvette and subjected to fluorescence reading by the instrument. To get blank counts, a blank sample was prepared using double distilled water with same amount of fluorescing reagent for measurement of U concentration. Both micropipettes and analytical balance were used simultaneously to avoid any error in pipetting. In standard addition method, U concentration in the sample was estimated by
where C s is the concentration of U in the sample (µg l−1), C st is the concentration of U standard solution (µg l−1), F s is the fluorescence due to sample only, F mix is the fluorescence due to sample and spiked U standard solution. V s is the volume of sample (ml), V st is the volume of U standard solution spiked (ml).
Quality assurance and quality control
The quality of the data was assured by cross method analysis. Ten water samples were analysed by both laser fluorimeter and LED based UV fluorimetry, which are the accepted and standard instruments and recommended method for ultra trace U analysis. The results were in good agreement with each other and the correlation coefficient was observed to be 0.99 (Fig. 2). Quality assurance was made by analysis of standard reference material, replicate analysis and cross method checking. All laboratory glassware used for sample processing was soaked in 10 % nitric acid for 15 days and then rinsed thoroughly with distilled and double distilled water, respectively before use. Reagent blank was taken along with each batch of sample preparation and concentrations observed in the reagent blank were subtracted from the same batch of samples.
Results and discussion
Distribution of U in the ground water
The statistical analyses data of measured U concentration in groundwater samples is presented in Table 1. Overall U concentration in the groundwater samples was found to vary from 0.38 to 79.7 µg l−1 with a mean value of 15.65 µg l−1. The distribution of measured U concentration was found to be following approximately close to the log-normal distribution and the same has been affirmed by χ 2 test. The geometric mean of U concentration was estimated to be 9.83 µg l−1. The available rocks in the study region are found to be granite and basic dyke, cherty and massive limestone, conglomerate, shale purple, dolomite and quartzite and further the area is known for uranium mineralisation. Therefore, a wide range of variation and marginally higher U concentration in groundwater has been assumed to be observed in the study area. Further to understand the behaviour of U concentration in ground waters at different distance from the U deposit, the study area was categorised to three zones such as Zone 1 (0–5 km), Zone 2 (6–10 km) and Zone 3 (11–15 km) assuming the mining site as the centre point of the study area and zone wises U concentrations were reported. The mean U concentrations in different zones were observed to be 4.45, 18.55 and 18.93 µg l−1 for Zone 1, Zone 2 and Zone 3, respectively. It has been observed slightly higher U concentration in two zones (Zone 2 and Zone 3) as compared to the Zone 1 and this may attributed to the local geology of the region and different types of rocks coming contact with the aquifer rather than any source of pollution. U concentrations in water depends on many factors such as U content in the host aquifer rocks and its chemical constituents, presence of oxygen and its complexion agent, chemical reaction with ions in solution and nature of contact between uraniferous minerals and water [20]. Further water samples collected from the area within the 5 km distance from the site (Zone 1) have shown less U concentration and this may be attributed to presence of many small hills within this area and the region is at higher altitude as compared to other two zones. Therefore, intensity of weathering and percolation of rain water was assumed to be less within the 5 km area and this may be a reason to observe less U concentration in water samples in the zone 1. Further water samples collected beyond 5 km radius (Zone 2 and Zone 3) showed U concentration slightly in the higher range. Frequency distribution of overall measured U concentration was studied and is presented in Fig. 3. Of the samples, 60 % were observed to be lower than the 2004 WHO’s guideline of 15 µg l−1 [21] and more than 85 % of the water samples showed U concentration lower than the USEPA and current WHO recommended drinking water guideline value of 30 µg l−1 [22, 23]. However, if we compare with the Indian regulatory limit of 60 µg l−1 as prescribed by Atomic Energy Regulatory Board [24], almost all samples showed U concentration below of this limit.
The first quartile, median and third quartile of the data were found to be 4.60, 10.89 and 22.88 µg l−1, respectively and all are within the current WHO recommended limit. Therefore, the available groundwater samples within the study region are assumed to be suitable for drinking purposes.
Comparison of U concentration with worldwide values
The values of U concentration observed in water samples in the present study were compared with other worldwide studies (Table 2). Uranium content in groundwater shows a wide variation in India and throughout the world. Variation in uranium concentration mainly depends on the geology of the area and other meteorological parameters. Sahoo et al., has been reported U concentration in the range of 0.1–19.6 µg l−1 in the drinking water samples collected from Orissa, Andhra Pradesh, Maharashtra, Madhya Pradesh, Kerala etc. [13], U concentration around uranium mining and processing facility of the Jaduguda, Jharkhand has been reported in the range of 0.5–28 µg l−1 [15] and around Narwapahar U mine in Jharkhand it has been in the range of 0.1–3.75 µg l−1 [14]. Further around the proposed U mining sites located at Lambapur and Pedagattu in the Nalgonda district of Andhra Pradesh, it has been reported in the range of 0.2–77.4 µg l−1 [18]. In Tiruvantapuram district of Kerla, U concentration has been reported in the range of 0.31–4.92 µg l−1 [25]. All of these areas showed uranium concentration in the lower range and most of them well below the WHO prescribed drinking water limits of intake of 30 µg l−1 and have been found to comparable with the results of the present study. However some higher values of U concentration have been reported in the Kolar district of Karnataka (0.3–1442.9 µg l−1) [17] and Bhatinda, Mansa, Faridakot and firojapur of Punjab (<2–644 µg l−1) [16]. Further the studies conducted in other countries like Switzerland (0.05–92.02 µg l−1) [26], France (0.37–75.3 µg l−1) and Germany (0.03–48.6 µg l−1) [6] and central Portugal (0.1–2.7 µg l−1) [27] have been reported U concentration in the lower range and also found to be comparable to the present study. However some higher values of U concentration were recorded in Kosovo in the range of 0.012–166 µg l−1 [28], in Korea in the range of 0.02–402.3 µg l−1 [29] and in Finland in the range of 0.02–6000 µg l−1 [6].
Assessment of annual effective dose
Natural occurring U having three isotopes, such as 238U, 235U and 234U, all these radionuclides decay via alpha emission. To estimate the ingestion dose it has been assumed that the peoples in the study area use only the groundwater sources for drinking purposes and annual ingestion dose due to intake of U through the drinking water pathway to the adults was calculated by using ingestion dose coefficient for different isotopes of U as recommended by ICRP publication [30] and they are reported to be 4.5 × 10−8 Sv Bq−1 for 238U, 4.7 × 10−8 Sv Bq−1 for 235U and 4.9 × 10−8 Sv Bq−1 for 234U, respectively. Here, average daily water of intake (DWI) of 4.05 l day−1 has been assumed for Indian adults for dose calculation [31]. The annual ingestion dose due to intake of only U through drinking water pathways was calculated by using the following relation [14].
where D is the annual effective ingestion dose (Sv year−1), C is the mean concentration of a particular radionuclide in the water (Bq l−1), DWI is the daily water of intake by the adults (l day−1) and DCF is the dose conversion factor for a particular radionuclide for the adults (Sv Bq−1).
In this study individual concentration 234U, 235U and 238U was estimated by multiplying the mean concentration of U with their specific activity and relative abundance in nature, respectively (Table 3). Further ingestion dose was estimated based on individual isotopic concentration by using Eq. (2) and total dose due to natural U through intake of water to the adults was estimated by adding doses calculated from individual isotopes. Taking the geometric mean of the measured U concentration, the annual effective dose due to intake of only U through drinking water pathway to the member of the public was estimated to be 16.75 µ Sv year−1, which is found to be well below the recommended dose limits of 100 µ Sv year−1 as per WHO [21] and it is about 5.6 % of the global average annual ingestion dose [6]. In India, [13–15] reported mean annual ingestion doses in the different parts of the country are 3.5, 0.99 and 2 µ Sv year−1, respectively which are slightly less in comparison to the present study.
Assessment of radiological and chemical toxicity risk
In this study two types of risk of U were evaluated as human health effects due to exposure of U can be classified as radiological risk by radiation of U isotopes and chemical risk as a heavy metal. The radiological risk was evaluated according to the USEPA standard method [22] and the excess life time cancer risk was evaluated by the following equation.
where C is the U concentration (µg l−1), CF is the mass to activity conversion factor (0.025 Bq µg−1), RC is the risk coefficient (1.19 × 10−9 Bq−1), DWI is daily water of intake (4.05 l day−1), LE is the average life expectancy (23,250 days).
For Indian reference man, 4.05 l day−1 is considered as DWI [31] and 63.7 years is taken as average life expectancy for both male and female and 23,250 days is the average time for the Indians [32]. Statistical analysis data on radiological risk is presented in Table 4. The excess life time cancer risk was found to vary from 1.07 × 10−6 to 2.23 × 10−4 with a geometric mean of 4.38 × 10−5, which is found to be lower than the maximum acceptable value of 1.6 × 10−4 [24].
Further the chemical toxicity risk as life time average daily dose (LADD) and hazard quotient (HQ) were estimated with the help of Eq. (4) and (5) [2, 22, 33] and was compared with the reference dose (RfD) of 4.48 µg kg−1 day−1 which has been calculated on the basis of the maximum acceptable level of U (60 µg l−1) in drinking water [24]. 350 days has been assumed as the exposure frequency [34] and 51.5 ± 8.5 kg is considered as the average body weight for Indians [35].
where, C is the U concentration (µg l−1), DWI is daily water of intake (l day−1), EF is the exposure frequency (day−1), LE is the average life expectancy (years), AT is the average time (23,250 days), BW is the body weight (kg), RfD is the reference dose (µg kg−1 day−1), LADD is the lifetime average daily dose (µg kg−1 day−1), HQ is the hazard quotient.
Statistical analysis data on chemical toxicity risk as LADD and hazard quotient are presented in Table 4. The chemical toxicity risk was found to vary from 0.03 to 6.01 with a geometric mean of 0.74, which is observed to be lower than the Indian reference dose of 4.48 µg kg−1 day−1. The mean lifetime average daily dose of the present study was found to be about 16.5 % of the Indian reference dose. The hazard quotient was found to vary from 0.01 to 1.34 with a geometric mean of 0.17. The mean hazard quotient of this study is also found to be less than unity for the study area.
Conclusions
-
In the study region U concentration in 106 water samples has been found to vary between 0.38 and 79.70 μg l−1 with a geometric mean of 9.83 μg l−1. Data analysis revealed about 15 % of the samples have U concentration higher than the WHO, 2011 recommended guideline value of 30 µg l−1 [23] and almost all samples have U concentration well within the prescribed limit of 60 µg l−1 as given by AERB, DAE, India, 2004.
-
A marginal higher U content in groundwater samples has been observed in the study region than the natural background areas and this may be attributed to regional geology of the rock formation and U mineralisation of the area.
-
The estimated annual ingestion dose due to intake of U through drinking water to the local population has been observed to be low and mean dose has been estimated to be 16.75 µ Sv year−1, which is well below the recommended dose limit of 100 µ Sv year−1 as prescribed by WHO [21].
-
The hazard quotient, radiological and chemical toxicity risks were observed to be well below recommended limit. The groundwater in the study region has been assumed to be suitable for consumption from radiological and chemical toxicity aspects of U.
-
This study gives a brief background radiological status about uranium in groundwater around the Tummalapalle uranium mining and processing site and in future all other raionuclides can also be studied in ground water around the site.
References
ICRP (2007) International Commission on Radiological Protection, The recommendations of international commission on radiological protection, ICRP Publication 103, Ann. ICRP 37(2/4), Pergamon Press, Oxford
WHO (1998) World Health Organisation, guidelines for drinking water quality. Addendum to: health criteria and other supporting information, WHO/EOS/98.1. vol 2, 2nd, Geneva, p 283
Kurttio P, Auvinen A, Salonen L, Saha H, Pekkanen J, Makelainen I, Vaisanen SB, Penttila IM, Komulainen H (2002) Renal effects of uranium in drinking water. Environ Health Perspect 110:337–342
Zamora ML, Tracy BL, Zielinski JM, Meyerhof DP, Moss MA (1998) Chronic ingestion of uranium in drinking water: a study of kidney bioeffects in humans. Toxicol Sci 43:68–77
Lussenhop AJ, Gallimore JC, Sweet WH, Struxness EG, Robinson J (1958) Am J Roentgenol 79:83
UNSCEAR (2000) Sources and effects of ionizing radiation united nations scientific committee on the effects of atomic radiation, New York
Bhatt KB, Saklani S (1996) Hydro geochemistry of the upper Ganges river, India. J Geol Soc India 48(3):171–182
Nisi B, Buccianti A, Vaselli O, Perini G, Tassi F, Minissale A et al (2008) Hydro geochemistry and strontium isotopes in the Arno river basin (Tuscany, Italy): constraints on natural controls by statistical modelling. J Hydrol 360:166–183
Schot PP, Van der Wal J (1992) Human impact on regional groundwater composition through intervention in natural flow patterns and changes in land use. J Hydrol 134:297–313
Leijnse A, Van De Weerd H, Hassanizadeh SM (2001) Modelling uranium transport in Koongarra, Australia: the effect of a moving weathering zone. Math Geol 33(1):1–29
Gómez P, Garralón A, Buil B, Turrero MJ, Sánchez de la Cruz B (2006) Modelling of geochemical processes related to uranium mobilization in the groundwater of a uranium mine. Sci Total Environ 366:295–309
Neves O, Matias MJ (2008) Assessment of groundwater quality and contamination problems ascribed to an abandoned uranium mine (Cunha Baixa region, Central Portugal). Environ Geol 53:1799–1810
Sahoo SK, Mohapatra S, Chakrabarty A, Sumesh CG, Trpathi RM, Puranik VD (2009) Distribution of uranium in drinking water and associated age-dependent radiation dose in India. Radiat Prot Dosim 136(2):108–113
Rana BK, Tripathi RM, Sahoo SK, Sethy NK, Sribatav VS, Shukla AK, Puranik VD (2010) Assessment of natural uranium and 226Ra concentration in ground water around the uranium mine at Narwapahara, Jharkhand., India and its radiological significance. J Radioanal Nucl Chem 285:711–717
Sethy NK, Tripathi RM, Jha VN, Sahoo SK, Shukla AK, Puranik VD (2011) Assessment of natural uranium in ground water around Jaduguda uranium mining complex, India. J Environ Prot 2:1002–1007
Ajay Kumar, Usha N, Sawant PD, Tripathi RM, Raj Sanu S, Manish Mishra, Sabyasachi Rout, Supreeta P, Jaspal Singh, Sanjeev Kumar, Kushwaha HS (2011) Risk assessment of natural uranium in surface water of Punjab State, India. Hum Ecol Risk Assess 17(2):381–393
Babu MNS, Somashekar RK, Kumar SA, Shivanna K, Krishnamurthy K, Eappaen KP (2008) Concentration of uranium levels in ground water. Int J Environ Sci Tech 5(2):263–266
Brinda K, Elango L, Nair RN (2011) The new uranium mining boom. DOI. doi:10.1007/978-3-642-22122-4@springer
Sahoo SK, Tripathi RM, Chakrabarty A, Mohapatra S, Sumesh CG and Puranik VD (2008) Optimization of method parameters for estimation of uranium at nanogram level in drinking water samples by laser fluorimeter. In: Proceedings of the Indian analytical science congress. pp 111–112
Hess CT, Michel J, Harton TR, Procured HM, Coniglio WA (1985) The occurrences of radioactivity in public water supplies in United States. Health Phys 48(5):553–586
WHO (2004) World health organization, guidelines for drinking-water quality, 3rd edn. WHO, Geneva
USEPA (2000) United States environmental protection agency, national primary drinking water regulations, radionuclides final rule, 40 CFR Parts 9, 141, and 142, pp 76708–76712
WHO (2011) World health organization, guidelines for drinking-water quality, 4th edn. WHO, Geneva
AERB, DAE (2004) Atomic energy regulatory board, department of atomic energy, drinking water specifications in India. Atomic Energy Regulatory Board, Mumbai
Byju Ben S, Sunil A, Reeba MJ, Christa EP, Vaidyan VK, Prasad R, Jojo PJ (2012) Uranium in drinking water from the south coast districts of Kerala. India, Iran J Radiat Res 10(1):31–36
Stalder E, Blanc A, Haldimann M, Dudler V (2012) Occurrence of uranium in Swiss drinking water. Chemosphere 86:672–679
Pereira AJSC, Neves LJP (2012) Estimation of the radiological background and dose assessment in the areas with naturally occurring uranium geochemical anomalies-a case study in the Iiberian Massif (Central Portugal). J Environ Radioact 112:96–107
Berisha F, Goessler W (2013) Uranium in Kosvo’s drinking water. Chemosphere 93:2165–2170
Kim Y, Park H, Kim J, Park S, Cho B, Sung I, Shin D (2004) Health risk assessment for uranium in Korean groundwater. J Environ Radioact 77:77–85
ICRP (1993) International Commission on Radiological Protection, Age-dependent doses to members of the public from intake of radionuclides: Part 2 Ingestion dose coefficients. ICRP Publication 67, Ann ICRP 23(3/4), Pergamon Press, Oxford
Jain SC, Mehta SC, Kumar B et al (1995) Formulation of the reference Indian adult anatomic and physiological data. Health Phys 68(4):509–522
HDR (2009) Human Development Report, National Resource Centre for Urban Poverty and All India Institute of Local Self Government. Mumbai
Health Canada (1999) Summary of guidelines for canadian drinking water quality. Prepared by federal- provincial subcommittee on drinking water of the federal-provincial-territorial committee on environmental and occupational health. Ottawa
USEPA (1991) US environmental protection agency, EPA standard default exposure factors. USEPA, Washington
Dang HS, Jaiswal DD, Parmeshwran M et al (1994) Physical, anatomical, physiological and metabolic data for reference indian man-a proposal. BARC/1994/E/03. Board of Research in Nuclear Science, Mumbai
Acknowledgments
The authors are thankful to Dr. K. S. Pradeep Kumar, Associate Director, Health, Safety & Environment Group, BARC, for his keen interest and constant encouragement. The authors express their sincere thanks to UCIL authorities for their interest and inspiration in the work and for extending the infrastructural facilities and support to carry out the work. Cooperation received from other colleagues is fully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rana, B.K., Dhumale, M.R., Lenka, P. et al. A study of natural uranium content in groundwater around Tummalapalle uranium mining and processing facility, India. J Radioanal Nucl Chem 307, 1499–1506 (2016). https://doi.org/10.1007/s10967-015-4350-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4350-8