Abstract
A study was undertaken to measure the dissolved radon (222Rn) concentration in parts of western India (Southwest Punjab) in order to evaluate its hydrological and radiological implications. Radon was monitored using Smart Radon Monitor in 25 locations. The dissolved 222Rn concentrations ranged from 618 to 3210 Bq/m3 in the groundwater and were well below the USEPA maximum contaminant level of 11,100 Bq/m3 and an alternate maximum contaminant level of 148,000 Bq/m3. Radiological dose due to dissolved radon was calculated and the results indicated that the estimated total annual effective dose (both from ingestion and inhalation) varied between 6.07 and 31.52 μSv/year, which is within the recommended limit of 100 μSv/year prescribed by WHO and EU Council (1998). This infers that there is no significant radiological risk due to dissolved 222Rn for the inhabitants of this region. 222Rn showed a negative correlation with corresponding U concentration while with other hydrochemical parameters the correlations were either weak or insignificant. The depth variation of dissolved 222Rn concentration indicated that shallow zone has a wider range of radon values as compared to deeper zone. Spatial and hydrochemical trends signify that 222Rn concentration is not only controlled by a variety of geochemical processes but also by surface processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Radon (222Rn) is an odourless, colourless and radioactive noble gas with half-life of 3.82 days. It occurs naturally in air, water, rocks and soil on earth. 222Rn is a part of the U decay series and its decay to daughter nuclides results in the release of alpha particles. It is an inert gas which moves through porous media such as soil or fragmented rock [1, 2]. For radon gas to escape from the mineral grain into the pore space, the decay must occur within the recoil distance (displacement due to alpha emission) of the grain surface. Recoil distance for 222Rn in common minerals ranged from 20 to 70 nm, 100 nm in water, and 63 μm in air [1, 3]. Radon gas which enters the pore space is then transported by diffusion and advection processes through this space until it decays or gets released into the atmosphere (exhalation).
The most significant contributors to human exposure from natural sources include radon and its short-lived decay products in the atmosphere. Environmental radon exposure through indoor radon is one of the main causes of health risks besides high radon exposures in underground mines. 222Rn generation and its decay inside the rocks do not contribute to health risk until released to the groundwater systems [2]. Exposure to radon are the result of the radon gas that enters indoor air from soil under homes and other buildings and also through de-emanation when the radon dissolved water is used mainly for household purposes. Three exposure pathways for waterborne radon were put forward by EPA which includes; (1) ingesting radon dissolved in water (2) inhaling radon released from water during household use and (3) inhaling radon progeny derived from radon released from water. Inhalation of the short-lived decay products of 222Rn, mainly the emitted alpha particles and to a marginal extent the decay products of 220Rn (Thoron) and their subsequent deposition along the walls of the various airways of the bronchial tree provides the major pathway for radiation exposure of the lungs [3]. Natural exposure to radon concentration varies worldwide, usually by a factor of about 3. The average annual exposure to natural radiation sources is 2.4 mSv globally where internal exposure through inhalation mainly from 222Rn comprises 1.2 mSv. The US Environmental Protection Agency (USEPA) proposes new directives to safeguard the people/public from radon exposure with two alternatives for the maximum level of radon that is acceptable in community water supplies. The recommended maximum contaminant level (MCL) is 300 picoCuries per liter (pCi/L) which corresponds to 11,100 Bq/m3 and in case of the above alternate maximum contaminant level (AMCL) it is 4000 pCi/L which corresponds to 148,000 Bq/m3. It is estimated by EPA that lifetime exposure to drinking water at 4000 pCi/L would commensurate to an incremental lifetime cancer risk of 26 in 10,000 to the general population, which overshoots the risk range of 1 in 10,000 to 1 in 1 million (10−4–10−6) [4].
Groundwater which caters drinking water needs in many parts of the world is shown to contain relatively higher levels of dissolved 222Rn as compared to other water sources like rain, river and other surface waters [5,6,7,8,9,10,11,12,13]. Low 222Rn concentration is normally observed in sedimentary formations while higher values are noted in granitic formations as well as U enriched phosphate bearing rocks [2, 5,6,7,8,9,10,11,12,13,14,15,16,17,18]. Reports suggest that exposure to radon through inhalation or ingestion can increase the risk of lung cancer in human as well as radiation to the stomach [4, 19]. Studies on radon and its possible health effects were carried out by various workers across India [5,6,7,8,9,10,11,12,13, 18]. Radon concentration in groundwater of Punjab, Haryana, Jammu & Kashmir, Karnataka, Rajasthan and Uttarakhand are reported by earlier researchers [7, 9,10,11,12,13]. Radon concentration up to 3,050,000 Bq/m3 was observed in groundwater of Garhwal Himalayas [13] while in other places the maximum values were up to 63,640 Bq/m3 [10]. Studies also reported radon concentration up to 31,500 Bq/m3 in hard rock aquifers of Andhra Pradesh [5] and 85,700 Bq/m3 in Rajasthan [12]. A comparison of the radon values worldwide with the present study is provided in Table 1. Review of the published literature on dissolved radon concentration indicates that alluvial formations show low radon concentration as compared to other hard rock formations.
Recent reports have indicated the presence of dissolved radon in groundwater in northwest part of India (Punjab) where high uranium concentration in groundwater is already reported [7, 8, 22, 23]. Detailed studies have been carried out on U distribution and its sources in this region including isotope tracers, which are widely employed in water resources and industry [24, 25]. However, no systematic studies were conducted to identify the link between high U groundwater and dissolved radon content in them. In this study we estimated the dissolved radon (222Rn) activity in groundwater and corresponding radiological dose. In addition, U and other hydrochemical parameters were also measured to evaluate the possible controls on radon distribution in groundwater of this region.
Study area description
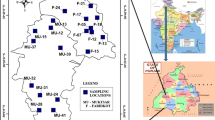
Two districts of Southwest Punjab, India viz., Mansa and Bathinda were chosen for the present investigation. This region covers a total area of 5538 km2 and falls between 29°32′–30°36′ north latitudes and 74°37′–75°46′ east longitudes as shown in Fig. 1. The study area comprises of Quaternary alluvial deposits of Recent to Sub-Recent age and the alluvial deposits were formed by the sediments transported from adjoining areas comprising of Siwaliks, Granites and other Metamorphic rocks [25,26,27]. The study area forms part of the Indo-Gangetic alluvial plain and is devoid of any outcrops of hard rock, the depth of the alluvium is fairly deep going down to hundreds of meters. The alluvial sediments of the study area consist of alternating beds of fine to medium sand, silt and clay [28, 29]. Older Alluvium (Middle to Upper Pleistocene age) and Newer Alluvium (Upper Pleistocene to Recent) are the main Quaternary sediments present in the region.
Groundwater exits under both unconfined and confined conditions. Two major aquifers are present in this region, viz., shallow aquifer with depth up to 60–70 m bgl (below ground level) and deep aquifer below 70 m bgl [25, 30, 31]. The general slope of the water table is towards SW from North, NE, East and SE. Since the region has extensive canal system, 80% of the study area is irrigated by major canals and their streams which include Bathinda branch, Kotla branch and Abohar branch canals originating from Satluj River. The climate can be classified as semi-arid and hot which is mainly dry except in rainy months and characterized by intensely hot summer and cold winter with an average annual rainfall of 400–500 mm [25,26,27]. The southwest monsoon (July–September) contributes about 82% of annual rainfall while rest of the rainfall mostly occurs during non-monsoon months of the year.
Punjab state is a major contributor to food production in the country where 82.35% land area of the state comprises the net sown area i.e. agriculture. The net irrigated area to gross area sown has increased from 71% during 1970–1971–97.4% during 2008–09 and increased up to 99.2% during 2012–2013 [32]. The fertilizer (NPK) consumption rate also follows the similar trend of increase where during 1970–1971 it was 37.5 kg/ha which increased to 223 kg/ha in 2008–2009 and then to 239 kg/ha during 2012–2013 [33]. Thus the pattern of land use over the years has undergone a tremendous transformation resulting in unique and extreme ecosystem vulnerabilities [34].
Methodology
A total of 25 groundwater samples comprising tube wells, hand pumps and bore wells were collected during pre monsoon season (June 2016) from the alluvial formations of the study area for dissolved radon, uranium, physicochemical and hydrochemical parameters. The well depth ranged from 9 to 213 m bgl (below ground level). Representative water sample was collected from the well after purging. Physical parameters such as temperature (°C), pH, electrical conductivity (μS/cm) were measured in situ by hand-held water quality kit (Hanna Make). Alkalinity was measured by titrating 10 mL of water sample with 0.02 N H2SO4 by Gran titration method. For anions, water samples were filtered using 0.45-μm filter and stored in polyethylene bottles while for cations, samples were filtered and acidified to pH 2 using concentrated HNO3. Cation (Na+, K+, Mg2+ and Ca2+) and anion (F−, Cl−, NO3− and SO42−) analysis was carried out by ion chromatography (Dionex 500). Charge balance error (CBE) was calculated using Eq. (1) and the error was within the accepted limits of ± 5% [35].
Uranium analysis was carried out using Laser Fluorimeter. The details of measurement and validation protocols are given in Rishi et al. [23]. Radon (222Rn) in groundwater was measured using a portable continuous activity monitor (SRM—SMART RnDuo Monitor). The principle is based on detection of alpha particles, emitted from sampled radon and its decay products formed inside the detector volume by scintillation in ZnS:Ag detector [36]. The water samples were collected in leak proof glass bottles (volume ~ 60 ml) of low permeability material provided with the radon kit. While sampling, caution was taken to avoid formation of bubble/agitation in the liquid. No air volume should remain in the bottle so after filling the bottle completely, the complete volume of water is replaced 4–5 times with the sample water and the bottle cap is closed tightly. Before starting the radon counting, the gas present in system including detector was flushed for about 5 min by pumping then the sampling bottle is connected into the bubbler cautiously so that there is no bubble formation. The measurement cycle of 15 min is selected and before starting the monitor, pump is kept on for about 5 min so that dissolved radon can be transferred from liquid sample to detection chamber volume of the chamber. The monitor is started with 15 min cycles and measurement is continued for about 1 h (4 readings for each sample) to have the concurrent radon concentration values (Cair).
Estimation of radon concentration in liquid (Cliq) from the concentration measured in air (Cair) with SMART-Rn is given as Eq. (2):
where Vair and Vliq are the volume of air and water in the bottle respectively. The decay due to delay in the measurement is corrected by decay Eq. (3);
where Cmeas is the measured concentration after time t, Ctrue is initial concentration after the decay correction and t is the time elapsed since sample collection and λ is decay constant (0.181 per day).
The detection limit, instrument sensitivity and upper limit of detection are 8 Bq/m3 (1σ confidence) for 1 h counting, 1.2 CPH/Bq/m3 and 50 MBq/m3 respectively. The schematic flow diagram of radon measurement by SRM is shown in Fig. 2. The radon analysis was completed immediately after sample collection, so that the variation in the meteorological parameters, such as temperature, pressure and humidity is minimum.
Results
Water quality
The statistical summary of the dissolved radon (222Rn), U and other hydrochemical data is given in Table 2. Total uranium in the groundwater ranged from 22.34 to 240 µg/L with a mean of 83.5 µg/L. Electrical conductivity values range from 650 to 5790 µS/cm in the groundwater of the study area while pH is in neutral condition (6.8–8.5). Groundwater also show high nitrate (384 mg/L), high potassium (67.8 mg/L and high fluoride (6.88 mg/L) in the study area.
Radon in groundwater
The radon concentration in groundwater of the study area ranged from 618 to 3210 Bq/m3 with a median value of 1530 Bq/m3 and mean value of 1619 Bq/m3. The highest radon concentration was recorded from the bore well with a depth of 167 m bgl while the lowest value was measured from bore well with depth of 30 m bgl, both from Mansa district. Radon concentration was observed in all the studied wells and the measured radon values are within the recommended MCL and AMCL proposed by US Environment Protection Agency (USEPA) [4]. Figure 3a shows the radon distribution in the study area. A Box-Whisker plot of radon is shown in Fig. 3b to depict overall spread in the data. From the figure it can be observed that there is almost an equal spread of radon data in the samples with a median value of 1530 Bq/m3 with an outlier towards the upper whisker showing a maximum radon value of 3210 Bq/m3. The United Nations Scientific Committee on the effects of Atomic Radiation (UNSCEAR) have suggested radon concentration in the range of 4–40 Bq/L for human consumption [37] and all the measured values (0.62–3.21 Bq/L) lies below the suggested range. It is observed from the present study that radon concentrations are lesser in shallow wells located near canals or open water bodies signifying dilution effect. The other reason can be radon exhalation from shallow aquifer as it is very close to the atmospheric conditions.
From the present investigation it was found that the observed radon concentration range was lower than the other reported range in Punjab region [7, 12]. Groundwater from alluvial aquifers of Haryana also reported higher radon concentration as compared to the present study [13, 20]. Other neighboring states like Himachal Pradesh, Jammu & Kashmir and Rajasthan with mixed formations also indicated very high radon concentration [8, 10, 12] than the present findings while comparable values were reported from river basin area of Karnataka [11]. In the case of hard rock formations, states like Andhra Pradesh and Tamil Nadu have shown higher radon values in groundwater than the values observed in this study [5, 6]. In other countries higher radon concentrations was reported as compared to the present study area [14,15,16,17, 21].
Radiological dose estimation
Ingestion and inhalation are the two main processes through which radon can enter human body and causes damage to the stomach and lungs through radiation. Radiation dose studies were carried out by several researchers in India which include studies by Bajwa et al. [8] in Punjab and Himachal Pradesh, Rani et al. [12] in Rajasthan, Duggal et al. [9] and Sharma et al. [20] in Haryana, Kumar et al. [10] in Jammu and Kashmir, Somashekar and Ravikumar [11] in Karnataka [8,9,10,11,12, 20]. Since groundwater is the major source of drinking water in the study area, the radiation dose was also calculated to assess the radiological risk to the inhabitants of the region due to consumption of the groundwater.
Calculation for the annual effective dose for ingestion was carried out using the Eq. (4) as per UNSCEAR [3];
where AEDig is the annual effective dose from ingestion, C is the concentration of radon in Bq/L, DWI is the daily water intake (2 L/day) as per WHO [38], DCF is the ingesting dose conversion factor of 222Rn (10−8Sv/Bq) as per UNSCEAR [3] and EF is the exposure frequency (365 days/year).
Further the estimation of the annual effective dose for inhalation was calculated using with the following equation:
where AEDih is the annual effective dose from inhalation, C is the concentration of radon in Bq/L, Raw is the ratio of radon activity in air to water (10−4). F is the equilibrium factor between radon and its progenies (0.4), O is the average indoor occupancy time per individual (7000 h/year) as per ICRP [39] and UNSCEAR [3], and DCF is the dose conversion factor for radon exposure (9 nSv/(Bqhrm−3) as per UNSCEAR [3]. The EU Council [40] and WHO [38] recommended a 100 μSv/year annual effective dose for drinking water to be safe limit from the 3H, 40K and radon radio isotopes.
Results from the above calculation shows that the annual mean effective dose for radon ingestion and inhalation varies from 4.51 to 23.43 μSv/year and 1.56 to 8.09 μSv/year respectively (Fig. 4). The estimated total annual effective dose due to both ingestion and inhalation together through drinking water ranges from 6.07 to 31.52 μSv/year. The total annual effective dose from all the studied locations is found to be within the recommended dose limit for drinking water (100 μSv/year) given by WHO as well as EU Council [38, 40]. This indicates no significant radiological risk to the inhabitants of this region from drinking water due to dissolved radon.
Discussion
In order to assess the impact of high U on radon concentration in groundwater, a scatter plot was constructed between 222Rn and U data (Fig. 5). The plot shows an inverse trend, when there is an increasing U concentration there is a decrease in the Radon values in the groundwater (Fig. 5). Overall a negative correlation with R2 value of 0.6 was observed with few deviations (sample nos. 4, 5 and 15). The highlighted square box shows samples with high U and low Radon values. Higher U values are observed more in shallow wells compared to deeper wells [25, 34]. The inverse relationship signifies that 222Rn concentration is more in the groundwater in contact with underlying sediments due to the emanation process rather than in the shallow zone where exhalation of Radon gas is more dominant. Similar findings were reported by Keesari et al. [5] while poor positive relationship was established by Thivya et al. [6].
Similarly, correlation between 222Rn and other ions was also attempted in this study. Physico chemical parameters such as EC, Temperature, pH and well depth were correlated with 222Rn along with major cations and anions.
EC versus 222Rn plot (Fig. 6a) indicates that very high EC corresponds to lower radon values with maximum samples showing random distribution. Temperature versus 222Rn plot indicates that samples are more clustered in the range of 26–30 °C (Fig. 6b). Increase in diffusion rate with corresponding increase in temperature results in higher 222Rn concentration in groundwater [6, 41, 42]. Overall weak correlation is noted between the two parameters [6, 7]. In case of pH versus 222Rn plot (Fig. 6c), it is observed that there is no significant correlation between the two parameter as observed in similar studies carried out by Thivya et al. and Badhan et al. [6, 7]. The pH of the study area samples ranges from near neutral towards alkaline side where low 222Rn concentration was observed in low or near neutral pH and maximum samples are scattered in the pH range of 7–8. Radon samples were taken from aquifer depth ranging from 9 to 213 m bgl in the study area. Correlation of depth versus 222Rn (Fig. 6d) shows that samples are scattered randomly with no significant correlation. Shallow zone showed greater variation compared to deeper zone which may be due to different geochemical processes in addition to the influence of surface processes. Shallow aquifers are particularly vulnerable to changes in radon distribution owing to their interaction with surface waters, impact of rainfall and increased human interventions through groundwater exploitation. Similar results were noticed in other alluvial aquifers [9, 20].
An inverse relationship was observed in the case of 222Rn versus Cl− concentration plot (Fig. 8a). The study area is reported to have high salinity due to water logging, application of fertilizers and wastewater discharges from various industrial units [43,44,45]. Maximum 222Rn concentration is observed in Cl− range up to 300 mg/L and the lowest 222Rn value corresponds to the highest Cl− value. Low dissolved 222Rn values in the shallow zone may be due to the contact with the atmospheric conditions through the porous subsurface where 222Rn emanates to the air while maximum anthropogenic contamination is observed in shallow zone leading to high Cl− values. Similar trends were observed by other researchers [6, 46]. With regard to correlation of radon with cations and anions weak to insignificant correlation was established (Figs. 7a–d, 8b–d). These observations are in agreement with the studies undertaken elsewhere which also highlight that radon and hydrochemical parameters are not related in the study area [6].
Summary and conclusion
Radon concentration (222Rn) in the groundwater of western parts of India (SW Punjab) was measured to estimate the radiation dose due to dissolved radon to the local population consuming this water. 25 locations from Mansa and Bathinda districts of SW Punjab were covered in the study, which are impacted by high uranium in groundwater. The radon concentration was found to vary from 618 to 3210 Bq/m3. These values were found to be within the USEPA [4] MCL of 11,100 Bq/m3 and AMCL of 148,000 Bq/m3 and also well below the UNSCEAR [37] recommended range for drinking purposes. The estimated total annual effective dose (both from ingestion and inhalation) ranges from 6.07 to 31.52 μSv/year which is within the recommended limit of 100 μSv/year given by WHO and EU Council [40]. This infers that there is no significant radiological risk to the inhabitants of this region due to dissolved 222Rn. Depth profile of dissolved radon indicates that shallow zone has wide variations in 222Rn concentration than deep zone. This may be due to different geochemical processes occurring in shallow zone as well as the influence of surface sources. The narrow distribution of 222Rn in deep groundwater signifies that there is no active hydraulic interconnection between shallow and deep groundwaters and the impact of surface processes on deep groundwater is minimal. Correlations among 222Rn, U and Cl− show inverse trends indicating the role of surface sources on diluting the dissolved radon while increasing the U and Cl− content in groundwater. Radon shows weak or insignificant correlations with other hydrochemical ions. The spatial trends and hydrochemical correlations of radon in groundwater infer that the distribution of radon in groundwater of this area is controlled by hydrochemical nature of groundwater as well as impact from surface sources. Further study on Ra2+ in groundwater is needed to confirm the exact geochemical process/es controlling uranium–radon correlations in groundwater of this region.
References
Tanner AB (1980) Radon migration in the ground: a supplementary review. In: Gesell TF, Lowder WM (eds) The natural radiation environment III. Symposium proceedings Houston, April 1978, CONF-780422, pp 5–56
Cecil LD, Green JR (2000) Radon-222, In: Environmental tracers in subsurface hydrology. Springer, Boston, pp 175–194
UNSCEAR (2000) Sources, effects and risks of ionizing radiation. United Nations, New York, pp 1–592
US EPA (2012) Report to congress: radon in drinking water regulations. https://archive.epa.gov/water/archive/web/pdf/epa815r12002.pdf. Accessed 7 Dec 2018
Keesari T, Mohokar HV, Sahoo BK, Mallesh G (2014) Assessment of environmental radioactive elements in groundwater in parts of Nalgonda district, Andhra Pradesh, South India using scintillation detection methods. J Radioanal Nucl Chem 302(3):1391–1398
Thivya C, Chidambaram S, Thilagavathi R, Tirumalesh K, Nepolian M, Prasanna MV (2017) Spatial and temporal variations of radon concentrations in groundwater of hard rock aquifers in Madurai district, India. J Radioanal Nucl Chem 313(3):603–609
Badhan K, Mehra R, Sonkawade RG (2010) Measurement of radon concentration in ground water using RAD7 and assessment of average annual dose in the environs of NITJ, Punjab, India. Indian J Pure Appl Phys 48:508–511
Bajwa BS, Mahajan S, Singh H, Singh J, Singh S, Walia V, Virk HS (2005) A study of groundwater radon concentrations in Punjab and Himachal Pradesh States, India. Indoor Built Environ 14(6):481–486
Duggal V, Sharma S, Mehra R (2017) Radon levels in drinking water of Fatehabad district of Haryana, India. Appl Radiat Isot 123:36–40
Kumar A, Sharma S, Mehra R, Kanwar P, Mishra R, Kaur I (2018) Assessment of radon concentration and heavy metal contamination in groundwater of Udhampur district, Jammu & Kashmir, India. Environ Geochem Health 40(2):815–831
Somashekar R, Ravikumar P (2010) Radon concentration in groundwater of Varahi and Markandeya river basins, Karnataka State, India. J Radioanal Nucl Chem 285(2):343–351
Rani A, Mehra R, Duggal V (2012) Radon monitoring in groundwater samples from some areas of northern Rajasthan, India, using a RAD7 detector. Radiat Prot Dosimetry 153(4):496–501
Prasad G, Prasad Y, Gusain GS, Ramola RC (2008) Measurement of radon and thoron levels in soil, water and indoor atmosphere of Budhakedar in Garhwal Himalaya, India. Radiat Meas 43:S375–S379
Moreno V, Bach J, Baixeras C, Font L (2014) Radon levels in groundwaters and natural radioactivity in soils of the volcanic region of La Garrotxa, Spain. J Environ Radioact 128:1–8
Pereira AJSC, Pereira MD, Neves LJPF, Azevedo JMM, Campos ABA (2015) Evaluation of groundwater quality based on radiological and hydrochemical data from two uraniferous regions of Western Iberia: Nisa (Portugal) and Ciudad Rodrigo (Spain). Environ Earth Sci 73(6):2717–2731
Fonollosa E, Peñalver A, Borrull F, Aguilar C (2016) Radon in spring waters in the south of Catalonia. J Environ Radioact 151:275–281
Moldovan M, Niţă DC, Cucos-Dinu A, Dicu T, Bican-Brişan N, Cosma C (2013) Radon concentration in drinking water and supplementary exposure in Băiţa-Ştei mining area, Bihor county (Romania). Radiat Prot Dosim 158(4):447–452
Hess CT, Michel J, Horton TR, Prichard HM, Coniglio WA (1985) The occurrence of radioactivity in public water supplies in the United States. Health Phys 48(5):553–586
Auvinen A, Salonen L, Pekkanen J, Pukkala E, Ilus T, Kurttio P (2005) Radon and other natural radionuclides in drinking water and risk of stomach cancer: a case-cohort study in Finland. Int J Cancer 114(1):109–113
Sharma S, Duggal V, Srivastava AK, Mehra R, Rani A (2017) Radon concentration in groundwater and associated effective dose assessment in Western Haryana, India. Internat J Innov Res Sci Eng 3:69–78
Mustapha AO, Patel JP, Rathore IV (2002) Preliminary report on radon concentration in drinking water and indoor air in Kenya. Environ Geochem Health 24:387–396
Pant D, Keesari T, Sharma D, Rishi M, Singh G, Jaryal A, Sinha UK, Dash A, Tripathi RM (2017) Study on uranium contamination in groundwater of Faridkot and Muktsar districts of Punjab using stable isotopes of water. J Radioanal Nucl Chem 313(3):635–639
Rishi MS, Keesari T, Sharma DA, Pant D, Uday Sinha K (2017) Spatial trends in uranium distribution in groundwaters of Southwest Punjab, India-a hydrochemical perspective. J Radioanal Nucl Chem 311(3):1937–1945
Kalaga DV, Kulkarni AV, Acharya R, Kumar U, Singh G, Joshi JB (2009) Some industrial applications of gamma-ray tomography. J Taiwan Inst Chem Eng 40:602–612
Sharma DA, Rishi MS, Keesari T, Pant D, Singh R, Thakur N, Sinha UK (2017) Distribution of uranium in groundwaters of Bathinda and Mansa districts of Punjab, India: inferences from an isotope hydrochemical study. J Radioanal Nucl Chem 313(3):625–633
CGWB (2013a) Groundwater information booklet. Bathinda District, Punjab A report. http://cgwb.gov.in/District_Profile/Punjab/Bathinda.pdf. Accessed 7 Dec 2018
CGWB (2013b) Groundwater information booklet. Mansa District, Punjab A report. http://www.cgwb.gov.in/District_Profile/Punjab/Mansa.pdf. Accessed 7 Dec 2018
CGWB (2014) Water quality issues and challenges in Punjab, Central Groundwater Board, p. 182. http://cgwb.gov.in
Gupta S, Thakur RS (1989) Hydrogeology of district Bathinda, Punjab CGWB (NW region). Unpublished report, 75
Dhiman SC (2012) Aquifer systems of India. Central Ground Water Board, Ministry of Water Resources, Government of India, Guwahati, p 111. Accessed 7 Dec 2018
Keesari T, Sharma DA, Rishi MS, Pant D, Mohokar HV, Jaryal AK, Sinha UK (2017) Isotope investigation on groundwater recharge and dynamics in shallow and deep alluvial aquifers of southwest Punjab. Appl Radiat Isot 129:163–170
Statistical Abstract of Punjab (2015) Government of Punjab, Economic and Statistical Organisation. Publication no. 951
http://punenvis.nic.in/index3.aspx?sslid=5862&subsublinkid=4973&langid=1&mid=1
Sharma DA, Keesari T, Rishi MS, Pant D (2018) A study on the role of hydrogeology on the distribution of uranium in alluvial aquifers of northwest India. Environ Monit Assess 190(12):746
Hounslow AW (1995) Water quality data analysis and interpretation. CRC Press, Florida
Gaware JJ, Sahoo BK, Sapra BK, Mayya YS (2011) Indigenous development and networking of online radon monitors in the underground uranium mine. Radiat Prot Environ 34:37–40
UNSCEAR (2008) Sources, effects and risks of ionizing radiation, vol I. United Nations, New York, pp 1–683
WHO (2004) Guidelines for drinking-water quality. World Health Organization vol. 1. Recommendations, third ed., Geneva
ICRP (1993) International commission on radiological protection, age-dependent doses to members of the public from intake of radionuclides: part 2 ingestion dose coefficients. ICRP Publication 67, Ann ICRP 23(3/4), Pergamon Press, Oxford
EU (1998) European drinking water directive 98/83/EC of 3rd November 1998 on the quality of water intended for human consumption. Off J Eur Comm (European Commission)
Singh M, Ramola RC, Singh S, Virk HS (1988) The influence of meteorological parameters on soil gas radon. J Assoc Explor Geophys 9:85–90
Sharma AK, Walia V, Virk HS (2000) Effect of meteorological parameters on radon emanation at Palampur (HP). J Assoc Explor Geophys 21(1):47–50
Sharma DA, Rishi MS, Keesari T, Sinha UK (2016) Assessment of groundwater quality of Bathinda district, Punjab with reference to nitrate contamination. J Appl Geochem 18(4):480
Sharma DA, Rishi MS, Keesari T (2016) Understanding the factors responsible for groundwater contamination in parts of southwest Punjab, India. J Energy Environ Carbon Credits 6(2):7–23
Sharma DA, Rishi MS, Keesari T (2017) Evaluation of groundwater quality and suitability for irrigation and drinking purposes in southwest Punjab, India using hydrochemical approach. Appl Water Sci 7(6):3137–3150
Brutsaert WF, Norton SA, Hess CT, Williams JS (1981) Geologic and hydrologic factors controlling radon-222 in ground water in Maine. Groundwater 19(4):407–441
Acknowledgements
The authors would like to acknowledge the Board of Research in Nuclear Sciences, Department of Atomic Energy, Government of India for providing the necessary financial support to carry out this work (Letter No. 35/14/11/2014-BRNS-193). Authors sincerely acknowledge the constant support and encouragement by Dr. P.K. Pujari, Associate Director, Radiochemistry and Isotope Group, Bhabha Atomic Research Centre, Mumbai. Special thanks for the support provided by Dr. Bijay Sahoo of RPAD, BARC and the scholars of Department of Environment Studies, Panjab University during fieldwork.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, D., Keesari, T., Rishi, M. et al. Radiological and hydrological implications of dissolved radon in alluvial aquifers of western India. J Radioanal Nucl Chem 323, 1257–1267 (2020). https://doi.org/10.1007/s10967-019-06619-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06619-1