Abstract
Subterranean radioiodine contamination at the Hanford Site in Washington State is believed to be present as iodide, iodate, and organo-I species, with iodate being the predominant form. Because these species have different sediment-sorption characteristics, understanding their distribution is important for developing an accurate understanding of iodine migration in the subsurface. Herein, we report a novel, rapid technique for simultaneous iodine speciation (iodide/iodate) and isotopic ratio (129I/127I) measurements using ion chromatography (IC) joined with collision/reaction cell inductively coupled plasma mass spectrometry (ICP-MS), collectively referred to as IC-ICP-MS. This approach employs online dynamically regenerated eluent suppression post chromatographic separation of the samples and collision cell technology, with pure oxygen as a collision gas for the active suppression of 129Xe (which naturally exists in the argon supplied to the ICP source) to rapidly (< 15 min) achieve precise and reproducible results. Speciated standard reference materials yielded detection limits for 127I of approximately 23.8 ng/L for iodate and 24.3 ng/L for iodide, and for 129I of approximately 1.81 ng/L for iodate and 2.62 ng/L for iodide. The method was demonstrated by analyzing groundwater samples from six wells from 129I-contaminated regions of the Hanford Site; iodate was the primary species for both 127I and 129I. Small quantities of 127I-iodide were also detected in most of the samples, but all 129I-iodide results were below the detection limit. An interference from molybdenum prevented the estimation of organo-iodine concentrations but did not affect the iodate and iodide results. This new analytical capability will enable rapid, simultaneous characterization of speciated inorganic iodine in vadose zone sediments and groundwater samples at levels below the US federal drinking water standard for 129I of 1 pCi/L (~ 5.6 ng/L).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Long-lived 129I (t1/2 = 15.7 million years) is a highly mobile, radioactive isotope of iodine and a contaminant of interest in the vadose zone and groundwater at many nuclear facilities [1]. Radioiodine is a significant health risk due to the bioaccumulation factor of iodine in the thyroid gland, which can result in thyroid cancer, as has been epidemiologically confirmed after the Chernobyl accident [2, 3]. The Safe Drinking Water Act of 1974, mandated by the Environmental Protection Agency, established maximum contaminant levels for beta emitters, such as 129I, which is currently regulated at 10 pCi/L (≈ 56 ng/L). The US Department of Energy (US DOE) set an even lower limit of 1 pCi/L (≈ 5.6 ng/L) based on a different dose conversion factor derived from the International Council on Radiation Protection Publication 30 [4], which is the lowest among all radionuclides in the Federal Register.
At the US DOE’s Hanford Site in southeastern Washington State, large quantities of iodine isotopes, including 131I and 129I, were formed as a byproduct of nuclear fission in the site’s nine reactors. The short-lived 131I (t1/2 = 8 days) released from the fuel to the atmosphere in the 1940s is no longer a public health concern due to radiological decay over time [5]. However, the long-lived 129I remains present in several large, dilute groundwater plumes resulting from production operation wastes that were discharged to the soil and subsurface [6]. These dilute 129I groundwater plumes encompass an area of over 50 km2 in the Hanford Central Plateau (Fig. 1). The highest groundwater concentrations (up to 22.8 pCi/L [≈ 128 ng/L]) have been measured in samples from the 200 West Area [6], while levels up to approximately 10 pCi/L (≈ 56 ng/L) have been observed in samples from the 200 East Area (Fig. 1) [7].
a Map of 129I groundwater contamination at the Hanford Site, located along the Columbia River near Richland, WA. The red dashed box denotes the sampling area for this study, as detailed in (b). b Well locations for groundwater iodine speciation measurements sampled in 2019–2020 by CH2M Hill Plateau Remediation Company for this study. Light blue shading denotes activity in solution between 1 and 10 pCi/L (5.6 ng/L and 56 ng/L). Dark blue denotes activity levels > 10pCi/L (> 56 ng/L). (Color figure online)
Radioiodine (129I) fate and transport in both the vadose zone and groundwater is dependent on speciation due to differences in co-precipitation and sorption mechanisms for the various forms of iodine [8]. Therefore, identifying the species distribution is important for understanding subsurface radioiodine migration. At Hanford, the radioiodine is believed to be present as iodate (IO3−), iodide (I−), and organo-iodine. Iodate is presumed to be the major species based on a limited characterization study [9]. In contrast, at the Savannah River Site in South Carolina, radioiodine is primarily in the form of iodide near the source term (F-Area Seepage Basins), but as the 129I migrates away from the source, the distribution includes more iodate and organo-iodine [10,11,12]. Due to the biogeochemical differences of each iodine species, speciation data can also be used to support the selection of optimal remediation strategies for iodine.
Presently, there exists no established analytical method for the rapid, direct determination of speciated 129I/127I analyses of natural samples at or below the federal drinking water standard. Due to an isobaric interference of 129Xe (present in quantifiable amounts within the source argon (Ar) supply) on 129I, inductively coupled plasma mass spectrometry (ICP-MS) is an analytical platform generally ill-equipped to analyze low-level perturbations of 129I/127I ratios. Because of this and the low levels at which 129I must be quantified in groundwater, stable iodine (127I) has been used as a proxy in past geochemical investigations of radioiodine fate and transport ([6, 8]; and references therein). However, because 127I and 129I have different sources, and their complex biogeochemical environments may not be in thermodynamic equilibrium, the species distributions may differ for the two isotopes. Therefore, independent 127I and 129I speciation measurements are required for the most accurate understanding of iodine speciation. When equipped with collision cell technology, ICP-MS has excellent potential to suppress isobaric interferences to manageable levels in order to discern deviations of 129I/127I in natural samples.
In this paper, we present a new, simple method using ion chromatography (IC) coupled to collision cell ICP-MS (IC-ICP-MS) that enables the rapid and simultaneous species separation and quantification of 129I/127I ratios in iodate and iodide in environmental samples at levels below the 129I drinking water standard. Other researchers have described similar methods of iodine speciation analysis [13,14,15,16,17]; however, none have achieved the high resolution, particularly with respect to speciated 129I, within natural samples as is demonstrated in this study. Moreover, the method reported here is unique in that samples require no pre-treatment, as has been discussed by other authors (e.g., [18]). This method may be readily adapted to analyze a wide variety of sample types at concentrations higher than what is required for the current application. Furthermore, the small sample volume (100 μL) needed for speciation analysis is considerably less than that required by traditional chemical speciation separation methods [19] while maintaining high precision at low concentrations. This novel method was tested on a small set of groundwater samples from the Hanford Site.
Experimental
Instrumentation
A Thermo Scientific iCAP RQ™ ICP-MS operating in collision cell (CCT) mode was coupled to a Thermo Scientific Dionex Ion Chromatography System (ICS6000) for iodine speciation measurements (Table 1). This coupled analytical platform is also referred to as an IC-ICP-MS (Fig. 2). The system uses high-pressure ion chromatography (> 2000 psi) to separate chemical anionic species prior to injection into the ICP-MS for isotopic analysis. Background signals for masses 127 and 129 in CCT mode were approximately 1500 cps and 200–400 cps on average, respectively, for the suppressed eluent [50 mM, potassium hydroxide (KOH)] being delivered by the IC to the ICP-MS. By contrast, backgrounds for masses 127 and 129 in standard operation mode yielded intensities of approximately 1000–2000 cps and 10,000–20,000 cps on average, respectively. The inflation of background signal of mass 129 in standard operation mode results from the xenon (Xe) isotope (129Xe) contained within the Ar tank supply to the ICP-MS, and this signal can fluctuate on a day-to-day basis, depending on the volume of Ar remaining in the tank. In CCT mode, research grade oxygen (> 99.998% purity) is used to eliminate > 99% of 129Xe from the Ar supply feed by a charge-exchange reaction, allowing for high precision analysis of 129I, effectively eliminating the daily fluctuations with the 129Xe isobaric interference. To ensure that Xe has been effectively removed from the analysis and the contribution to mass 129 is solely from iodine, 131Xe is also monitored during all analytical sessions.
The ICS6000 hosts an auto-sampler, injection line system, eluent generator, and chromatographic columns for separation (Fig. 2). Prior to sample injection, the in-line pumps are primed and set at a flow rate of 300 μL/min and left to stabilize for at least 1 h, and the column compartment temperature is set and left to stabilize at 40 °C. The sample is then loaded into an autosampler, and 100 µL is injected by a 250 μL syringe onto a Thermo Scientific Dionex AG-19 IonPac guard column (4 μm 2 × 50 mm) followed by loading onto a Thermo Scientific Dionex AS-19 IonPac analytical column (4 μm 2 × 250 mm) (Fig. 2). The AS-19 is a high capacity, hydroxide selective anion exchange column. In-line electrolytic suppression, with the current set to 38 mA, is used to remove the 50 mM KOH from the sample eluent prior to passing through the conductivity detector cell and direct nebulization into the ICP-MS, rendering the carrier solution as water. A bypass of the normal function of the online Anion Electrolytically Regenerated Suppressor (AERS) of the ICS6000 was required due to the diversion of the sample containing effluent to the ICP-MS for trace level iodine analysis. To mitigate the loss of necessary membrane hydration (which typically occurs by recycling the electrolytically suppressed sample solution), an external regenerator (regen) pump supply of water was installed to regenerate the AERS membrane (Fig. 2). This external pump water supply flow is maintained at a level ~ 20% higher than the flow rate of the internal pump (360 μL/min) on the ICS6000 to maintain a slight positive back pressure on the system (Table 1). Following chromatographic separation of the desired anionic species, the sample is delivered to the ICP-MS and analyzed for isotopics using a time-resolved analytical method, with a total analysis time of < 15 min. Peak retention times of the desired anionic species are mapped using pure reference materials, and the peak areas of each separated species are integrated, compared to certified reference materials, and converted into concentrations reported as ng/L (Figs. 3, 4). Standard reference materials are detailed in the following section.
Speciated 127I calibration using High Purity Standards iodate and iodide. a–b 20 and 1000 ng/L calibration standards. c–d Species specific calibration curves. Typical calibration range for 127I speciation: 20–1000 ng/L with detection limits for iodate and iodide 127 reported as 23.8 ng/L and 24.3 ng/L, respectively
Speciated 129I calibration with Amersham RS-218 iodide. Iodate was quantitatively oxidized from the certified iodide standard (see main text for details). a–b 2 and 100 ng/L calibration standards. c–d Species specific calibration curves. Typical calibration range for 129I speciation: 20–100 ng/L with detection limits for iodate and iodide 129 reported as 1.81 ng/L and 2.62 ng/L, respectively. Note the presence of 127I in the Amersham 129I standard, due to this impurity, both 127I and 129I are calibrated independently of each other
Sample and standard preparation
Preparation of iodate-129 standards
Both 127IO3− and 127I− are commercially available for purchase and were used in this study for calibration standards (High Purity Standards) and independent calibration verification standards (iodate: SPEX CertiPrep and iodide: Inorganic Ventures). Two commercially available 129I− standard solutions were also used in this study for calibration (Amersham RS-218) and independent calibration verification standards (NIST SRM 4949). There are no commercial entities that currently produce 129IO3− standard reference materials. As such, this study required the oxidation of commercially available 129I− standards, with quantitative recovery. It has been shown previously that I− can be quantitatively oxidized to IO3− using sodium hypochlorite (NaClO) at pH ≥ 8 (to avoid oxidative loss via volatilization of I2), followed by being scrubbed of excess oxidant using sodium sulfite (Na2SO3). This procedure was based on a method described in Takayanagi and Wong [20].
To achieve quantitative oxidation, a volume of 10 mL containing 100 ng/mL 127I− (High Purity Standards) suspended in 0.1 mM NaOH was treated with 0.053 mL of 0.5% (vol) NaClO, allowed to react for 30 min at room temperature, and then treated with 0.053 mL of 500 mM Na2SO3. Fresh reagents were mixed the same day of the oxidation experiment. This 100 ng/mL solution served as the stock for the following analysis. An independent 100 ng/mL 127IO3− (High Purity Standards) solution was also prepared for comparison. All three of the 127I standard solutions, including the newly oxidized 127IO3−, stock 127I−, and stock 127IO3− were diluted to 1 ng/mL, suspended in 0.01% triethylamine (Inorganic Ventures) to stabilize the iodine species in solution, and analyzed using IC-ICP-MS to compare peak retention times and peak areas. Additionally, the newly oxidized 127IO3−, and stock 127I− standards were mixed in equal proportions in order to assess the 127IO3−/127I− peak ratios and confirm unity (≈ 1 ± 0.02).
Following the confirmation of quantitative oxidation of the 127I− standard, the same method was applied to the 129I− standard solutions (Amersham RS-218 and NIST SRM 4949). Both 129I− standard solutions were treated with the previously described quantitative oxidation method in order to create 129IO3− 100 ng/mL standard stock solutions. Post standard preparation, the IO3− solutions were stored in the dark to avoid photodegradation.
Preparation of samples
Groundwater samples were collected from six monitoring wells located within 129I plume areas in the Central Plateau of the Hanford Site (Fig. 1). The sample solutions were kept in cold storage until analysis. Approximately a 0.5–1 mL aliquot from each sample was added to a 1.5 mL glass syringe vial and loaded into the IC autosampler. For analysis, 100 μL was pulled from each sample, injected into the ICS6000 for chromatographic speciation, and directly fed into the ICP-MS for trace iodine analysis, the details of which were described in “Instrumentation” section.
Results
The IC-ICP-MS analytical technique demonstrated well-resolved separation of IO3− and I− peaks with peak retention times spaced > 250 s apart. Speciated standard reference materials yielded detection limits of approximately 23.8 ng/L for 127IO3− and 24.3 ng/L for 127I−. For 129I, the detection limits were approximately 1.81 ng/L for 129IO3− and 2.62 ng/L for 129I−. These levels are well below the federal drinking water standard for 129I and were obtained within a 15-min time frame, using a small sample volume of only 100 μL (Table 2). Standards produced reliable and reproducible calibrated results for both 127I and 129I species, with retention times for the IO3− and I− peaks separated by several minutes (Figs. 3, 4). Analytical reproducibility was demonstrated by repeated injection of the independent calibration verification standards (described in “Preparation of iodate-129 standards” section) over the course of this work at concentrations of 200 ng/L for the 127I species and 20 ng/L for the 129I species. These injections yielded values for 127IO3− and 127I− of 202 ± 20 ng/L (2σ, n = 10) and 206 ± 20 ng/L (2σ, n = 10), respectively. For 129IO3− and 129I− results yielded 20.2 ± 1.8 ng/L (2σ, n = 10) and 18.5 ± 1.8 ng/L (2σ, n = 10), respectively. However, analytical methods must demonstrate proficiency when dealing with real samples in order to truly validate performance.
Seven Hanford groundwater samples from wells in 129I-contaminated regions of the 200 East and 200 West Areas (Fig. 1) were selected for 127I and 129I iodine speciation analysis for this study. Splits of the samples had been previously analyzed for total radioiodine concentrations by General Engineering Laboratories (GEL; Charleston, SC). In the GEL method, sodium bisulfite (NaHSO3) is added to an aqueous sample to reduce the total iodine in solution to iodide, and the resulting solution is passed through an anion exchange column to separate and concentrate the iodide. Radioiodine on the resin is then determined by low energy gamma counting (with a detection limit of ~ 6 ng/L). The IC-ICP-MS results demonstrated that nearly all groundwater samples contained 127I in both the iodate (2800–13,500 ng/L) and iodide (28–328 ng/L) species, with the majority existing as iodate. Two samples contained 127I iodide at levels below the detection limit. For 129I, iodate was the only species detected; concentrations ranged from 31 to 130 ng/L (Table 2). The total radioiodine concentration (reported by GEL) showed excellent agreement (average 10% difference) with the 129I-iodate concentration as determined by IC-ICP-MS, which provides an independent validation of the IC-ICP-MS analytical method (Table 2). Observations during chromatographic separation demonstrated a potential issue with molybdenum (Mo) interfering with the analysis; this is discussed in detail in the following section.
Discussion
Molybdenum interference
Molybdenum anionic species within the groundwater samples at concentrations of several ng/L resulted in phantom 127 and 129 peaks in the ion chromatograms (Fig. 5). Injection tests of groundwater samples into the IC-ICP-MS in both standard mode and CCT while monitoring all stable isotopes of Mo (including 95Mo and 97Mo at natural abundances of 15.9% and 9.6%, respectively) demonstrate that the oxygen in the collision cell of the ICP-MS creates 95MoO2 (mass 127) and 97MoO2 (mass 129) during the analysis, resulting in false iodine peaks at a retention time between the iodate and iodide peaks (Fig. 5). Fortunately, the anionic Mo species in the sample solution poses no direct interference with the iodate and iodide peak recovery as it conveniently elutes between the two iodine species peaks (Figs. 5, 6). Unfortunately, due to this interference, total 129I cannot be quantified in samples containing Mo concentrations at high Mo/129I ratios (> 20). An attempt was made to develop a mathematical correction for this interference based on the measured response of iodine standards spiked with known amounts of Mo, using the raw intensity of 129I and the % abundances of 97MoO2 and 100MoO2. The correction was effective for single element standards, but it was found to be inadequate for correcting sample 129I concentrations due to the high Mo concentrations and the interactions of other matrix analytes. Molybdenum concentrations in Hanford groundwater are variable, but typical concentrations in the Central Plateau are 2–10 µg/L, which results in a Mo/129I ratio that is significantly higher than our correction could handle. A sample cleanup step that removes Mo would overcome this issue, but such a process would need to be developed for routine use.
Test injection of Hanford groundwater sampled from well 299-W21-3. a Analyzed in standard (STD) mode and b analyzed in collision cell (CCT) mode. All other instrument parameters remain the same. Note the peaks for each mass of molybdenum appearing in STD mode. In CCT mode, the molybdenum peaks diminish and masses 127 and 129 appear in their place, resulting from the reaction of 95Mo and 97Mo with oxygen forming 95,97MoO2 in the collision cell. Fortunately, the instrument generated 127 and 129 peaks do not overlap with iodate or iodide. Molybdate peak retention times are shifted from ~ 450 s (Fig. 6) to 300 s due to the aging of the column. Both guard and analytical columns were replaced and retention times re-mapped prior to analyzing the samples reported in Table 2
Stable and radioiodine speciation in Hanford groundwaters
The first definitive report of stable and radioactive iodine species in Hanford groundwater was published by Zhang et al. [9]. Samples from seven wells in the 200 West Area (Fig. 1) were analyzed by gas chromatography-mass spectrometry (GC–MS) after derivatization to 4-iodo-N,N-dimethylaniline. This methodology enabled the determination of iodide, iodate, and organo-iodine (the latter was calculated from the difference of a total iodine measurement minus the iodate and iodide fractions). Detection limits for both 129I iodide and 129I iodate were 10 ng/L and 14 ng/L (approximately two times higher than the drinking water standard). The new IC-ICP-MS method presented in this study has lower detection limits by a factor of 2–5, requires fewer manual sample manipulations, and requires less sample volume (100 μL). However, the Mo interference discussed previously limits the new method’s ability to quantify organo-iodine by difference from total 129I.
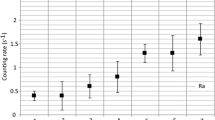
Despite limitations in estimating total iodine concentrations, inorganic iodine species are easily quantified using IC-ICP-MS. The method was demonstrated through the analysis of samples from the Hanford 200 West and 200 East Area sites (Fig. 1). In the 200 West Area, well 299-W21-3 (Fig. 1), which has had the highest radioiodine concentrations in the Central Plateau since the well was completed in 2016, was sampled in duplicate (collected at the same time from same well) and analyzed by IC-ICP-MS (Fig. 6, Table 2). Similar to the trends from Zhang et al. [9], iodate was the primary species for both 127I and 129I, the ratio of 129I-iodate/127I-iodate was 0.0096, and no 129I iodide was detected (Fig. 6). Three other studies on 200 West Area groundwater samples provide further evidence that iodate is the predominant form of iodine [6, 21, 22]. However, the speciation information in these latter studies is for 127I only.
In contrast to the 200 West Area, groundwater from the 200 East Area has not been as extensively characterized. Sample results from the five wells analyzed in this study demonstrate similar trends to the 200 West Area. Again, the predominant form of 129I in the groundwater of the 200 East Area is iodate. Iodate 129I concentrations agree very well with the total 129I concentrations as reported by GEL (Table 2). Future analysis of groundwater from wells spatially distributed throughout the 200 East and West Areas using IC-ICP-MS would provide a more complete picture of iodine speciation at the Hanford Site. Coupled with analyses of sediment extracts, these data would help validate fate and transport models that predict plume behavior and serve as the basis for 129I remedy decisions.
Conclusion
A relatively fast (< 15 min), simple, and sensitive method for the low-level determination of both 127I and 129I as speciated iodate and iodide in groundwaters has been developed using only 100 μL of sample. The IC-ICP-MS detection limits for 129I in iodate and iodide species were 1.81 and 2.62 ng/L (0.32–0.47 pCi/L), respectively. Analysis of Hanford groundwater samples yielded iodate as the predominant species for 129I, quantified at values ranging from 31 to 130 ng/L (5.54–23.2 pCi/L), and closely matching total 129I concentrations reported by a radiochemistry method. These results hold promise for the further quantification and speciation of 129I contaminated waters at and below the drinking water standard (5.6 ng/L), with the added potential for isotopic species mapping that could lead to more finely tuned fate and transport studies and informed remediation decisions.
References
Kaplan DI, Denham ME, Zhang S, Yeager C, Xu C et al (2014) Radioiodine biogeochemistry and prevalence in groundwater. Crit Rev Environ Sci Technol 44:2287–2335. https://doi.org/10.1080/10643389.2013.828273
Brenner AV, Tronko MD, Hatch M, Bogdanova TI, Oliynik VA et al (2011) I-131 Dose response for incident thyroid cancers in Ukraine related to the Chernobyl accident. Environ Health Perspect. https://doi.org/10.1289/ehp.1002674
Ivanov VK, Tsyb AF, Khait SE, Kashcheev VV, Chekin SY et al (2012) Leukemia incidence in the Russian cohort of Chernobyl emergency workers. Rad Environ Biophys. https://doi.org/10.1007/s00411-011-0400-y
International Council on Radiation Protection (1979) Annals of the ICRP, limits for intakes of radionuclides by workers (Ed. Sowby FD). Pergamon Press, New York. Part 1, Publication 30:2(3–4)
Kantelo MV, Bauer LR, Marter WL, Murphy Jr CE, Zeigler CC (1993) Radioiodine in the Savannah River Site environment. United States. https://doi.org/10.2172/10122501
Truex MJ, Lee BD, Johnson CD, Qafoku NP, Szecsody JE et al (2017) Conceptual model of iodine behavior in the subsurface at the Hanford Site, PNNL-24709, Pacific Northwest National Laboratory, Richland, WA. https://doi.org/10.2172/1224515
DOE/RL-2018-66 (2019) Hanford Site Groundwater Monitoring Report for 2018, Rev. 0, US Department of Energy, Richland Operations Office, Richland, WA
Neeway JJ, Kaplan DI, Bagwell CE, Rockhold ML, Szecsody JE et al (2019) A review of the behavior of radioiodine in the subsurface at two DOE sites. Sci Tot Environ. https://doi.org/10.1016/j.scitotenv.2019.07.146
Zhang S, Xu C, Creely D, Ho Y-F, Li H-P et al (2013) Iodine-129 and Iodine-127 speciation in groundwater at the Hanford Site, U.S: Iodate Incorporation into Calcite. Environ Sci Technol 47:9635–9642
Otosaka S, Schwehr KA, Kaplan DI, Roberts KA, Zhang S et al (2011) Factors controlling mobility of 127I and 129I species in an acidic groundwater plume at the Savannah River Site. Radiochim Acta. https://doi.org/10.1016/j.scitotenv.2011.05.018
Kaplan DI, Roberts KA, Schwehr KA, Lilley MS, Brinkmeyer R et al (2011) Evaluation of a radioiodine plume increasing in concentration at the Savannah River Site. Environ Sci Tech. 45:489–495
Kaplan DI, Zhang S, Roberts KA, Schwehr K, Xu C et al (2014) Radioiodine concentrated in a wetland. J Environ Radioact 131:57–61
Stärk H-J, Mattusch J, Wennrich R, Mroczek A (1997) Investigation of the IC-ICP-MS determination of iodine species with reference to sample digestion procedures. Fresenius J Anal Chem 359:371–374
Yoshida S, Muramatsu Y, Katou S, Sekimoto H (2007) Determination of the chemical forms of iodine with IC-ICP-MS and its application to environmental samples. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-007-0738-4
Zhang LY, Hou XL (2013) Speciation analysis of 129I and its applications in environmental research. Radiochim Acta. https://doi.org/10.1524/ract.2013.2077
Takeda A, Tsukada H, Takaku Y, Satta N, Baba M et al (2016) Determination of iodide, iodate and total iodine in natural water samples by HPLC with amperometric and spectrophotometric detection, and off-line UV irradiation. Anal Sci. https://doi.org/10.2116/analsci.32.839
Humphrey OS, Young SD, Crout NMJ, Bailey E, Ander EL et al (2020) Short-term iodine dynamics in soil solution. Environ Sci Technol. https://doi.org/10.1021/acs.est.9b02296
Hou X (2019) Radioanalysis of ultra-low level radionuclides for environmental tracer studies and decommissioning of nuclear facilities. J Radioanal Nucl Chem 322:1217–1245. https://doi.org/10.1007/s10967-019-06908-9
U.S. EPA, EMSL (1980) Method 901.1: Gamma emitting radionuclides in drinking water. Prescribed procedures for measurement of radioactivity in drinking water, EPA/600/4/80/032
Takayanagi K, Wong GTF (1986) The oxidation of iodide to iodate for the polarographic determination of total iodine in natural waters. Talanta 33(5):451–454
Xu C, Kaplan DI, Zhang S, Athon M, Ho Y-F et al (2015) Radioiodine sorption/desorption and speciation transformation by subsurface sediments from the Hanford Site. J Environ Radioact 139:43–55
Lee BD, Szecsody JE, Qafoku NP, McElroy EM, Baum SR et al (2017) Contaminant attenuation and transport characterization of 200-UP-1 operable unit sediment samples, PNNL-26894, Pacific Northwest National Laboratory, Richland, WA
Acknowledgements
This research was conducted as part of the Deep Vadose Zone—Applied Field Research Initiative at the Pacific Northwest National Laboratory (PNNL). Funding for this work was provided by the US Department of Energy (DOE) Richland Operations Office. PNNL is operated by Battelle Memorial Institute for the DOE under Contract DE-AC05-6RL01830. We thank Andrew Plymale for help with standard preparation and Michelle Snyder for assistance with sample preparation, Steve Shen for help with the interference corrections, as well as Hilary Emerson, James Szecsody, and Eirik Krogstad for discussions pertaining to iodine in natural samples that led to improvements of this work. Finally, we gratefully acknowledge the anonymous reviewer whose comments helped improve the quality of this manuscript.
Funding
This study was funded by the US Department of Energy (DOE) Richland Operations Office. PNNL is operated by Battelle Memorial Institute for the DOE under Contract DE-AC05-6RL01830.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kimmig, S.R., Thompson, C., Baum, S. et al. Evaluation of iodine speciation and 129I/127I ratios at low concentrations in environmental samples using IC-ICP-MS. J Radioanal Nucl Chem 327, 929–937 (2021). https://doi.org/10.1007/s10967-020-07537-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07537-3