Abstract
Speciation analysis of long-lived 129I in seawater can provide useful information on the source of water masses. This paper presents an improved method for speciation analysis of 129I based on coprecipitation of iodide as AgI with Ag2SO3 and AgCl. By adding a small amount of 127I carrier, the separation efficiency of iodine species and the accuracy and precision of 129I measurement are remarkably improved. 129I species in depth profiles of seawater from the Antarctic were analyzed for investigation of water circulation in the Antarctic.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iodine is a conservative element in the ocean and mainly exists as iodide and iodate with a minor organic iodine [1]. Iodine is also a biophilic element and is highly concentrated in algae [2, 3]. Meanwhile, iodine is a redox sensitive element, and in the marine environment biological activities significantly influence the species of iodine present. 129I (15.7 Ma) is the only long-lived radioisotope of iodine. In the nuclear age, 129I is dominantly originated from human nuclear activities, mainly nuclear reprocessing plants, nuclear weapons testing in 1940s–1980s and nuclear accidents [3]. Anthropogenic 129I therefore provides us an excellent oceanographic tracer for water circulation and marine environment [2, 4].
Solvent extraction is the most commonly used method for separation of iodine from solution [5, 6]. Due to low concentration of iodine and high salinity in seawater, iodine (127I) has to be added as carrier to enhance the extraction efficiency and provide a physical sample for AMS target preparation. This is not suitable for the determination of low level 129I in seawater, such as those collected in the southern hemisphere including the Antarctic and deep seas because of the interference of minor 129I in the iodine carrier. In addition, solvent extract cannot be used to separate single species of iodine from water. A coprecipitation method based on selective precipitation of iodide as AgI has been proposed for separation of iodide from iodate in seawater for speciation analysis of 129I in seawater using accelerator mass spectrometry (AMS) measurement [7]. AMS is the most suitable method for determination of ultralow level 129I/127I in natural samples with a 129I/127I atomic ratio <10−10 [2, 7–11], such as those collected from the southern hemisphere, the deep seas and the Antarctica water. However, due to the low concentration of iodine in samples, the 127I signal in the AMS measurement of a target prepared using the carrier-free coprecipitation method is very weak, causing a high uncertainty in the AMS measurements of the 129I/127I ratio. This work aims to improve the method for speciation analysis of 129I by increasing the 127I signal in AMS measurement thereby reducing the uncertainty in the 129I/127I ratio. Meanwhile, depth profiles of seawater collected from the Amundsen Sea in the Antarctic were analyzed for species of 129I, in order to use it as an oceanographic tracer of water circulation and marine environment in this region.
Materials and methods
Samples and chemicals
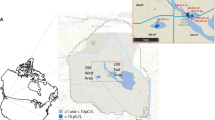
Three depth profiles of seawater were collected using a CTD rosette consisting of Niskins bottles and CTD sensors in the research vessel N.B. Palmer during cruise in the Amundsen Sea, Antarctica (73°–74°S, 111°–114°E) in Dec., 2010. Figure 1 shows the sampling locations. Salinity, seawater temperature and concentrations of chlorophyll, phosphate and nitrite were measured by on-line detecting system of the research vessel (Table 1). One seawater sample was collected in open sea of the Yellow Sea (43°29.5′N, 121°59.1′E) in Jan., 2013 and South China Sea in 2013 (not shown in Fig. 1). All seawater samples were filtered through a 0.45 membrane to remove suspended particles immediately after collection. All seawater samples were collected in a pre-cleaned polyethylene bottle (using deionized water in the lab and original seawater on site) and stored in the dark at room temperature until analysis. A certified seawater reference material for 129I (IAEA-418), which was collected at the DY-FAMED station (43°25.117′N, 7°50.040′E) in the Mediterranean Sea on 18 Feb., 2001, was provided by International Atomic Energy Agency.
129I standard solution (NIST-SRM-4949c) was purchased from the National Institute Standard and Technology (Gaithersburg, MD). 127I carrier solution with a low 129I level (129I/127I atomic ratio <2 × 10−14) was prepared by dissolving iodine crystals (Woodward iodine, Woodward Iodine Corporation, Oklahoma, U.S.A.) in 0.40 mol/L NaOH–0.05 mol/L NaHSO3 solution. 127I− carrier (KI, 129I/127I atomic ratio of (2.0 ± 0.3) × 10−13) was purchased from Shantou Xilong Chemical Factory in Shantou, China. All chemical reagents used were of analytical grade, and all solutions were prepared using deionized water (18.2 MΩ·cm).
Separation of iodine and its chemical species in seawater
0.60 L of seawater was transferred to a beaker, 0.5 kBq of 125IO3 − tracer was spiked, 0–1.0 mg of 127I carrier and 0.50 mL of 2.0 mol/L NaHSO3 solution were added into the beaker, and then 3.0 mol/L HNO3 was added to adjust pH 1–2 to convert all iodine species to iodide. 30 mg Ag+ (28 mL of 0.01 mol/L AgNO3 solution) was dropwise added to the sample solution under stirring to form AgI–AgCl–Ag2SO3–AgBr coprecipitate. The precipitate was separated by centrifuge and sequentially washed with 3.0 mol/L HNO3, H2O, 30 % and 20 % NH4OH to remove Ag2SO3 and most of the AgCl and AgBr until 1–3 mg of coprecipitate was obtained.
1.20 L seawater was transferred to a beaker for separation of iodide. 0.5 kBq of 125I− tracer and 0–1.0 mg of 127I− carrier (KI, 129I/127I atomic ratio of <2.0×10−13 carrier) were spiked, NaHSO3 was added into the sample to a final concentration of 0.30 mmol/L, and then 0.5 mol/L HNO3 was slowly added under stirring to adjust pH 4.2–5.5 (measured using a pH meter). 150 mg Ag+ (45 mL of 0.03 mol/L AgNO3) was dropwise added to the solution to form AgI–AgCl–Ag2SO3–AgBr coprecipitate. The precipitate was separated by centrifuge and the supernatant was used for separation of iodate. The separated precipitate was sequentially washed with 3.0 mol/L HNO3, H2O, 30 % and 20 % NH4OH until 1–3 mg of precipitate were obtained. To the supernatant, 0.5 kBq 125IO3 − tracer was spiked, 0.1–0.2 mg of 127I carrier, 0.5 mL of 2.0 mol/L NaHSO3 solution were added, and then 3.0 mol/L HNO3 was added to adjust pH 1–2 to convert all iodine species to iodide. The following procedure was the same as that for total iodine. The diagram of the analytical procedure is schematically shown in Fig. 2.
125I in the precipitate was measured using a NaI gamma detector (Model FJ-2021, Xi’an Nuclear Instrument factory, Xi’an, China) for monitoring the chemical yield of iodine in the procedure. The recoveries of iodine species in the entire procedure for total iodine and species of iodine are higher than 80 %.
Two 129I standard solutions with a total iodine concentration of 1.00 mg/mL and 129I/127I atomic ratios of 9.954 × 10−12 and 1.138 × 10−10, respectively, were first prepared by dilution of 129I standard solution (NIST-SRM-4949c) with 127I carrier solution [12]. Two 129I working solutions were prepared by mixing the above prepared 129I standard solutions with NaCl solution in Cl/I mass ratio of 2:1. From each standard, 1.0 mL of working solution was taken to a 15 mL centrifuge tube, 0.5 kBq of 125I− tracer, 0.10 mL of 2.0 mol/L NaHSO3, 0.20 mL of 3.0 mol/L HNO3 were added and the solution is mixed. 0.20 mL of 1.0 mol/L AgNO3 was then added to coprecipitate iodine as AgI–AgCl. After centrifuging, the precipitate was sequentially washed with 3.0 mol/L HNO3 and deionized water. For 129I standards in AgI form, the 129I/127I standard solution with a total iodine concentration of 1.0 mg/mL was first converted to iodide by NaHSO3 in acidic medium, and then AgNO3 was added to directly precipitate iodide as AgI.
The procedure blank samples were prepared using the same procedure as for separation of total iodine, iodide and iodate in seawater.
Iodine in the commercial 125I tracer exists as iodide (NaI). To synthesize 125IO3 − tracer, 125I− solution was taken to a beaker, NaClO was added, then HCl is added to adjust pH 1–2 to oxidize iodide to iodate. The remained NaClO in the solution was decomposed by heating at 80 °C. The residue is dissolved in water, and the solution passed through a small anion exchange column (AG1- × 4 resin, NO3 − form, 1.0 cm in diameter and 5 cm in height). The effluent containing iodate was collected and used as 125IO3 − tracer.
50 mL of seawater was taken to a beaker and 125I− was spiked. The sample was loaded to an anion exchange column (1.0 × 5.0 cm, AG1- × 4 resin, NO3 − form), the column is rinsed with 10 mL of 0.2 mol/L NaNO3. The influent and rinse solution were collected and combined for 127IO3 − determination. Iodide on the column was eluted with 5 % NaClO, and the eluate was used for determination of 127I−. 125I in the iodide fraction was measured by gamma spectrometer to monitor chemical recovery of iodide during column separation.
AMS measurement of 129I
The separated AgI–AgCl coprecipitate was dried in an oven at 60–70 °C for 3–6 h, the dried precipitate was ground to fine powder and mixed with five times by mass of niobium powder (325-mesh, Alfa Aesar, Ward Hill, MA), which was finally pressed into a copper holder using a pneumatic press (Zhenjiang Aode Presser Instruments Ltd.). 129I/127I atomic ratios in the prepared targets were measured by AMS using 3MV Tandem AMS system (HVEE) in the Xi’an AMS center. I5+ ions were chosen for the measurement, where 127I5+ was measured as charges (current) using a Faraday cup and 129I5+ was measured using a gas ionization detector. All samples were measured for 6 cycles and 5 min per sample in each cycle. A detailed description of AMS system and measurement of 129I has been reported elsewhere [13].
Measurement of 127I concentration by ICP-MS
1.0 mL sample solution of the iodide fraction and the iodate fraction separated using anion exchange column and the original seawater were taken to a vial, Cs solution (CsNO3) was spiked to a concentration of 2 ng/mL and used as internal standard of ICP-MS measurement, and the samples were diluted for 10 times using 1 % NH4OH solution. 127I in the prepared samples was measured using ICP-MS (X-series II, Thermo Scientific, USA). A detection limit of 0.02 ng/mL for 127I was obtained. Iodide concentration in the samples was corrected for chemical yield during column separation.
Results and discussion
Influence of the amount of 127I carrier on 129I measurement
Figure 3 shows the variation of measured concentration of 129I with the amounts of added 127I carrier in samples. Sample-1 and Sample-2 were collected in open sea of the South China Sea and the Yellow Sea, respectively. They were analyzed after addition of different amount of 127I carrier, as well as using carrier free method as described in the literature. The measured concentration of 129I (13.9 × 106 atoms/L for sample-1 and 38.7 × 106 atoms/L for sample-2) by the carrier free AgI–AgCl coprecipitation method is 1.5–3.5 times higher than that in the target prepared by the carrier addition (0.1–1.0 mg) and AgI–AgCl coprecipitation method, in which concentrations of 129I measured range from 4.08 × 106 atoms/L to 5.53 × 106 atoms/L for sample-1 and 26.1 × 106 atoms/L to 29.7 × 106 atoms/L for sample-2. The difference between the measured 129I concentrations by two methods in the sample-1 is much bigger than that in sample-2, indicating that unreliable measurement results might be obtained when the target was prepared by carrier free method, especially for the low 129I samples.
Figure 4 shows the variation of the measured 127I current with the amounts of 127I carrier added in samples. The intensity of 127I current (4.4 nA) in the sample-2 prepared by carrier free copreciptiation method is only about 5 times higher than that in the blank (0.77 nA). In the AMS facilities using in Xi’an AMS center, the 127I intensity is measured by Faraday cup while 129I is counted by ionization detector. According to the deflection of 127I measuring position by Faraday cup, terminal voltage of AMS can automatically adjust to take 129I5+ through 115° magnet as much as possible before reach to the detector. Therefore, the 129I was adjusted according to the measured 127I intensity, the low iodine concentration in sample causes a higher measurement uncertainty of 127I current, which consequently influence the stability of 129I counts measured in AMS.
For sample-2, addition of 127I carrier significantly enhanced the 127I current intensity by factors of 30–140 to 137–620 nA compared to the carrier free target (4.4 nA). Meanwhile the measurement stability and reliability of 129I can be significantly improved.
The amount of 127I carrier is therefore a key parameter for the measurement accuracy and precision of 129I by AMS. Figure 5 shows variation of the measured 129I/127I ratios with the amount of added 127I carrier in samples. The difference between the 129I/127I ratio in the samples and procedure blank decreases as the amount of 127I carrier added increases. When 1.0 mg 127I carrier was added, the measured 129I/127I value in sample-1 [(0.65 ± 0.02) × 10−12] is close to the procedure blank [(0.26 ± 0.02) × 10−12], causing an increased analytical uncertainty of 129I in the sample after a correction for the blank. Therefore, addition of higher amount of 127I carrier is not suitable for the determination of low level 129I in seawater because of contribution of 129I in the iodine carrier to the sample. When the amount of 127I carrier was reduced to 0.1–0.2 mg, the measured 129I/127I value in the sample-1 is more than one order of magnitude higher than that in the procedure blank, making the measurement results of 129I more reliable. These results reveal that addition of a small amount of carrier (0.1–0.2 mg) can significantly improve the measurement uncertainty in AMS measurement, especially in low level samples.
Reliability of the measurement of 129I and its species in seawater
Many parameters affect the reliability of the measurement of 129I and its species. Besides the addition of 127I carrier presented above, the chemical yield of 129I during separation, procedure blank and instrument background are also important factors for the reliable measurement of low level 129I. The chemical yield of 129I in the overall separation procedure was monitored using 125I which was spiked into the sample at the beginning of the separation; a chemical yield for 125I of more than 80 % was obtained for total 129I and its species, which is sufficient to ensure accurate measurement of 129I in low level samples, such as those collected from the Antarctic.
The procedure blanks were prepared using the same procedure as the sample, and the instrumental background was assessed by directly pressed Nb powder into a target holder. The measurement results including the measured intensity of 127I and 129I signal in these blanks, as well as the standards and IAEA-418 reference materials are presented in Table 2. The measured 129I/127I atomic ratios in the procedure blanks are lower than 4 × 10−13, which are 2–3 orders of magnitude lower than those in the standards and IAEA-418 reference material. Meanwhile it is also more than one order of magnitude lower than the 129I/127I ratios in the seawater samples from the Antarctic (Fig. 6). This low procedure blank level and the high sensitivity of AMS for 129I measurement ensure the reliability of the analytical results of 129I in these low-level samples. However, it can be observed that the intensity of the 129I signals (4.6–5.4 counts/min) in the procedure blanks of seawater is higher than that in the instrumental background [(0.2 ± 0.1) counts/min] by a factor of more than 10. This might be contributed to the existence of tiny amount of 129I in the 127I carrier, which also confirms that it is critical to add suitable amount of 127I carrier in the analysis of low level seawater samples.
The measured 129I/127I ratios in two types of standards prepared by AgI precipitation and AgI–AgCl coprecipitation are (11.3 ± 0.2) × 10−11 and (1.02 ± 0.03) × 10−11, respectively (Table 2), which are in a good agreement (p > 0.05) with the known value of (11.38 ± 0.15) × 10−11 and (0.9954 ± 0.0150) × 10−11, respectively. All these features indicate that the analytical results of low level 129I in the Antarctic seawater samples by the improved method are reliable.
A certified reference material, IAEA-418 (Mediterranean Sea water) was analyzed using the improved method as well as the traditional solvent extraction method, i.e., after addition of iodine carrier and NaHSO3, the pH of solution was adjusted to 1–2 using HNO3 and then after the procedures of solvent extraction and back-extraction iodine was precipitated as AgI and finally measured by AMS. The measured concentrations of 129I and 129I/127I atomic ratios in IAEA-418 (Table 2) by the two methods are in agreement (p > 0.05). The measured 129I concentration in IAEA-418 seawater [(2.71 ± 0.01) × 108 atoms/L for coprecipitation method] also agrees well with the certified value [(2.16–2.73) × 108 atoms/L] [14]. This confirms the reliability of the presented method for 129I in seawater.
Due to lack of certified reference materials of seawater for 129I species, a seawater sample collected from Yellow Sea was analyzed for iodide and iodate using the improved coprecipitation method presented in this work and the conventional anion-exchange chromatography method [2, 15] for control of the analytical quality. The analytical results of 129I concentrations and 129I/127I atomic ratios for total 129I, 129I−, 129IO3 − obtained by the two methods are presented in Table 3. The results show a good agreement between the analytical results for 129I and its species obtained by two methods (p > 0.05). This confirmed that the improved coprecipitation method for speciation analysis of 129I presented here is reliable and suitable for analysis of 129I in seawater. In addition, the sample was analyzed twice (Table 3), and the results show good reproducibility for both 129I concentrations and 129I/127I ratios for both iodide and iodate species (RSD < 5 %). Meanwhile, the cross contamination was also investigated, iodide precipitated in the iodate fraction is less than 3 %, and less than 1 % of iodate is precipitated in the iodide fraction, therefore the cross contamination of iodine species is therefore negligible.
In addition, the improved method presented in this work is less chemicals consuming, and very easy to operate in field compared to the conventional anion-exchange chromatography method for separation of iodine species in seawater [15]. These features make this method suitable for in situ separation of iodine species on board sampling vessels during expedition. In particular, the method is apprciate for the separation of low level 129I from seawater collected in the area far away from nuclear activity such as the Antarctic.
Distribution of 129I and 127I species in seawater in the Antarctic
Seawater samples collected from three depth profiles in the Antarctic water were analyzed for species of 129I using the presented method. Figure 6 shows depth distribution of 129I concentrations and 129I/127I atomic ratios in these three seawater profiles and iodide/iodate molar ratios in one seawater profile. The concentrations of 129I ranged from 0.90 × 106 to 2.40 × 106 atoms/L, with an average of 1.71 × 106 atoms/L, which is significantly lower than that in the Northern Hemisphere (>1.0 × 107 atoms/L) [16]. The 129I/127I atomic ratios range from 3.4 × 10−12 to 9.0 × 10−12, with an average of 6.0 × 10−12. This ratio is 4 times higher than the pre-nuclear level (1.5 × 10−12) in the marine system [17–20], and indicates that anthropogenic 129I has not only reached into the Antarctic surface marine environment [21] but into deeper waters down to 1227 m. The main source of 129I in the investigated area might be attributed to the global fallout of atmospheric nuclear weapons testing [21].
129I concentrations and 129I/127I atomic ratios in profile 2 show a small but visible decreasing trend with increasing depth, which might reflect a downward migration of 129I in the ice shelf zone. However, vertical variation of profile 1 and 3 in the Amundsen Sea Polynya fluctuates smoothly with increase of the depth in some ranges, indicating strong vertical exchange/mixture of the water masses between the surface and the deep water. The trend of profile 2 is similar to that in the Gulf of Mexico and the Makarov Basin, Arctic Ocean, which shows a decrease with the increase of depth and the highest 129I concentration lies near the surface (<100 m) [22, 23]. The trend of 129I level in the profile 1 and 3 is different from that in the profile 2 and most of reported seawater profiles in other locations, where 129I level exponentially decreases with the increase of depth and the highest 129I level lies in subsurface water (<200 m) [24–26]. This indicates that a strong mixing of the water masses between the surface and subsurface layer occurred in this region in the Antarctic.
The iodide/iodate molar ratios of 127I and 129I show an obviously different distribution for 129I compared with 127I in the profile 1 (Fig. 6). The 127I−/127IO3 − values are normally below 0.35 and show a fairly small variation, while the ratios for 129I−/129IO3 − lie at 0.84–4.20, indicating that 129I exists predominantly as iodide. This difference of iodine species between 127I and 129I is likely attributed to the different sources of these two isotopes and the comparatively long time it takes to reach equilibrium between iodide and iodate in the open sea. It is well known that iodine mainly exists as iodate in the open sea, and iodide is formed in the coastal water and surface water by reduction of iodate through biological activities and photochemical and chemical reactions [2, 24]. Profile 1 was collected from an open sea, and the 127I−/127IO3 − ratios are typical values for the open sea. Meanwhile, there are higher concentrations of phosphate, nitrite and lower concentrations of chlorophyll in the whole profile (Table 1), indicating the relatively weak biological activity. Therefore, the 129I species should be controlled by the source water. The higher 129I−/129IO3 − values in the deep sea might reflect strong vertical mixture of water masses and originate from the upwelling of circumpolar deep water [27] that carried the higher 129I−/129IO3 − values. These results imply that the chemical speciation analysis of 129I can be used to investigate the mixing and circulation of the water masses.
Conclusions
Based on the results and discussion above, it can be concluded: (1) addition of a small amount of 127I carrier (0.1–0.2 mg) remarkably improved the accuracy and precision of 129I measurement; (2) Three seawater profiles collected from the Antarctic in 2011 were successfully analyzed utilizing the improved method; (3) The results show that anthropogenic 129I has reached into the Antarctic deep water down to 1227 m and its main source might be the global fallout of atmospheric nuclear weapons testing; (4) Depth distribution of 129I and its speciation indicate that a strong mixing of the water masses between the surface and subsurface layer occurred in the Antarctic. This is just a preliminary investigation for the dispersion and mixing of water in the Antarctic and a comprehensive investigation using 129I will provide detailed information on the water circulation in this region.
References
Wong GTF (1991) The Marine geochemistry of iodine. Rev Aqua Sci 4:45–73
Hou XL, Aldahan A, Nielsen SP, Possnert G, Nies H, Hedfors J (2007) Speciation of 129I and 127I in seawater and implications for sources and transport pathways in the North Sea. Environ Sci Technol 41:5993–5999
Hou XL, Hansen V, Aldahan A, Possnert G, Lind OC, Lujaniene G (2009) A review on speciation of iodine-129 in the environmental and biological samples. Anal Chim Acta 632:181–196
Raisbeck GM, Yiou F (1999) 129I in the oceans: origins and applications. Sci Total Environ 237–238:31–41
Nishiizumi K, Elmore D, Honda M, Arnold JR, Gove HE (1983) Measurements of 129I in meteorites and lunar rock by tandem accelerator mass spectrometry. Nature 305:611–612
Rao U, Fehn U (1999) Sources and reservoirs of anthropogenic iodine-129 in western New York. Geochim Cosmochim Acta 63:1927–1938
Luo MY, Hou XL, He CH, Liu Q, Fan YK (2013) Speciation analysis of 129I in seawater by carrier-free AgI–AgCl coprecipitation and accelerator mass spectrometric measurement. Anal Chim 85:3715–3722
Fehn U, Snyder G, Egeberg PK (2000) Dating of pore waters with 129I: relevance for the origin of marine gas hydrates. Science 289:2332–2335
Keogh SM, Aldahan A, Possnert G, Finegan P, Vintro LL, Mitchell PI (2007) Trends in the spatial and temporal distribution of 129I and 99Tc in coastal waters surrounding Ireland using fucus vesiculosus as a bio-indicator. J Environ Radioact 95:23–38
Reithmeier H, Lazarev V, Rühm W, Schwikowski TM, Gäggeler HW, Nolte E (2006) Estimate of European 129I releases supported by 129I analysis in an Alpine ice core. Environ Sci Technol 40:5891–5896
Santschi PH, Schwehr KA (2004) 129I/127I as a new environmental tracer or geochronometer for biogeochemical or hydrodynamic processes in the hydrosphere and geosphere: the central role of organo-iodine. Sci Total Environ 321:257–271
Hou XL, Zhou WJ, Chen N, Zhang LY, Liu Q, Luo MY, Fan YK, Liang W, Fu YC (2010) Determination of ultralow level 129I/127I in natural samples by separation of microgram carrier free iodine and accelerator mass spectrometry detection. Anal Chim 82:7713–7721
Zhou W, Hou X, Chen N (2010) Preliminary study of radioisotope 129I application in china using Xi’an accelerator mass spectrometer. INCS News 25:8–23
Pham MK, Betti M, Povinec PP, Alfimov V, Biddulph D, Gastaud J, Kieser WE, Lopez Gutierrez JM, Possnert G, Sanchez-Cabeza JA et al (2010) Certified reference material IAEA-418: 129I in Mediterranean Sea water. J Radioanal Nucl Chem 286:121–127
Hou XL, Dahlgaard H, Nielsen SP (2001) Chemical speciation analysis of 129I in seawater and a preliminary investigation to use it as a tracer for geochemical cycle study of stable iodine. Mar Chem 74:145–155
Snyder G, Aldahan A, Possnert G (2010) Global distribution and long-term fate of anthropogenic 129I in marine and surface water reservoirs. Geochem Geophys Geosyst 11:1–19
Fehn U, Moran JE, Snyder GT, Muramatsu Y (2007) The initial 129I/I ratio and the presence of ‘old’ iodine in continental margins. Nucl Instrum Methods Phys Res Sect B 259:496–502
Fehn U, Snyder G (2000) 129I in the Southern Hemisphere: global redistribution of an anthropogenic isotope. Nucl Instrum Methods Phys Res Sect B 172:366–371
Moran JE, Fehn U, Hanor JS (1995) Determination of source ages and migration patterns of brines from the U.S. gulf coast basin using 129I. Geochim Cosmochim Acta 59:5055–5069
Moran JE, Fehn U, Teng RTD (1998) Variations in 129I/127I ratios in recent marine sediments: evidence for a fossil organic component. Chem Geol 152:193–203
Xing S, Hou XL, Aldahan A, Possnert G, Shi KL, Yi P, Zhou WJ (2015) Iodine-129 in snow and seawater in the Antarctic: level and source. Environ Sci Technol 49:6691–6700
Alfimov V, Aldahan A, Possnert G, Winsor P (2004) Anthropogenic iodine-129 in seawater along a transect from the norwegian coastal current to the North Pole. Mar Pollut Bull 49:1097–1104
Schink DR, Santschi PH, Corapcioglu O, Sharma P, Fehn U (1995) 129I in Gulf of Mexico waters. Earth Planet Sci Lett 135:131–138
Hou XL, Povinec PP, Zhang LY, Shi KL, Biddulph D, Chang C-C, Fan YK, Golser R, Hou YK, Ješkovský M et al (2013) Iodine-129 in seawater offshore Fukushima: distribution, inorganic speciation, sources, and budget. Environ Sci Technol 47:3091–3098
Smith JN, Ellis KM, Kilius LR (1998) 129I and 137Cs tracer measurements in the Arctic Ocean. Deep Sea Res I 45:959–984
Suzuki T, Kabuto S, Amano H, Togawa O (2008) Measurement of iodine-129 in seawater samples collected from the Japan Sea area using accelerator mass spectrometry: contribution of nuclear fuel reprocessing plants. Quat Geochronol 3:268–275
Yabuki T, Suga T, Hanawa K, Matsuoka K, Kiwada H, Watanabe T (2006) Possible source of the antarctic bottom water in the Prydz Bay region. J Oceanogr 62:649–655
Acknowledgments
Financial supports from the Ministry of Science and Technology of China (2015FY110800), as well as State Key Laboratory of Loess and Quaternary Geology are gratefully acknowledged. S. Xing thanks Dr. Qi Liu in Xi’an AMS center for his help in AMS measurement of 129I. The sampling program was done as part of 2010/2011 Antarctica two-ship expedition through the international scientific cruise jointly funded by the Swedish Polar Research Secretariat and the US National Science Foundation (NSF). We thank the N.P. Palmar scientific team for providing the onlone measurement data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xing, S., Hou, X., Aldahan, A. et al. Speciation analysis of 129I in seawater using coprecipitation and accelerator mass spectrometry and its applications. J Radioanal Nucl Chem 311, 833–841 (2017). https://doi.org/10.1007/s10967-016-5060-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-016-5060-6